
Review Article
Austin J Womens Health. 2024; 10(1): 1065.
Lifestyle Concerns for Metabolic Syndrome in Present-day Society with Special Reference to Poly Cystic Ovary Syndrome (PCOS) in Employed Women
Rahal A¹*; Kumar A²
¹Division of Animal Health, ICAR-CIRG, Makhdum, Farah-281122, Mathura, India
²Division of Animal Biotechnology, College of Biotechnology, SVPUAT, Modipuram-250110, Meerut, India.
*Corresponding author: Rahal A Division of Animal Health, ICAR-CIRG, Makhdum, Farah-281122, Mathura, India. Email: rahalanu72@gmail.com
Received: November 29, 2023 Accepted: January 09, 2024 Published: January 16, 2024
Abstract
Chronic Vascular Disease (CVD), obesity, diabetes mellitus type II, insulin resistance, hyperinsulinemia, hyperandrogenism and infertility are intricately interwoven conditions and collectively form metabolic syndrome and significantly affect the psychological and physiological defensive performance of the body. In the current lifestyle scenario, metabolic syndrome is ubiquitous across the globe and mainly originate from stress induced by feeding habits, profession, social, psychological, nutritional and environmental factors. To further complicate the situation, the dimensions of the metabolic syndrome are successfully transferred to an individual’s progeny with considerable magnifications. The most important concerns are regarding infertility and infertility rate in adolescence mainly resulting from poor semen quality in male and polycystic ovary syndrome in females. Both the conditions might be the outcome of any of the metabolic disorders. Unless public awareness and astringent measures are taken to address all the dimensions of metabolic syndrome, it may continue to aggravate and pose a boundless challenge to the progress of society. Thus, it seems imperative to understand the mechanism and management strategies to minimize its impact on society. The current review is an attempt to give the insight of metabolic syndrome with special reference to Polycystic Ovary Syndrome (PCOS) in employed females as it makes a significant contribution to the worldwide pandemic of lifestyle-related persistent syndrome.
Keywords: Metabolic syndrome; Polycystic ovary syndrome (PCOS); Stress; Female
Introduction
Chronic Vascular Diseases (CVDs) contributed in about 32% of total global deaths in 2019, and 85% of that were due to heart attack and stroke with three quarters in low- and middle-income countries. In addition to this, the incidence of death due to hypertension, high blood sugar and obesity have been 10.85, 6.5 and 5.02 million in 2019 worldwide [1]. To further elaborate the global health status [2] almost 10 percent of men and 14 percent of women presently are obese and it is nearly double the rate of obesity in 1980. World Health Organization (WHO) reports that the share of overweight or obese children and adolescents has risen from 4% in 1975 to around 18% in 2016 [3] with unequal distribution around the globe, being skewed towards low- and middle-income countries, with numbers more than doubling across them, and tripling in low income countries, compared to 2010.
The global share of the population with overweight has been forecasted to incessantly increase by in total 2.3% from 2022 and estimated to reach 43.41% in 2028 [4]. Surprisingly, even under-nutrition countries like Bangladesh, Cambodia, China, India, Nepal, and Vietnam, have seen the prevalence of overweight and obesity in women increase by anywhere from 3.5 to 38.5 percent a year from the 1990s through the mid-2000 [5]. If nothing is done to reverse the epidemic, It is estimated that the number of children aged 5-19 years living with obesity worldwide will increase from 158 million in 2020 to 254 million by the year 2030 [6]. The World Obesity Atlas 2022, published by the World Obesity Federation (WOF), also predicts that one billion obese people globally [7], with an incidence rate of 20% in women and 14% in men by 2030. The shift from traditional diets to overeating on Western diets being blamed monotonously, one paradox of this alleged “nutrition transition” is that even as obesity rates rise, underweight persists, sometimes within the same household [8]. To further add to it, low- and middle-income countries often face a dual burden-the infectious diseases with malnutrition and, increasingly, the debilitating chronic diseases linked to obesity and Western lifestyles. The health significance of this dual burden can be emphasized by the fact that rates of many non-infectious health conditions, such as heart failure and stroke, were substantially higher in COVID-19 recovered people compared to their counterparts. These health issues may be considered as a reflection of globalization, urbanization and early ageing in the population as the major forces driving social, economic and cultural change.
Over the decades, the society has observed a massive transition in gender-role attitudes, work and family identity salience, and division of household labour over the generations. Although, with both husband–wife employed gives them a financial balance and various fulfilment in life by creating a great deal of satisfaction, contributing to a sense of well-being and self-worth. However, the supposed need to accomplish more and more in very limited time produces a false sense of urgency leading to negative physiological changes in the individuals and subjecting them to potentially harmful stress [9]. Moreover, some jobs are boring, unchallenging, socially isolating as well as poorly paid and without prestige/security and contribute to occupational stress leading to psychological ill health. Modern society seems to be governed by “survival of the fittest”, each trying their best to provide the best to their family and the employment, and all pleasure of living appear to completely disappear from their life, their mere existence burdened with stress and tension [10] leading them to face stress related illnesses.
Today stress is ubiquitous with a demanding work environment, a busy nuclear household with confined residential and work premises, fewer chances to interact socially and ease out, each adding to a racing heart, irritability and other psychosocial effects. Stress activates hypothalamus-pituitary-adrenal axis inducing a fear or flight response-fear not to lose and flight to surpass others in the race. During the entire process, physiological parameters undergo a change to meet the sudden outstanding demand of tissues. Apparently, it seems to be okay, the change being temporary in nature but what if they continue to become a part of the daily routine and exhaust the resources.
What is Stress- a Boon or Blight in Disguise
Stress is the Outcome of a Physiological, Psychological or Environmental Challenge or Change: A short-term stress is helpful. It increases alertness and work efficiency but long-term stress can lead to serious health troubles. Work stress is an important current problem because the rate of transitory incapacitation, frequent absenteeism and untimely retirement, among others in the workplace are alarming. The after effects of stress are manifested not only in the individual and his family, but the productivity of the work organisation as well, thereby making people resort to measures adopted either for prophylactic or therapeutic remedies to overcome health related issues with the annual monetary losses occurring due to reduced productivity linked to stress [11-12]. This holds true especially in the case of modern day females, who faces-“do it all” syndrome, especially in orthodox societies like India. At the work front also, the high achieving women tend to show perfectionism, a strong inner critic, and a desire to be approved of by others. In an attempt to excel professionally, the Indian females have placed them in a grave situation where they remain attached physically and psychologically to all their household duties along with their liabilities at professional front, often ignoring health markers until they are at absolute breaking point [13-14]. Stress is also associated to various behavioural and hormonal disorders like tom-boy behaviour [15], increased anti-Mullerian Hormone (AMH) levels in the serum during adolescence [16] and higher prevalence of disorders related to autism spectrum [17].
Childhood Stress Leads to Later Life Compromised Health and Fertility
“Early Life Stress” (ELS) is an independent risk factor for chronic disease, compromising the neuroendocrine, immune, metabolic, and cardiovascular systems [18]. Stress affects the flexibility of metabolically active tissues. Adverse childhood stress causes weight gain more rapidly in women compared with men [19]. Also stress-related eating is more prevalent among adolescent girls (43%) compared with boys (15%), presenting a strong association with obesity rates in females [20]. Obesity acts as a predisposing factor for the initiation or development of physical as well as mental disorders in females. There are published reports that associate obesity with cancers of breast, kidney, gallbladder, endometrium and oesophagus. Obesity directly affects reproductive health with a negative impact on the rate of fertility and chances of contraception. It has also been found associated with cases of miscarriage, improper labour pains leading to higher rate of caesarean and other high-risk conditions related to obstetrics along with higher rate of neonatal and maternal mortality, congenital abnormalities in new born. It is further linked with mental health as a major cause of depression and disorders like anxiety, neurodegenerative conditions and lack/ irregular sleep [21]. Further, diabetes is also considered as a major risk factor inviting stroke, heart attack and other coronary heart diseases with almost 44% greater prevalence in women [22]. Maternal separation modulates the adult life HPA axis sensitivity and behavioral and metabolic responses in a sex-dependent manner [23-24]. Feeding of omega-3-deficient diet leads to increased food intake, higher body weight gain, higher plasma leptin and insulin, more chances of impaired tolerance for glucose and increased level of PEPCK in the man liver [25]. Another study conducted on females provided a diet with high-sucrose content for the period of ten weeks showed a positive impact on insulin, leptin and visceral fat levels with significant levels of augmentation in insulin resistance.
