
Editorial
Austin J Biotechnol Bioeng. 2015;2(3): 1047.
Streamlining the Drug Discovery Process through Repurposing of Clinically Approved Drugs
Arnish Chakraborty and Vishal Trivedi*
Department of Biosciences & Bioengineering, Indian Institute of Technology-Guwahati, India
*Corresponding author: Vishal Trivedi, Malaria Research Group, Department of Biosciences and Bioengineering, Indian Institute of Technology-Guwahati, Guwahati-781039, Assam, India
Received: July 20, 2015; Accepted: July 22, 2015; Published: July 24, 2015
Editorial
Drug repurposing (also known as Drug repositioning) is an approach to drug design and development where known compounds are assigned to new indications. In this process one starts from already existing clinical drugs and assigns a new therapeutic target to the molecule. This approach is quickly gaining popularity both in industry as well as in academia as it banks upon the initial knowledge and investment which brought the drug to the market at the first instance. The major bottleneck of de novo drug development is that almost 90% of the identified novel molecules fail the clinical trials, resulting in the rise of the overall pharmaceutical R&D cost. The repurposing strategy helps to overcome such barriers. There are notable advantages of this approach over the traditional drug discovery process (Figure 1). Firstly, the repurposed drugs have already undergone clinical trials in the past and as a result their safety is ensured. Secondly, the repurposing strategy is cheap and takes much lesser time to develop a drug. Several pharmaceutical companies therefore endorse this low-risk repositioning strategy to improve their profits.
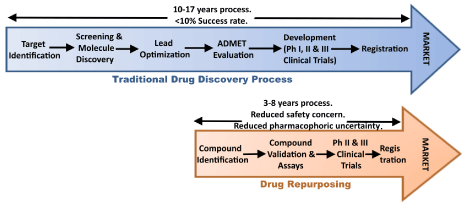
Figure 1: Schematic representation of the steps involved in Traditional drug
discovery process vs. Drug repurposing with the salient features of both the
processes.
The Drug Repurposing Work Flow
The starting materials for a repurposing process are drug molecules which (a) have been approved for clinical use (b) have passed safety trial (Phase I) but failed to demonstrate efficacy (Phase II) for a disease (c) have been replaced by better therapeutics and (d) have become generic due to patent expiry [1]. The drug repurposing process can be broadly classified into the following two strategies: (i) Existing compound-novel target approach: It is based on the observation that many drugs bind to multiple targets. These secondary targets could be related to a different disease or physiological condition and (ii) Known mechanism-new disease: It is based on the observation that several biological processes and signaling pathways are relevant for more than one disease and hence the same drug which inhibits a biological process can exert effect on two different disease states [1]. Once a secondary target has been assigned to a drug, proof-ofconcept experimentation has to be performed to study the effect of the drug on the newly identified target. Computational biology, chemical biology, in vitro/cell-based assays and in vivo analysis are frequently used to validate the repositioning. Upon validation of the hypothesis, the drug can leap directly into the phase II and III clinical trials. Moreover the availability of previous clinical and pharmacokinetic data along with the knowledge of the range of viable dose for that particular drug substantially reduces the risks associated with the further development of the molecule.
Case Studies
One of the most promising instances of a repurposed drug is Zidovudine, which was originally designed for cancer in 1964 [2]. The drug was later found to be potent against HIV in 1985. Released in 1987 by Glaxo Smithkline as AZT, it became the first drug to be approved for HIV treatment. Mifepristone, a glucocorticoid receptor antagonist, is another example of a repurposed drug which was initially synthesized in 1980 in France by Danco laboratories as an oral abortifacient and was licensed for use in France in 1988 and in the USA in 2000 [3]. The Drug has been repositioned for psychotic major depression and bipolar disorder under the trade name of Corlux by Corcept therapeutics [4]. Aspirin is the most frequently used analgesic and antipyretic drug in the world. It was licensed by the German company Bayer pharmaceuticals in 1897. The drug was later found to possess anti-cancer properties (Table 1). Clinical trials held in 2011 studied the risk of cancer death among patients who regularly took aspirin for 4 years and patients who did not take the drug. It was found out that, aspirin use lowered the overall risk of cancer by 20%. Another example of repositioning is that of the acetyl cholinesterase inhibitor Galantamine which was licensed as Nivalin by Sopharma in 1960 as a treatment for paralysis due to Polio. With the licensing of the polio vaccine in 1962 and the gradual eradication of polio, Galantamine remained abandoned for years until 2000 when it was repurposed for Alzheimer’s disease by Johnson & Johnson under the trade name Reminyl.