Chronic stress is also considered as a major risk factor associated with anxiety and depression. Change in behaviour due to mood disturbances is also associated with fatigue and physical inactivity, which in turn contribute to increased CVD risk [26]. Moreover, any depression or CVD may result in the activation of neurobiological pathways that are common in both diseases, such as the hypothalamic pituitary adrenal (HPA) axis, the autonomic nervous system, and the inflammatory response [27-28], encompassing a bidirectional relationship between depression and CVD [29]. Significant neuronal hyperactivation occurs in both sexes across multiple brain regions in response to repeated mild stress. Dramatically high induction (Fos as marker) occurs in amygdala and the piriform cortex along with tolerant frontal cortex following repeated exposure in females while modest sensitization in the frontal cortex with minimal affected other brain areas is observed in males. These differences in synaptic adaptations and patterns of brain activation are likely to contribute to observed sex differences in stress-induced behaviours.
The hypothalamic-pituitary-gonadal HPG hormonal cascadebegins with the release of gonadotropin-releasing hormone (GnRH), stimulating the synthesis and the pulsatile release of the gonadotropins, Luteinizing Hormone (LH) and Follicle-Stimulating Hormone (FSH), which in turn arouse the production of gametes and the release of sex steroids, including estrogen, progesterone, and testosterone. Both the HPA and the HPG axes well coregulate one another both centrally and peripherally, by complex positive and negative feedback loops, alongwith other neuroendocrine signals. Chronic stress downregulates the hypothalamic GnRH pulse generator, leading to hypothalamic amenorrhea [30], and can be accompanied by reproductive dysregulations associated with other targets along the HPG axis.
Infertility affects a remarkable one in four couples in developing countries. Stress is a common and commonly underappreciated cause of reproductive dysfunction. Stress influences reproductive function at all levels. Stress can interfere with reproductive function at all levels of the reproductive axis [31]. It can suppress libido, reward, and mating behavior at the level of the brain, particularly the ventral tegmental area. It interferes with the hypothalamic GnRH pulse generator and LH and FSH release from the anterior pituitary. It suppresses oocyte maturation, ovulation at the level of the gonads, as well as increasing the likelihood of ovarian cysts and affecting both ovarian and testicular steroidogenesis. Stress is also detrimental to pregnancy outcomes postconception, reducing the likelihood of successful blastocyst implantation. Infertility itself is stressful, and gets overloaded with its aftereffects like social pressures, testing, diagnosis, treatments, failures, unfulfilled desires and even economic costs with which it is associated.
Manoeuvring stress no matter whether it is accompanied with self-assured victory or aggressive survival fight, may negatively impact male fertility, by increasing adrenergic activation, leading to additional vasoconstriction in the testes [32], resulting in a lower testosterone level and decreased spermatogenesis. Acute stress may impair testicular function, with higher levels of cortisol causing apoptosis of both germ cells and Leydig cells [33]. The Leydig cell is the primary target of glucocorticoid regulation in the testes with direct inhibition of transcription of genes encoding testosterone biosynthetic enzymes such as cytochrome P450-dependent cholesterol side chain cleavage enzyme, and cytochrome P450-dependent 17a-hydroxylase/C17–C20lyase [34], causing a decline in testosterone production. The reduced Luteinizing Hormone (LH) and testosterone pulsing reduces both spermatogenesis and sperm quality [35] in a linear negative association [36]. Thus, overall a negative association of fecundability with stress score in men with low semen quality [37] represents a public health concern.
The glucocorticoids, Kisspeptin, ghrelin (hunger hormone released by gut) and GABA (inhibitory neurotransmitter) show an intricate interplay in reproductive functioning. Ghrelin, plausibly also acts via the activation of CRH neurons in these areas and indirectly through trees-regulatory areas in the medial and central nuclei to suppress LH pulsatility alongwith GnRH pulse frequency. Kisspeptin conveys information regarding systemic levels of sex steroids to GnRH neurons, and thus regulates both tonic and pulsatile GnRH release, playing a critical role in the onset of puberty [38-40]. The effects of glucocorticoids at the pituitary are possibly divergent for LH and FSH, being inhibitory and stimulatory, respectively in vitro [41].
In male gonads, 11b-hydroxysteroid dehydrogenase type 1 enzyme catalyses the oxidative inactivation of glucocorticoids and restricts their access to their receptors on Leydig cells. However, in case of excessive glucocorticoids as in severe or prolonged stress, it gets saturated and rapid repression of testosterone production may be observed. Ghrelin has also been shown to modulate testicular function both directly at the testicular level and through its systemic administration, suppressing Sertoli and Leydig cell proliferation [42]. Glucocorticoid receptors are also expressed in different cell types [43], during follicular maturation, ovulation, and pregnancy [44] within the ovary. In ovary, diminished 11b-hydroxysteroid dehydrogenase mediates cortisol-induced inhibition of ovarian steroidogenesis [45]. Ghrelin also suppresses ovarian steroidogenesis alongwith other steroid pathway enzymes, such as 3β-hydroxysteroid dehydrogenase, 17b-hydroxysteroid dehydrogenase, and cytochrome P450 aromatase [46]. Various studies revealed species specific impact of glucocorticoids on oocyte maturation and ovulatory cycle. Glucocorticoids suppress meiotic maturation in gilt oocytes [47]. In contrast, in mouse oocytes, only supraphysiological levels of glucocorticoids inhibit follicle differentiation and oocyte maturation [48]. In ewes, inconsistent effects of cortisol and dexamethasone on oocyte maturation have been demonstrated, with no effect of these glucocorticoids on the capacity of the oocytes to undergo fertilization [49].
GABAA and GABAB receptors in the mPOA are differentially involved in mediating the effects of stress on LH pulsatility, and antagonism of both receptors has been shown to block the CRH-induced inhibition of LH release in rats [50-51]. At the level of the median eminence GABA can act on the GnRH nerve terminals, leading to disruption of estrous cyclicity in rats) while blockade of GABAA receptor increases GnRH release and accelerates the onset of puberty [52-53].
Female Infertility Issues
Female infertility mainly occurs as ovulation disorders and tubal damage (~50%), while 10% to 30% of cases remain unexplained [54]. Polycystic ovarian syndrome is the most predominant heterogeneous endocrine disorder affecting 6-22% of all women globally [55]. Polycystic ovary syndrome is a complex multisystem condition with metabolic, endocrine, psychological, fertility and pregnancy-related implications at all stages of life [56-57]. According to the Rotterdam criteria, PCOS may be clinically observed as “classic PCOS” (excessive androgen secretion and irregular menstrual periods with or without ovarian cysts), “ovulatory PCOS” (augmented androgen secretion and multiple cysts), or “nonandrogenic PCOS” (irregular menstruation and multiple cysts) [58]. The underline indications for PCOS include ovarian cysts, anovulation, and endocrine variation (precisely hyperandrogenism) occurring mainly in the adolescence stage creating disturbances in hormonal balance and menstrual regularity. Anovulation (CA) is deeply rooted with an incidence of 2.2- 26% in Western countries, 2- 7.5% in China, 6.3% in Sri Lanka [59] and 9.13- 36% in India [60-61]. According to the World Health Organization (WHO), over 116 million women (3.4%) are affected by PCOS worldwide with higher prevalence (9.13%) in Indians compared to their Caucasian counterparts [62-63]. This multifactorial condition initially comorbids with obesity, type II diabetes, infertility, endometrial dysplasia, cardiovascular disorders, and/or psychotic disorders [64].
The main etiology and endocrine aspects of PCOS are the increased level of androgen, which is also known as “Hyperandrogenemia (HA)” and secondly the “Insulin Resistance (IR [65]. Androgen hike can impede the pulsatile release of LH:FSH, leading to follicular arrest and dysplasia [66]. There is dysregulation of the hypothalamic-pituitary-ovary axis leading to surplus gonadotropin, and in turn surplus production of LH over FSH. While LH facilitates androgen production in theca cells, FSH transforms androgens to estrogens in granulosa cells, which in turn promote follicile growth. Ultimately, the excess androgen promotes development of primordial follicles and rise in antral follicles at early GnRH stage [67].
Insulin resistance and compensatory hyperinsulinaemia are proposed as significant aetiological factors and are present in 75% and 95% of lean and overweight women with PCOS respectively [68-69].
Vicious Cycle of PCOS over Generations
The uterine environment of PCOS inflicted on pregnant women is most probably hyperandrogenic with higher 3β-HSD-1 activity and lower P450 aromatase activity in placenta tissue, which further facilitate androgengenesis [70]. Defective P450 aromatase gene and sex hormone-binding globulin gene, though infrequent, have been detected in female foetuses of such women, and have been linked to PCOS at puberty [71], perhaps altered placental steroidogenesis plays a key role in vicious cycle of PCOS pathogenesis.
Augmented androgen levels alter the placental function or metabolic profile of the females including development of hyperinsulinemia, pre-eclampsia and diminished nutrient transfer followed by progressive IR and an increased prevalence of metabolic syndrome when exposed to normal nourishment [72] and PCOS in the future. Again owing to hyperinsulinemia, augmented ovarian steroidogenesis leads to hyperandrogenemia in adulthood of female foetuses with Intrauterine Growth Restriction (IUGR), finally culminating into clinical expression of PCOS.
Evidence shows that the uterine environment can ‘programme’ reproductive efficiency prenatally. Detection of polycystic ovaries before the onset of puberty also signifies the origin of the syndrome during ‘programming’ of ovarian morphology and function at foetal maturity—perhaps during ovarian development and oogenesis under the influence of hyperandrogenemia of maternal uterus [73]. In fact maternal plasma testosterone at 18 weeks of gestation significantly correlates with early follicular-phase circulating AMH levels in female adolescent offspring [16]. The variable hormone levels during the gestation play a critical role at a specific time window during the time of system and organ differentiation. The animal model based studies related such abnormalities with pathogenesis of PolyCystic Ovarian Syndrome [16]. A study conducted on sheep developed phenotypically virilised female progeny after prenatal androgen exposure [74] while later exposure creates PCOS phenotypes with irregular ovulation and lower fertility rates [75].
The higher aromatase activity of placenta with higher plasma binding proteins protects human foetus from maternal hyperandrogenemia. Usually P450 aromatase produced by placenta metabolize androgens in to oestrogens. Thus, even in coexistence of androgen-secreting tumours, it is very unusual to see virilization of a female foetus [76]. The prenatally androgenized rats show higher expression of oestrogen and androgen receptors in placenta [77], suggesting higher placental sensitivity to sex steroids. Co-occurence of 21-hydroxylase deficient adrenal hyperplasia and prenatal androgenemia also show symptoms similar to PCOS (LH hypersecretion and reproductive dysfunction) even after postnatal therapies [71,78].
PCOS is directly associated with hereditary and environmental determinants [79]. The hereditary factors are mainly due to an hyperandrogenemia associated with premature fetal development, early puberty and adulthood, and ancestry of PCOS among close relatives [79-80]. Premature in utero development of the female facilitates more rapid onset of puberty with an increased risk of developing PCOS [81]. Occurrence of hyperinsulinemia in children of PCOS affected women prior to onset of puberty is also indicative of genetic susceptibility [81]. The environmental factors include endocrine disruptors, physical sluggishness, obesity, and the coupled insulin resistance which has a high prevalence in the urban Indian lifestyle [82].
Health Hazards of PCOS
In India, metabolic syndrome is quite prevalent (nearly 50 percent) in geriatrics, reaching an alarming 63.1% among urban members [83]. Metabolic syndrome clusters typically include abdominal obesity, insulin resistance, hypertension, and dyslipidemia, the presence of which confers higher risk of cardiovascular disease and diabetes mellitus. While all the metabolic syndromes favour the disruption of hypothalamic-pituitary-ovary axis, hyperinsulinemia particularly, goes hand-in hand with hyperandrogenemia along with insulin resistance for the progress of PCOS, thus, establishing a vicious cycle through which they stimulate each other. Various published reports associated PCOS with two fold higher rate of metabolic syndrome [84], 2.5 to 4.5 fold higher chances of impaired glucose tolerance and Type 2 diabetes [85], higher level of blood pressure and dyslipidaemia in almost 70% of PCOS cases [86-87]. The pathophysiology of PCOS is greatly exacerbated by obesity and diabetes. In obese people, PCOS facilitates the progress of IR and hyperinsulinemia [88-89]. Women with PCOS have a higher threat of Gestational Diabetes (GDM) [90] and mental and feeding disorders like anxiety, depression and bipolar disorders [91-92]. Both PCOS and GDM are predisposing factors for IR, weight gain [93], pregnancy-related hypertension, pre-eclampsia, and infant hypoglycemia. According to the International Diabetes Federation PCOS is a major and non-modifiable predisposing factor for Type 2 diabetes. Understanding of pathogenesis of PCOS revealed IR as a common component in between PCOS and T2D. The expression of T2D-related genes also play critical role in PCOS [94]. It results in the development of IGT in almost 20% of PCOS affected women [64]. The process of development of PCOS is also found associated with obesity [95] and pancreatic β-cell dysfunction [96].
Stress in Postmenopause Female
Indian women have a higher body fat percentage for a given BMI when compared with their western counterparts. Obesity accelerates CVD after menopause [97] owing to its association with autonomic dysregulation, with striking sympathetic hyperactivity and downregulated parasympathetic tone. The Registrar General of India has also projected CVD as the most important cause of death in women [98]. During menopause parasympathetic tone protection is lost leading to higher sympathetic control. Moreover, menopause is commonly associated with obesity, and metabolic syndromes owing to a decline in the levels of estrogens. Although obesity further helps in conversion of peripheral androgens to estrogens including Estradiol (E2) [99], unfortunately, these estrogens lack cardiovascular protectivity. Even after a myocardial infarction episode, women find it harder to pin down stress and discomfort and often experience lower quality of life compared with men.
Blood pressure regulation also shows gender variation, possibly due to influences of female sex hormones on the cardiovascular function [100] leading to higher prevalence of orthostatic intolerance in young women at premenopausal stage and higher levels of MSNA and increased blood pressure in older women at postmenopausal stage.
Urban/Rural Lifestyle Changes
Till date, urban development has been quite lucrative with better prospects of education, job opportunities, infrastructure, medical facilities contributing in the betterment of living standards. What we fail to recognise is the other side of the coin, which is an essential concurrence. Urban society lacks open spaces, is polluted, and has an inflated cost of living with hectic and stressful lives that makes its people susceptible to problems of obesity, infertility and depression. People living in urban areas tend to live in a hierarchical organisational setup and tend to build defence mechanisms trying hard not to reveal their real self. They live programmed lives constantly with no fixed eating schedule and lack of exercise which makes them impatient, and emotionally distressed to further add to the burden. The recent report of Centre for Urban Design and Mental Health (UD/MH), reported significantly higher rates of mental health related disorders in cities in comparison to rural areas with nearly 40% higher risk of depression, over 20% more of anxiety, and double the risk of schizophrenia, in addition to loneliness, isolation and stress [101]. The differences in health impact of urban vs rural lifestyle can clearly be appreciated from the covid era where urban society faced a greater morbidity with more fatal outcomes as compared to rural masses which had comparatively meagre awareness and medical aids.
Lifestyle is intricately interwoven with Generalised Chronic Stress, Obesity, Menopause, PCOS and Hormonal Imbalance (Figure 1)
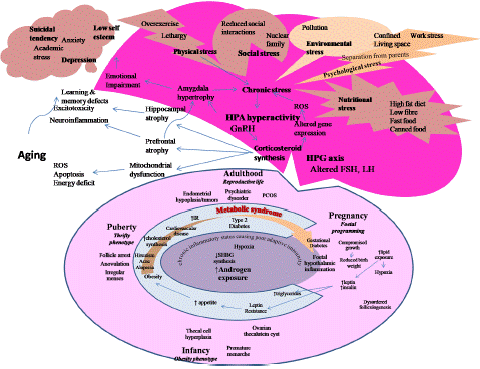
Figure 1: Lifestyle is intricately interwoven with generalised chronic stress, metabolic syndrome, aging and poor mental and physical health adversely affecting the work efficiency irrespective of age and reproductive status. It further goes down the generations in a cumulative mode further escalating the health issues.
Although past reviews have generally indicated the positive effects of lifestyle modulation on anthropometric, reproductive (biochemical and clinical hyperandrogenism, menstrual function, ovulation, pregnancy and conception), metabolic (fasting insulin, fasting glucose, glucose tolerance, lipid profiles, surrogate markers of insulin resistance) and quality of life endpoints, no direct fertility benefits have been claimed. However, addressing the secondary factors will certainly reduce the impact of stress and related syndromes on the overall health status. PCOS also affects quality of life by worsening anxiety and depression directly due to its symptoms or indirectly arising from its diagnosis and prognosis and apprehensions regarding social stigmata. PCOS, metabolic syndrome and cardiovascular risk share a complex relationship [102] along with risk factors for diabetes and cardiovascular disease including high levels of insulin or insulin resistance and abnormal cholesterol levels.
Obesity holds the major share in worsening the presentation of PCOS and weight management (weight loss, maintenance or prevention of excess weight gain) is proposed as a preliminary handling strategy, which can be best achieved through lifestyle changes incorporating diet, exercise and behavioural interventions. Insulin resistance and compensatory hyperinsulinemia are proposed as significant aetiological factors and are present in 75% and 95% of lean and overweight women with PCOS respectively [68-69].
Obese and overweight women with PCOS display worsened clinical reproductive and metabolic features [103-104]. Lifestyle intervention is therefore anticipated to work because a reduction in BMI and waist circumference is associated with a reduction in insulin resistance and ultimately improvement of PCOS associated metabolic and reproductive features. Weight loss achieved through lifestyle management decreases abdominal fat, hyperandrogenism and insulin resistance, and improves lipid profiles, menstrual cyclicity, fertility and risk factors for type 2 diabetes and cardiovascular disease in overweight women with PCOS [105-106]. A 2-3 kg weight loss has previously been reported to be associated with reductions in impaired glucose tolerance prevalence with improvements in risk factors for cardiovascular disease and Type 2 diabetes [107]. Additional evidence favour the use of physical exercise to improve metabolic risk factors, in both PCOS patients and the general population [108-109], even when there is no weight loss.