Original Indication
Repurposed Indication
Drug
Disease
Target/s
Disease
Target/s
(a) Drugs repurposed for Infectious diseases
Zidovudine
Cancer
Telomerase [2]
HIV/AIDS
HIV reverse transcriptase [4]
Amphotericin
Fungal infections
Cell membrane sterols [5]
Leishmaniasis
Signaling pathways for IFN-γ, IL-12 and TNF- α activation in host [6 ]
Cycloserine
Urinary tract infections
Peptidoglycan synthesis in E.Coli [7 ]
Tuberculosis
Peptidoglycan synthesis in M. tuberculosis [8 ]
Clindamycin
Skin infections/acne
Ribosomal peptidyl transferase [9 ]
Malaria
Plasmodium apicoplast [10]
Paromomycin
Amoebiasis
16S Ribosomal rRNA [11 ]
Leishmaniasis
Mitochondrion function [12]
(b) Drugs repurposed for Neurological diseases
Milnacipran
Depression
Serotonin–Norepinephrine re-uptake [13]
Fibromyalgia
Serotonin–Norepinephrine re-uptake [14]
Atomoxetine
Parkinson’s disease
Noradrenaline re-uptake [15 ]
Attention deficit hyperactivity disorder
Noradrenaline re-uptake [16]
Galantamine
Polio, paralysis
Acetylcholinesterase [17 ]
Alzheimer’s disease
Acetylcholinesterase [18]
Ropinirole
Hypertension
Dopamine D2 receptor [19 ]
Parkinson’s disease/ restless leg syndrome
Dopamine D2 receptor [20]
Mifepristone
Pregnancy termination
Progesterone receptor [3 ]
Psychotic major depression
Glucocorticoid receptors [3 ]
(c) Drugs repurposed for Cancer
Aspirin
Analgesic and antipyretic
COX-1 and COX-2 [21]
Colorectal cancer
COX-2 inhibition and down-regulation of NF-κB and AP-1 signaling [22]
Rapamycin
Immunosuppressant
mTOR signaling [23 ]
Lymphoma and leukemia
mTOR pathway/VEGF signaling [24]
Methotrexate
Acute leukemia
Dihydrofolate reductase [25 ]
Osteosarcoma, breast cancer and Hodgkin lymphoma
NF-κBandTNF-α signaling [26 ]
Nitroxoline
Urinary tract infections ( P. aeruginosa)
Bacterial biofilm formation [27 ]
Bladder and breast cancer
Cathepsin B [28 ]
Minocycline
Acne vulgaris
Bacterial protein synthesis [29]
Ovarian cancer
NF-κB and TGF-β1 signaling [30 ]
Table 1: List of repurposed clinical drugs for the treatment of various diseases.
Conclusion and Future Directions
The Drug repositioning strategy is widely used as an alternative approach to drug development since it lowers the risk of safety and toxicity and at the same time saves millions of dollars worth of pharmaceutical R&D. Over the decades many drugs have been repositioned and licensed for use in alternate indications and many more repurposed drugs are currently at the phase II and III clinical trials. The Drug repositioning approach is thus a simple yet powerful strategy to fuel pharmaceutical research and streamline the drug discovery process.
References
- Sleigh SH, Barton CL. Repurposing strategies for therapeutics. Pharmaceutical Medicine. 2010; 24: 151-159.
- Gomez DE, Armando RG, Alonso DF. AZT as a telomerase inhibitor. Front Oncol. 2012; 2: 113.
- Fiala C, Gemzel-Danielsson K. Review of medical abortion using mifepristone in combination with a prostaglandin analogue. Contraception. 2006; 74: 66-86.
- Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 2004; 3: 673-683.
- Foglia F, Rogers SE, Webster JR, Akeroyd F, Gascoyne K, Lawrence MJ, et al. Neutron scattering studies of the effects of formulating amphotericin B with cholesteryl sulfate on the drug's interactions with phospholipid and phospholipid-sterol membranes. Langmuir: the ACS journal of surfaces and colloids. 2015.
- Mondal S, Bhattacharya P, Rahaman M, Ali N, Goswami RP. A curative immune profile one week after treatment of Indian kala-azar patients predicts success with a short-course liposomal amphotericin B therapy. PLoS Negl Trop Dis. 2010; 4: e764.
- Lambert MP, Neuhaus FC. Mechanism of D-cycloserine action: alanine racemase from Escherichia coli W. J Bacteriol. 1972; 110: 978-987.
- Prosser GA, de Carvalho LP. Kinetic mechanism and inhibition of Mycobacterium tuberculosis D-alanine:D-alanine ligase by the antibiotic D-cycloserine. FEBS J. 2013; 280: 1150-1166.
- Schlünzen F, Zarivach R, Harms J, Bashan A, Tocilj A, Albrecht R, et al. Structural basis for the interaction of antibiotics with the peptidyl transferase centre in eubacteria. Nature. 2001; 413: 814-821.