Lifestyle modifications may improve the free androgen index with a decline in total testosterone and a good increase in Sex Hormone-Binding Globulin (SHBG), leading to appreciable reduction in hirsutism. There may be a greater reduction in fasting insulin, total cholesterol and low density lipoprotein cholesterol with negligible change to high-density lipoprotein cholesterol and triglycerides. This is consistent with the widespread international recommendations that lifestyle treatment improves fertility and reproductive outcomes in PCOS [57]. Lifestyle intervention may improve quality of life scores in the domains of emotions and infertility in PCOS. Even factors like dress are found to affect reproductive health e.g. tight fitting underwear and pants showed a relative risk of impaired semen quality [110].
In females change of hormones during the shift from pre to postmenopausal period disturb autonomic functions that regulates cardiac functioning. The obesity further aggravates the condition. During the time the body tries to make balance between innate immune system and the HPA axis to maintain and regulate the homeostasis. However, metabolic diseases like inflammatory disorders are common due to accumulation of free reactive oxidative radicals due to increased blood glucocorticoids and proinflammatory cytokines like IL-1,6 and TNF-a [111-112]. Moreover, the release of arginine vasopressin and Corticotrophin Releasing Hormone (CRH) from the hypothalamus during the stage of anxiety and stress lead to the production of glucocorticoid from activated adrenal cortex. Glucocorticoids regulate foetal growth, development and mobilization of glucose and fat. It further control and modulates inflammation and immune response [113-114]. Thus, any alteration or modification of these pathways during foetal programming induces altered metabolic function that makes individuals susceptible to different metabolic disorders in adulthood.
Environmental Changes and Stress
Gender equality, social rights for sexual and reproductive health, and macro and microclimate change are inextricably correlated. Adverse climate setting offers increasing social, economic and gender inequalities. Global warming, with forecasts of extreme weather events like floods, droughts, and heat waves injudiciously threaten the health and rights of girls and women [115-117] owing to their greater burden of stress in the social setting. In addition, they often experience limited access to financial resources, more restricted rights, and limited access to participation in decision-making. Girls and women face gender discrimination to the basic determinants of physical and mental health viz. education, economic status, food security, shelter and other social characteristics, all of which play a crucial role in determining their vulnerability and adaptability [118-119]. Female mental health is a taboo in societies like India and unfortunately, people find it quite difficult to discuss and continue to be stressed on minor issues which could have otherwise been handled effectively. The latest report on the State of Mental Health in Delhi (2008) says “The significant stressors indicated were crowded roads, larger distances, traffic problems, disturbed and erratic routines, migrations of people resulting in a diluted culture and weak community links, lawlessness and fear for safety, especially of women, children and the elderly”. These conditions are aggravated by natural or man-made disasters such as social conflict, natural disasters like floods, and global pandemics like Covid era when temporary breakdown of governance led to gaps in accessibility, and quality of even the routine health services, thereby increasing gender biased vulnerability [120]. This with apprehension, stigma, and detrimental social customs flared up the reproductive stress in females as compared to males. Further, reduced male fertility due to advancing age, improper lifestyles, work stress and environmental factors also plays a critical role on fatality, and its consequences will definitely affect the future human population making it an important public health concern in the current century particularly in western world.
Lifestyle Adaptation Failures Lead to PCOS and Metabolic Syndrome
Lifestyle and dietary habits have an important effect on general health [121]. The mismatching of ancient genetic related survival mechanisms with modern lifestyle practices are supposed to be the predisposing factors for the expression of PCOS phenotypes.
Metabolic syndrome is a multifactorial condition, with the interplay of dysregulated lipid metabolism, insulin resistance, and inflammation paving the way to PCOS, cardiovascular disease, type 2 diabetes and atherosclerosis mortality [122-123]. Detrimental lifestyle factors such as obesity, smoking, physical inactivity, and unhealthy eating patterns, along with aging, are major risk factors [124-125]. PCOS is viewed as a mismatch between our rapid human cultural evolutions of the modern day society with comparatively sluggish biological evolution [126-127]. The multiple predisposing factors of PCOS are directly correlated to various factors associated with lifestyle like improper diet and feeding schedule, lack or no physical inactivity, disturbed or compromised gut microbiota and dysbiosis, exposure to endocrine-disrupting chemical substances. These all are correlated to the initiation and transgenerational inheritance starting from evolutionary intrauterine genetic or epigenetics along with conditions like insulin resistance, obesity and poor phenotypic, reproductive disorders including sub-fertility.
Insulin resistance, a major etiological factor of PCOS, is linked with increased severity of PCOS [128]. The reports showed that improvements in insulin resistance are directly linked to improved clinical manifestation of PCOS [129]. Thus, these are recommended as potential surrogate markers to study or intervene in lifestyles affected with PCOS. The adipose tissue functions as endocrine for energy balance and glucose homeostasis through leptin, adiponectin, cytokine (TNFa, IL6) and fatty acid release. It is also responsible for enzymatic conversion of steroids, thereby influencing the cardiovascular, immune and reproductive activities. Excessive fat is always associated with dysfunctioning of the immune system and loss or reduced productivity. Adipose dysfunction causes ectopic lipid depositions in tissues and contributes to iIR and metabolic diseases [130].
To highlight the significance of adipose tissues in reproductive fertility, it needs to be mentioned that onset of menarche and reproductive cycle require mandatorily presence of a critical adipose tissue in the female body to ensure sufficient energy reserve in case of pregnancy [130-131]. Obesity on the other hand, causes menstrual irregularities, diabetes mellitus, lack or loss of ovulation, sub-fertility, frequent abortions, gestational diabetes and preeclampsia. The sex steroids influence the fat deposition and sensitivity to insulin in different tissues. While estrogen favours peripheral fat deposition and improves insulin sensibility, androgen promotes central fat deposition and reduces insulin sensitivity [132].
The inverse relationship is observed between sitting time and metabolic health [133]. Greater awareness and higher standards of living, associated with higher education levels, generally lead to better health. The most intriguing relationship is the sleep and the risk associated with metabolic disorders [134]. In females, a U-shaped relationship exists between the severity of risk and duration of sleep, while it is a semi-linear association in males [135]. Sleeping for 7–8 hours significantly reduces the risk of metabolic disorders in females. Excessively long sleep duration (=9 hours) is mainly associated with abdominal obesity with lower energy expenditure and minimal physical activity, which increases the risk of obesity and linked syndromes [136]. Prolonged sleeping time usually has interrupted sleep and it leads to adverse impact on metabolic health. Such issues are more often observed in women suffering from sleep disorders related to emotions [137].
Physical exercise is an integral part of routine lifestyle interventions. However, over exercising can increase the circulating androgens including testosterone. The intervention of a low-calorie diet coupled with increased physical activity might seem simple, but the success lies in compliance and sustainability of this advice. The past decade has witnessed an explosion of research on the effects of dietary macronutrients on weight and CVD risk factors. Though dietary fat faces avoidance unanimously, high protein intake is thought to be associated with better satiety, and thus enable energy restriction [138-139]. Long term efficacy and sustenance of weight loss is challenging. Obesity is a complex disorder with a combination of multiple etiological factors and its management is often step-wise behavioural lifestyle modification. Interventions may be targeted to specifically induce weight loss, or towards weight maintenance with significantly improved triglycerides [140]. WHO and NIH have recommended that obese adults (ie, body mass index =30 kg/m2), as well as those who are overweight (body mass index of 25–29.9 kg/m2) and have comorbid conditions, lose 10% of their initial weight [141-142]. A reduction of 5% to 10% in weight significantly improves the psychological, metabolic and finally reproductive health of an individual [57].
Use of the Contraceptive Pill is Quite Common in Females: The contraceptive pill suppresses many of our hormones including testosterone, so the pill withdrawal will naturally cause testosterone levels to spike and can take some time to rebalance. The overexerting females often find relief with tea and coffee but caffeine easily crosses biologic membranes and impairs prenatal male gonadal development and thus adulthood gonadal function [143].
The semen quality is also positively correlated with high intakes of antioxidants and polyunsaturated fatty acids (among which omega-3) as fruits and vegetables [144], legumes [144] and fish [145-146], and negatively with diets including meats (processed meat in particular) and full-fat dairy products [147]. The studies established amelioration of impaired sperm quality and fertility in rat progeny from obese dams by adulthood exercise [148]. Even a low intensity swimming exercise improves reproductive performance without affecting adiposity in obese mice, which is indicative of only partial dependence on adiposity [149]. Exhausting exercise like continuous bicycling can negatively affect, semen parameters and testicular function owing to testicular heating [150], oxidative stress (ROS formation) [151], DNA fragmentation [152] and gonadotropin suppression [153]. Coping with various lifestyles may also affect fertility. As compared to sedentary controls, continuous higher numbers of motile spermatozoa with normal morphology with an improvement of sperm parameters has been found after reducing the exposure time of TV-watching [154-155].
To further add to this burden, there is increasing concern regarding low-level Radiofrequency Electromagnetic Fields (RF-EMF) of mobile phones associated with decreased semen quality [156]. RF-EMF may lead to histological changes to the testes, disrupted spermatogenesis, and increases in rectal temperature, but, again, the results are also conflicting [157]. Prolonged sitting in the car is another risk factor for the rise of testicular temperature, which increases by about 2°C after 2h of sitting [158].
Individuals consuming excessive processed foods, coffee, tea, or sugar are generally chromium deficient and need to include romaine lettuce, onions, tomatoes, whole grains, and potatoes, preferably with vitamin C that increases the absorption of chromium. Magnesium is required for proper glucose utilization and insulin signalling, both of which are impaired in people with insulin resistance.
Use of black coffee to kill appetite or hunger pangs is quite rampant in the younger generation to stay up all night to finish assignments. Its caffeine content increases wakefulness and low-calorie helps in weight loss. Numerous studies have shown a significant inverse relationship between coffee consumption and prevalence of metabolic syndrome [159-160] in women. However, we can conclude that there is no clear association between caffeine and fertility indexes, so this relationship remains unclear and, in some ways, contrasting.
Metabolic Syndrome and Immunity
Metabolic syndrome is multidimensional and each of its dimensions is capable of t causing directly as well as indirectly immunity dysfunction. To be particular, immune dysfunction or suppression is a direct bearing of a chronic inflammation, which “is a common feature of the metabolic syndrome. The intimate architectural proximity of metabolic and immune cells closely integrate the metabolic and immunologic pathways in adipose tissue and intestine and serve for symbiotic relationship between the host and the intestinal microbiota [161]. Cells participating in adaptive immunity are critical for an effective response against evading infections and for the initiation of immune reminiscence. Vaccination induces a short term (acute) inflammation which is indispensable for the body’s gift to battle tissue damage-and repair as well as assault pathogens. Immune cells like antibodies, complements, and lymphokines either initiate or suppress host inflammation by secreting pro-inflammatory or inhibitory cytokines, usually in a time prescribed manner. Abnormal chronic and prolonged immune cell activity can lead to immune cell dysfunction or an imbalance in immune-related factors. Obesity alters the composition of intestinal immune cells as well as its immune cell-secreted factors regulate intestinal microbiota [162-163] leading to a dysbiotic microbiome [164]. It downregulates the production of intestinal IgA antibodies which play a vital role in taking care of infections during metabolic disease [162]. The T cell dependent intestinal IgA thwart obesogenic microbiota and thus normalize lipid absorption [163]. In the presence of a disturbed microbiota, adipokines and cytokines amend insulin signaling and immune response leading to adipose tissue inflammation and systemic insulin resistance [165].
The low-grade chronic inflammation in PCOS is mainly attributed to visceral adipocytes undergoing hypoxic necrosis. During anovulatory PCOS, the low progesterone levels overstimulate the immune system, leading to higher estrogens levels and generation of related autoantibodies [166].
Lifestyle Schedule and Dietary Management for Correcting the Metabolic Iimbalance
A healthy lifestyle is always associated with a healthy metabolic life. Lifestyle interventions (dietary, exercise, behavioural or combined) are recommended as first-line management in an international evidence-based guideline on PCOS [57] with the target to optimise healthy weight, improve underlying hormone disturbances, prevent the future complications related to reproduction and metabolism and improve the quality of life. Modifications in lifestyle are effective in prevention of single metabolic risk factors and ultimately the metabolic syndrome. The synergistic role of such multiple components of the day today's life is more important than the effect of individual factors, separately. These include 3 primary components: diet, exercise, and behaviour therapy [167].
These can be practised as a reduced calorie, low-fat diet of conventional foods (1200–2000 kcal/d, depending on body weight) and 150 min/wk of physical activity (typically brisk walking), with the goal of losing 7% of initial weight [168]. Adopting this lifestyle has been reported to reduce the weight by 5.6 kg over a period of 2.8 years and 58% reduction in risk of developing type 2 diabetes. Even modest weight loss followed by weight regain can be beneficial to long-term health.
Lifestyle modification is the backbone of PCOS treatment to prevent excess weight gain, manage weight and prevent future reproductive and metabolic complications [57]. They not only help in improving symptoms but also help enhance the results of medicines. Prevention opportunities are particularly relevant as women with PCOS have a greater prevalence of overweight and obesity [169-170]. Such intervention may also improve insulin resistance or other features of PCOS independent of weight loss [171]. The lifestyle, ideal weight lead to a good metabolic life cycle and a healthy hormonal cycle with less or no chance of proportion PCOS. It involves six main components-low glycemic index diet, mandatory breakfast, befriend fats, repel carbohydrates, favour lean protein and regular exercise. While a low index diet with minimal carbohydrates helps in weight control and improves menstrual regularity, eating half of the daily calories at breakfast reduces insulin and testosterone levels and improves ovulation, suggesting an improvement in fertility. Regular supply of omega3 fatty acids is essential to resolve hormonal imbalance.
Four well-studied diets include low-carbohydrate, low-fat, Mediterranean, and low-glycemic load regimens [172-174]. Low-carbohydrate diet prescribes as few as 20 g/d of carbohydrate with high protein and fat content. Low-fat diets provide 10% to 20% of calories from fat and recommend plant-based foods (grains, fruits, and vegetables) and aims to provide satiety through large volume [175-176]. Mediterranean diets prefers intake of unsaturated fats such as olive oil, nuts, and fish, in lieu of saturated fats (eg, red meat and butter) [177] along with fruits, vegetables, and whole grains. The most commonly practised by weight control programmes are the portion-controlled diets which provide a fixed portion of food, and simplifies meal preparation and calorie counting. Psychological barriers in the patient's environment must also be addressed to achieve sustainable weight loss. Behavioural techniques to manage diet deviations closely associated with household environment and social setting like snacking, food cravings, and emotional eating should be progressively addressed [178]. Socializing and eating out is an unavoidable part of lifestyle today but fat containing foods containing “cream,” “fried,” “mayonnaise,” “pesto,” “mozzarella,” “basted,” “casserole,” “refined flour” and “honey-mustard sauce” etc. need to be avoided. Rather salads, soups, or protein-based dishes like grilled chicken, poached fish should be preferred.
The use of a plant-based diet is widely recommended to reduce the risk of cardio-metabolic diseases. However, all plant based diets are not equally beneficial to health. The plant based diets constituted with a high proportion of low or poor quality plant foods including refined grains, high salt diet, high calories and sugar containing diets may predispose to increased risk of type 2 diabetes, lipid disorder and coronary artery disease [179]. Previous analyses have confirmed the presence of a “traditional-carbohydrate” dietary pattern in our population, which is characterized by, among others, high consumption of potatoes and refined grains and was linked to more chances of abdominal obesity leading to triglyceridemia [180]. The study recorded the inverse relationship between the absence of MetS components and the consumption of vegetables and fruits. High intake of red meat is a widely accepted risk factor of cardiovascular disease and mortality. Furthermore, lower mortality risk was observed when SFA, trans fats, or refined carbohydrates were replaced by monounsaturated fatty acids from plants, but not animal products [181].
Management of IR requires dietary intervention with a combination of sodium reduction, fat reduction, calorie restriction and reduction of high glycemic index carbohydrates [182-183] along with physical activity to improve both calorie expenditure and insulin sensitivity in muscle tissue. High fiber foods like cruciferous vegetables, like cauliflower, broccoli, and brussels sprouts, greens, bell peppers, beans and lentils, almonds, berries, sweet potatoes, winter squash and pumpkin can help overcome insulin resistance through reduced digestion rate and ultimately and reducing level of sugars in blood.
The naturally circulating androgens level can be reduced by replacing inflammatory foods such as processed food, food materials carrying gluten, refined sugar, caffeine and alcohol with testosterone reducing herbs such as spearmint, alfaalfa leaf, lady mantle, nettle leaf etc. Higher consumption of coffee is associated with higher plasma concentrations of TG and HDL in women leading to higher female sex hormone plasma concentrations [184]. Coffee intake also reduces the levels of ghrelin, a hunger hormone [185]. However, it leads to disruption in pattern and duration of sleep predisposing weight gain. Less or irregular sleep is reported to be associated with increased appetite, more inclement to processed food leading to body weight gain [186-187].