- Lell B, Kremsner PG. Clindamycin as an antimalarial drug: review of clinical trials. Antimicrob Agents Chemother. 2002; 46: 2315-2320.
- Vicens Q, Westhof E. Crystal structure of paromomycin docked into the eubacterial ribosomal decoding A site. Structure. 2001; 9: 647-658.
- Croft SL, Coombs GH. Leishmaniasis--current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 2003; 19: 502-508.
- Lopez-Ibor J, Guelfi JD, Pletan Y, Tournoux A, Prost JF. Milnacipran and selective serotonin reuptake inhibitors in major depression. Int Clin Psychopharmacol. 1996; 11 Suppl 4: 41-46.
- Staud R, Lucas YE, Price DD, Robinson ME. Effects of Milnacipran on Clinical Pain and Hyperalgesia of Fibromyalgia Patients: Results of a 6 Week Randomized Controlled Trial. J Pain. 2015.
- Kehagia AA, Housden CR, Regenthal R, Barker RA, Müller U, Rowe J, et al. Targeting impulsivity in Parkinson's disease using atomoxetine. Brain. 2014; 137: 1986-1997.
- Haynes V, Lopez-Romero P, Anand E. Attention-deficit/hyperactivity disorder Under Treatment Outcomes Research (AUTOR): a European observational study in pediatric subjects. Atten Defic Hyperact Disord. 2015.
- Xin L, Yamujala R, Wang Y, Wang H, Wu WH, Lawton MA, et al. Acetylcholineestarase-inhibiting alkaloids from Lycoris radiata delay paralysis of amyloid beta-expressing transgenic C. elegans CL4176. PLoS One. 2013; 8: e63874.
- Buendia I, Parada E, Navarro E, León R. Subthreshold Concentrations of Melatonin and Galantamine Improves Pathological AD-Hallmarks in Hippocampal Organotypic Cultures. Mol Neurobiol. 2015.
- Parker SG, Raval P, Yeulet S, Eden RJ. Tolerance to peripheral, but not central, effects of ropinirole, a selective dopamine D2-like receptor agonist. Eur J Pharmacol. 1994; 265: 17-26.
- Wijemanne S, Jankovic J. Restless legs syndrome: clinical presentation diagnosis and treatment. Sleep Med. 2015; 16: 678-690.
- Bartfai T, Conti B. Fever. ScientificWorldJournal. 2010; 10: 490-503.
- Li H, Zhu F, Boardman LA, Wang L, Oi N, Liu K, et al. Aspirin Prevents Colorectal Cancer by Normalizing EGFR Expression. EBioMedicine. 2015; 2: 447-455.
- Xie X, Jiang Y, Lai X, Xiang S, Shou Z, Chen J. mTOR inhibitor versus mycophenolic acid as the primary immunosuppression regime combined with calcineurin inhibitor for kidney transplant recipients: a meta-analysis. BMC Nephrol. 2015; 16: 91.
- Cella CA, Minucci S, Spada F, Galdy S, Elgendy M, Ravenda PS, et al. Dual inhibition of mTOR pathway and VEGF signalling in neuroendocrine neoplasms: From bench to bedside. Cancer Treat Rev. 2015.
- Rajagopalan PT, Zhang Z, McCourt L, Dwyer M, Benkovic SJ, Hammes GG. Interaction of dihydrofolate reductase with methotrexate: ensemble and single-molecule kinetics. Proc Natl Acad Sci U S A. 2002; 99: 13481-13486.
- Spurlock CF, Tossberg JT, Matlock BK, Olsen NJ, Aune TM. Methotrexate inhibits NF-κB activity via long intergenic (noncoding) RNA-p21 induction. Arthritis Rheumatol. 2014; 66: 2947-2957.
- Sobke A, Klinger M, Hermann B, Sachse S, Nietzsche S, Makarewicz O, et al. The urinary antibiotic 5-nitro-8-hydroxyquinoline (Nitroxoline) reduces the formation and induces the dispersal of Pseudomonas aeruginosa biofilms by chelation of iron and zinc. Antimicrob Agents Chemother. 2012; 56: 6021-6025.
- Mirkoviĉ B, Renko M, Turk S, Sosiĉ I, Jevnikar Z, Obermajer N, et al. Novel mechanism of cathepsin B inhibition by antibiotic nitroxoline and related compounds. ChemMedChem. 2011; 6: 1351-1356.
- Laux B. [Treatment of acne vulgaris. A comparison of doxycycline versus minocycline]. Hautarzt. 1989; 40: 577-581.
- Ataie-Kachoie P, Badar S, Morris DL, Pourgholami MH. Minocycline targets the NF-κB Nexus through suppression of TGF-β1-TAK1-IκB signaling in ovarian cancer. Mol Cancer Res. 2013; 11: 1279-1291.