It is well established that the use of dietary restriction to reduce fat of weight using apple cider vinegar, citrus juices, green tea and detox drinks rapidly reduces the weight but may cause nutritional deficiencies on prolonged use. Plasma testosterone level can be reduced by using glycyrrhizin found in licorice root. B complex group vitamins are critical for many vital parameters like vitamin B2 support in energy generation whereas, vitamin B3 and vitamin B5 support to maintain sugar level in blood and metabolism of fat. Vitamin B6 supports proper absorption of zinc that plays a critical role in maintaining the redox and curbing the excess of stress. These are also required to maintain proper thyroid function and metabolic activities and may help in reduction of fat. Chromium helps in the fight against insulin resistance and promotes weight loss due to its ability to help control cravings, reduce hunger, and control fat in the blood. Further, medical treatments target biochemical, clinical hyperandrogenism, reproductive and metabolic features. The PCOS specific treatment includes targeted symptomatic treatment involving combination of oral contraceptives, anti-androgens and intermittent progestins. These have a three dimensional approach as improvement of hyperandrogenism, hirsutism and regulation of menstrual cycles. With the advancement, now three tier treatment is used to overcome PCOS. The first step is to cure anovulatory infertility using therapeutic protocol to induce ovulation, second step involves the use of gonadotropins and third step is the use of vitro fertilisation or laparoscopic ovarian surgery [57]. These include co-administered with insulin-sensitising drugs to address metabolic disorders [57,188, 189]. If we go through all possible cure and treatment aspects, the change of lifestyle and application of lifestyle based interventions seems to be an easy, safe, cost effective method during the initial stages of PCOS [190].
Conclusions
The hardship of life is prevailing over the physical, mental and metabolic health. The career and duties are more important in individual or family life. These are directly related to stress created on working person and ultimately affects his/her life through metabolic, physical, psychological disorders. These all are interlinked and PCOS is one of them that directly affect an individual and indirectly affect its working capabilities. Once the condition is created, it becomes difficult to get it rid off. However, it can be reverted with disciplines, regular and stress free life.
Depending upon the severity the possibility of reversion and time taken in the process may vary. As prevention is always better than cure, working woman has to prioritise between health and career. Without health, there is no career so choice is limited. To achieve anything in career, one must behave responsibly so that entry into vicious cycle of stress can be avoided. For better and metabolic disorder free life, lifestyle intervention like structured physical activity, a combined dietary and exercise intervention, or behavioural interventions can be used effectively. Polycystic ovary syndrome is a reversible metabolic condition and a healthy lifestyle consisting of a regular exercise, a complete healthy diet with healthy mind may lead to reversal. These are prime factors for emotional, psychological and social well-being and handling crises, lowering chronic stress exposure and ultimately preventing undesirable weight gain. Based on the available literature and information collected from various studies regulation of sleep, feeding and drinking habits, work culture can have desirable impact.
The deep and sound sleep of 7-8 hours, limiting animal fat consumption to less than two serving per day, use of more than two cups of coffee per day, no or minimum use of alcohol and reduction in sitting time to less than 6 hours per day may reduce the chances. The overall outcome depends upon the regular, sustainable changes in life style and these can be achieved by proper educational awareness at work places, desirable changes in working environment and nutritional changes in individuals. Physical exercise on regular basis and psychological support along with proper use of antioxidant neutraceutical in diet can minimize the issues related to infertility and help working women to live and enjoy better quality of life and career. It will naturally improve the chances of conception and carriage of full term of pregnancy.
Author Statements
Competing Interests
The authors declare no conflict of interest.
References
- Available from: http://www.healthdata.org. IHME, Global Burden of Disease. 2019.
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980, systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011; 377: 557-67.
- WHO. Fact sheet – obesity and overweight; 2018.
- Degenhard J. Overweight population share forecast in the World 2013-2028. 2022.
- Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012; 70: 3-21.
- Elflein J. Predicted number of children with obesity worldwide in 2020, 2025, and 2030. 2020.
- World Obesity Atlas 2023. Available from: http://www.worldobesityday.org on World Obesity Day 4 March 2023).
- Doak CM, Adair LS, Bentley M, Monteiro C, Popkin BM. The dual burden household and the nutrition transition paradox. Int J Obes (Lond). 2005; 29: 129-36.
- Pojwan MA, Ojo SS. Challenges of work stress and psychological well-being of workers in national development. African Journal of Social Sciences and Humanities Research. 2020; 3: 10-24.
- Roy S. Managing stress, Handle, control and prevent. India: New Dawn Press Inc. 2006.
- Lee DW, Lee J, Kim HR, Kang MY. Health-related productivity loss according to health conditions among workers in South Korea. Int J Environ Res Public Health. 2021; 18: 7589.
- Sucharitha M, Amzad Basha Md. A Study on Impact of Stress employee productivity and job performance Implications for Stress Measurement and Management. Ilkögretim Online. 2020; 19: 823-83.
- Padmanabhan V, Veiga-Lopez A, Sir-Petermann T, Maliqueo M, Angel B, Lara HE, et al. Sheep models of polycystic ovary syndrome phenotype. Mol Cell Endocrinol. 2013; 373: 8-20.
- Wu XY, Li ZL, Wu CY, Liu YM, Lin H, Wang SH, et al. Endocrine traits of polycystic ovary syndrome in prenatally androgenized female Sprague-Dawley rats. Endocr J. 2010; 57: 201-9.
- Hines M, Golombok S, Rust J, Johnston KJ, Golding J, Avon Longitudinal Study of Parents and Children Study Team. Testosterone during pregnancy and gender role behavior of preschool children, a longitudinal, population study. Child Dev. 2002; 73: 1678-87.
- Hart R, Sloboda DM, Doherty DA, Norman RJ, Atkinson HC, Newnham JP, et al. Circulating maternal testosterone concentrations at 18 weeks of gestation predict circulating levels of antiMullerian hormone in adolescence, a prospective cohort study. Fertil Steril. 2010; 94: 1544-7.
- Kosidou K, Dalman C, Widman L, Arver S, Lee BK, Magnusson C. Gardner RM2 maternal polycystic ovary syndrome and the risk of autism spectrum disorders in the offspring, a population-based nationwide study in Sweden. Mol Psychiatry. 2016; 21: 1441-8.
- Agorastos A, Pervanidou P, Chrousos GP, Kolaitis G. Early life stress and trauma, developmental neuroendocrine aspects of prolonged stress system dysregulation. Hormones (Athens). 2018; 17: 507-20.
- Liu H, Umberson D. Gender, stress in childhood and adulthood, and trajectories of change in body mass. Soc Sci Med. 2015; 139: 61-9.
- Jääskeläinen A, Nevanperä N, Remes J, Rahkonen F, Järvelin MR, Laitinen J. Stress-related eating, obesity and associated behavioural traits in adolescents, a prospective population-based cohort study. BMC Public Health. 2014; 14: 321.
- Weschenfelder J, Bentley J, Himmerich H. Physical and mental health consequences of obesity in women. Adipose tissue. London, United Kingdom: Edited by Leszek Szablewski, IntechOpen Limited. 2018.
- Peters SA, Huxley RR, Sattar N, Woodward M. Sex differences in the excess risk of cardiovascular diseases associated with Type 2 diabetes, potential explanations and clinical implications. Curr Cardiovasc Risk Rep. 2015; 9: 36.
- Jaimes-Hoy L, Romero F, Charli JL, Joseph-Bravo P. Sex dimorphic responses of the hypothalamus-pituitary-thyroid axis to maternal separation and palatable diet. Front Endocrinol (Lausanne). 2019; 10: 445.
- Nishi M. Effects of early-life stress on the brain and behaviors, implications of early maternal separation in rodents. Int J Mol Sci. 2020; 21: 7212.
- Bernardi JR, Ferreira CF, Senter G, Krolow R, de Aguiar BW, Portella AK, et al. Early life stress interacts with the diet deficiency of omega-3 fatty acids during the life course increasing the metabolic vulnerability in adult rats. PLOS ONE. 2013; 8: e62031.
- Glassman AH. Depression and cardiovascular comorbidity. Dial Clin Neurosci. 2007; 9: 9-17.
- Grippo AJ, Johnson AK. Stress, depression and cardiovascular dysregulation, a review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress. 2009; 12: 1-21.
- McNeal N, Scotti MA, Wardwell J, Chandler DL, Bates SL, Larocca M, et al. Disruption of social bonds induces behavioural and physiological dysregulation in male and female prairie voles. Auton Neurosci. 2014; 180: 9-16.
- Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, et al. Adverse childhood experiences and adult risk factors for age-related disease, depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med. 2009; 163: 1135-43.
- Meczekalski B, Niwczyk O, Bala G, Szeliga A. Stress, kisspeptin, and functional hypothalamic amenorrhea. Curr Opin Pharmacol. 2022; 67: 102288.
- Sominsky L, Hodgson DM, McLaughlin EA, Smith R, Wall HM, Spencer SJ. Linking stress and infertility, a novel role for ghrelin. Endocr Rev. 2017; 38: 432-67.
- Pook M, Tuschen-Caffier B, Kubek J, Schill WB, Krause W. Personality, coping and sperm count. Andrologia. 2005; 37: 29-35.
- Chen Y, Wang Q, Wang FF, Gao HB, Zhang P. Stress induces glucocorticoid mediated apoptosis of rat Leydig cells in vivo. Stress. 2012; 15: 74-84.
- Payne AH, Sha LL. Multiple mechanisms for regulation of 3â-hydroxysteroid dehydrogenase/Ä5-Ä4-isomerase, 17alpha-hydroxylase/C17-20 lyase cytochrome-P450, and cholesterol side-chain cleavage cytochrome-P450 messenger ribonucleic acid levels in primary cultures of mouse Leydig cells. Endocrinology. 1991; 129: 1429-35.
- Corona G, Giagulli VA, Maseroli E, Vignozzi L, Aversa A, Zitzmann M et al. Testosterone supplementation and body composition, results from a meta-analysis study. Eur J Endocrinol. 2016; 174: R99-116.
- Janevic T, Kahn LG, Landsbergis P, Cirillo PM, Cohn BA, Liu X, et al. Effects of work and life stress on semen quality. Fertil Steril. 2014; 102: 530-8.
- Hjollund NH, Bonde JP, Henriksen TB, Giwercman A, Olsen J, Danish First Pregnancy Planner Study Team. Reproductive effects of male psychologic stress. Epidemiology. 2004; 15: 21-7.
- Gottsch ML, Popa SM, Lawhorn JK, Qiu J, Tonsfeldt KJ, Bosch MA, et al. Molecular properties of Kiss1 neurons in the arcuate nucleus of the mouse. Endocrinology. 2011; 152: 4298-309.
- Li XF, Kinsey-Jones JS, Cheng Y, Knox AM, Lin Y, Petrou NA, et al. Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLOS ONE. 2009; 4: e8334.
- Smith JT. Sex steroid regulation of kisspeptin circuits. Adv Exp Med Biol. 2013; 784: 275-95.
- Suter DE, Schwartz NB. Effects of glucocorticoids on secretion of luteinizing hormone and folliclestimulating hormone by female rat pituitary cells in vitro. Endocrinology. 1985; 117: 849-54.
- Kheradmand A, Roshangar L, Taati M. The role of ghrelin on the morphometry and intracellular changes in the rat testis. Tissue Cell. 2009; 41: 105-11.
- Schreiber JR, Nakamura K, Erickson GF. Rat ovary glucocorticoid receptor, identification and characterization. Steroids. 1982; 39: 569-84.
- Tetsuka M, Milne M, Simpson GE, Hillier SG. Expression of 11beta-hydroxysteroid dehydrogenase, glucocorticoid receptor, and mineralocorticoid receptor genes in rat ovary. Biol Reprod. 1999; 60: 330-5.
- Michael AE, Pester LA, Curtis P, Shaw RW, Edwards CR, Cooke BA. Direct inhibition of ovarian steroidogenesis by cortisol and the modulatory role of 11 beta-hydroxysteroid dehydrogenase. Clin Endocrinol (Oxf). 1993; 38: 641-4.
- Rak-Mardyla A, Wrobel A, Gregoraszczuk EL. Ghrelin negatively affects the function of ovarian follicles in mature pigs by direct action on basal and gonadotropin-stimulated steroidogenesis. Reprod Sci. 2015; 22: 469-75.
- Yang JG, Chen WY, Li PS. Effects of glucocorticoids on maturation of pig oocytes and their subsequent fertilizing capacity in vitro. Biol Reprod. 1999; 60: 929-36.
- Van Merris V, Van Wemmel K, Cortvrindt R. In vitro effects of dexamethasone on mouse ovarian function and pre-implantation embryo development. Reprod Toxicol. 2007; 23: 32-41.
- Gonzalez R, Ruiz-Léon Y, Gomendio M, Roldan ER. The effect of glucocorticoids on ERK-1/2 phosphorylation during maturation of lamb oocytes and their subsequent fertilization and cleavage ability in vitro. Reprod Toxicol. 2010; 29: 198-205.
- Lin YS, Li XF, Shao B, Hu MH, Goundry AL, Jeyaram A, et al. The role of GABAergic signalling in stress-induced suppression of gonadotrophin-releasing hormone pulse generator frequency in female rats. J Neuroendocrinol. 2012; 24: 477-88.
- Li X, Shao B, Lin C, O’Byrne KT, Lin Y. Stress-induced inhibition of LH pulses in female rats, role of GABA in arcuate nucleus. J Mol Endocrinol. 2015; 55: 9-19.
- Bilger M, Heger S, Brann DW, Paredes A, Ojeda SR. A conditional tetracycline-regulated increase in gamma amino butyric acid production near luteinizing hormone-releasing hormone nerve terminals disrupts estrous cyclicity in the rat. Endocrinology. 2001; 142: 2102-14.
- Keen KL, Burich AJ, Mitsushima D, Kasuya E, Terasawa E. Effects of pulsatile infusion of the GABA(A) receptor blocker bicuculline on the onset of puberty in female rhesus monkeys. Endocrinology. 1999; 140: 5257-66.
- Gelbaya TA, Potdar N, Jeve YB, Nardo LG. Definition and epidemiology of unexplained infertility. Obstet Gynecol Surv. 2014; 69: 109-15.
- Li J, Wu Q, Wu XK, Zhou ZM, Fu P, Chen XH, et al. Effect of exposure to second-hand smoke from husbands on biochemical hyperandrogenism, metabolic syndrome and conception rates in women with polycystic ovary syndrome undergoing ovulation induction. Hum Reprod. 2018; 33: 617-25.
- Teede H, Deeks A, Moran L. Polycystic ovary syndrome, A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010; 8: 41.
- Teede H, Misso M, Costello M, Dokras A, Laven J, Moran L, et al. International evidence-based guideline for the assessment and management of polycystic ovary syndrome 2018, National Health and Medical Research Council [NHMRC], Canberra, Australia. 2018; 1-198.
- Wijeyaratne CN, Seneviratne RD, Dahanayake S, Kumarapeli V, Palipane E, Kuruppu N, et al. Phenotype and metabolic profile of South Asian women with polycystic ovary syndrome (PCOS), results of a large database from a specialist Endocrine Clinic. Hum Reprod. 2011; 26: 202-13.
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to Polycystic Ovary Syndrome (PCOS). Hum Reprod. 2004; 19: 41-7.
- Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States, A prospective study. J Clin Endocrinol Metab. 1998; 83: 3078-82.
- Diamanti-Kandarakis E, Kouli CR, Bergiele AT, Filandra FA, Tsianateli TC, Spina GG, et al. A survey of the polycystic ovary syndrome in the Greek island of Lesbos, hormonal and metabolic profile. J Clin Endocrinol Metab. 1999; 84: 4006-11.
- Nidhi R, Padmalatha V, Nagarathna R, Amritanshu R. Prevalence of polycystic ovarian syndrome in Indian adolescents. J Pediatr Adolesc Gynecol. 2011; 24: 223-7.
- Kalra P, Bansal B, Nag P, Singh JK, Gupta RK, Kumar S, et al. Abdominal fat distribution and insulin resistance in Indian women with polycystic ovarian syndrome. Fertil Steril. 2009; 91: 1437-40.
- El Hayek S, Bitar L, Hamdar LH, Mirza FG, Daoud G. Poly cystic ovarian syndrome, an updated overview. Front Physiol. 2016; 7: 124.
- Ding H, Zhang J, Zhang F, Zhang S, Chen X, Liang W, et al. Resistance to the insulin and elevated level of androgen, A major cause of polycystic ovary syndrome. Front Endocrinol. 2021; 12: 741764.
- Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015; 36: 487-525.
- Rosenfield RL, Ehrmann DA. The pathogenesis of Polycystic Ovary Syndrome (PCOS), the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016; 37: 467-520.
- Alebic MŠ, Bulum T, Stojanovic N, Duvnjak L. Definition of insulin resistance using the homeostasis model assessment (HOMA-IR) in IVF patients diagnosed with polycystic ovary syndrome (PCOS) according to the Rotterdam criteria. Endocrine. 2014; 47: 625-30.
- Behboudi-Gandevani S, Ramezani Tehrani F, Rostami Dovom M, Farahmand M, Bahri Khomami M, Noroozzadeh M, et al. Insulin resistance in obesity and polycystic ovary syndrome, systematic review and meta-analysis of observational studies. Gynecol Endocrinol. 2016; 32: 343-53.
- Maliqueo M, Lara HE, Sánchez F, Echiburú B, Crisosto N, Sir-Petermann T. Placental steroidogenesis in pregnant women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2013; 166: 151-5.
- Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab. 1995; 80: 3689-98.
- Dumesic DA, Abbott DH, Padmanabhan V. Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Disord. 2007; 8: 127-41.
- Webber LJ, Stubbs S, Stark J, Trew GH, Margara R, Hardy K, et al. Formation and early development of follicles in the polycystic ovary. Lancet. 2003; 362: 1017-21.
- Steckler TL, Roberts EK, Doop DD, Lee TM, Padmanabhan V. Developmental programming in sheep, administration of testosterone during 60-90 days of pregnancy reduces breeding success and pregnancy outcome. Theriogenology. 2007; 67: 459-67.
- Wood RI, Foster DL. Sexual differentiation of reproductive neuroendocrine function in sheep. Rev Reprod. 1998; 3: 130-40.
- McClamrock HD, Adashi EY. Gestational hyperandrogenism. Fertil Steril. 1992; 57: 257-74.
- Sun M, Maliqueo M, Benrick A, Johansson J, Shao R, Hou L, et al. Maternal androgen excess reduces placental and fetal weights, increases placental steroidogenesis, and leads to long-term health effects in their female offspring. Am J Physiol Endocrinol Metab. 2012; 303: E1373-85.
- Barnes RB, Rosenfield RL, Ehrmann DA, Cara JF, Cuttler L, Levitsky LL, et al. Ovarian hyperandrogynism as a result of congenital adrenal virilizing disorders, evidence for perinatal masculinization of neuroendocrine function in women. J Clin Endocrinol Metab. 1994; 79: 1328-33.
- Yii MF, Lim CE, Luo X, Wong WS, Cheng NC, Zhan X. Polycystic ovarian syndrome in adolescence. Gynecol Endocrinol. 2009; 25: 634-9.
- Bronstein J, Tawdekar S, Liu Y, Pawelczak M, David R, Shah B. Age of onset of polycystic ovarian syndrome in girls may be earlier than previously thought. J Pediatr Adolesc Gynecol. 2011; 24: 15-20.
- Ibáñez L, Díaz R, López-Bermejo A, Marcos MV. Clinical spectrum of premature pubarche, links to metabolic syndrome and ovarian hyperandrogenism. Rev Endocr Metab Disord. 2009; 10: 63-76.
- Das RR, Mangaraj M, Nayak S, Satapathy AK, Mahapatro S, Goyal JP. Prevalence of insulin resistance in urban Indian school children who are overweight/obese: A cross-sectional study. Front Med (Lausanne). 2021; 8: 613594.
- Sundarakumar JS, Stezin A, Menesgere AL, Ravindranath V, SANSCOG and TLSA Collaborators. Rural-urban and gender differences in metabolic syndrome in the aging population from southern India: two parallel, prospective cohort studies. EClinicalmedicine. 2022; 47: 101395.
- Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome, a consensus statement by the androgen Excess and polycystic ovary syndrome (AE–PCOS) Society. J Clin Endocrinol Metab. 2010; 95: 2038-49.
- Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome, a systematic review and meta-analysis. Hum Reprod Update. 2010; 16: 347-63.
- Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome, a consensus statement by the androgen Excess and polycystic ovary syndrome (AE–PCOS) Society. J Clin Endocrinol Metab. 2010; 95: 2038-49.
- Rizzo M, Longo RA, Guastella E, Rini GB, Carmina E. Assessing cardiovascular risk in Mediterranean women with polycystic ovary syndrome. J Endocrinol Invest. 2011; 34: 422-6.
- Poretsky L, Bhargava G, Saketos M, Dunaif A. Regulation of human ovarian insulin receptors in vivo. Metabolism. 1990; 39: 161-6.
- Meirow D, Yossepowitch O, Rösler A, Brzezinski A, Schenker JG, Laufer N, et al. Insulin resistant and nonresistant polycystic ovary syndrome represent two clinical and endocrinological subgroups. Hum Reprod. 1995; 10: 1951-6.
- Khan IA, Jahan P, Hasan Q, Rao P. Genetic confirmation of T2DM meta-analysis variants studied in gestational diabetes mellitus in an Indian population. Diabetes Metab Syndr. 2019; 13: 688-94.
- Rassi A, Veras AB, dos Reis M, Pastore DL, Bruno LM, Bruno RV, et al. Prevalence of psychiatric disorders in patients with polycystic ovary syndrome. Compr Psychiatry. 2010; 51: 599-602.
- Dokras A, Clifton S, Futterweit W, Wild R. Increased prevalence of anxiety symptoms in women with polycystic ovary syndrome, systematic review and meta-analysis. Fertil Steril. 2012; 97: 225-30.e2.
- Mustaniemi S, Vääräsmäki M, Eriksson JG, Gissler M, Laivuori H, Ijäs H, et al. Polycystic ovary syndrome and risk factors for gestational diabetes. Endocr Connect. 2018; 7: 859-69.
- Reddy BM, Kommoju UJ, Dasgupta S, Rayabarapu P. Association of type 2 diabetes mellitus genes in polycystic ovary syndrome aetiology among women from southern India. Indian J Med Res. 2016; 201: 400-8.
- Christopoulos P, Mastorakos G, Gazouli M, Panidis D, Deligeoroglou E, Katsikis I, et al. Genetic variants in TCF7L2 and KCNJ11 genes in a Greek population with polycystic ovary syndrome. Gynecol Endocrinol. 2008; 24: 486-90.
- Day F, Karaderi T, Jones MR, Meun C, He C, Drong A, et al. Large-scale genome-Wide Meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLOS Genet. 2018; 14: e1007813.
- Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003; 88: 2404-11.
- Gupta R, Mohan I, Narula J. Trends in coronary heart disease epidemiology in India. Ann Glob Health. 2016; 82: 307-15.
- Donato GB, Fuchs SC, Oppermann K, Bastos C, Spritzer PM. Association between menopause status and central adipos¬ity measured at different cutoffs of waist circumference and waist-to-hip ratio. Menopause. 2006; 13: 280-5.
- Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamimcs balance, implications for human blood pressure regulation. Hypertension. 2009; 53: 571-6.
- Adlakha N. How urban design impacts mental health Poorly designed cities and buildings impact us in more ways than one. We could use the post-Covid era as a chance to make changes in our living environment. 2020.
- Gunning MN, Fauser BCJM. Are women with polycystic ovary syndrome at increased cardiovascular disease risk later in life? Climacteric. 2017; 20: 222-7.
- Lønnebotn M, Natvig GK, Benediktsdóttir B, Burgess JA, Holm M, Jógi R, et al. Polycystic ovary syndrome, body mass index and hypertensive disorders in pregnancy. Pregnancy Hypertens. 2018; 11: 32-7.
- Kakoly NS, Khomami MB, Joham AE, Cooray SD, Misso ML, Norman RJ, et al. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS, a systematic review and meta-regression. Hum Reprod Update. 2018; 24: 455-67.
- Huber-Buchholz MM, Carey DG, Norman RJ. Restoration of reproductive potential by lifestyle modification in obese polycystic ovary syndrome, role of insulin sensitivity and luteinizing hormone. J Clin Endocrinol Metab. 1999; 84: 1470-4.
- Lass N, Kleber M, Winkel K, Wunsch R, Reinehr T. Effect of lifestyle intervention on features of polycystic ovarian syndrome, metabolic syndrome, and intima-media thickness in obese adolescent girls. J Clin Endocrinol Metab. 2011; 96: 3533-40.
- Aziz Z, Absetz P, Oldroyd J, Pronk NP, Oldenburg B. A systematic review of real-world diabetes prevention programs, learnings from the last 15 years. Implement Sci. 2015; 10: 172.
- Hutchison SK, Stepto NK, Harrison CL, Moran LJ, Strauss BJ, Teede HJ. Effects of exercise on insulin resistance and body composition in overweight and obese women with and without polycystic ovary syndrome. J Clin Endocrinol Metab. 2011; 96: E48-56.
- Roberts CK, Little JP, Thyfault JP. Modification of insulin sensitivity and glycemic control by activity and exercise. Med Sci Sports Exerc. 2013; 45: 1868-77.
- Parazzini F, Marchini M, Luchini L, Tozzi L, Mezzopane R, Fedele L. Tight underpants and trousers and risk of dyspermia. Int J Androl. 1995; 18: 137-40.
- Cai D, Liu T. Hypothalamic inflammation, a double-edged sword to nutritional diseases. Ann N Y Acad Sci. 2011; 1243: E1-39.
- Weiss TW, Seljeflot I, Hjerkinn EM, Arnesen H. Adipose tissue pro-inflammatory gene expression is associated with cardiovascular disease. Int J Clin Pract. 2011; 65: 939-44.
- Ramírez F, Fowell DJ, Puklavec M, Simmonds S, Mason D. Glucocorticoids promote a TH2 cytokine response by CD4+ T cells in vitro. J Immunol. 1996; 156: 2406-12.
- Jensen LE, Whitehead AS. Regulation of serum amyloid A protein expression during the acute-phase response. Biochem J. 1998; 334: 489-503.
- Bedoya F. Climate change, an emergent issue of public health in Peru. HPHR. 2018; 17: 1-6.
- Hashim JH, Hashim Z. Climate change, extreme weather events, and human health implications in the Asia Pacific region. Asia Pac J Public Health. 2016; 28: 8S-14S.
- Watts N, Amann M, Arnell N, Ayeb-Karlsson S, Belesova K, Boykoff M, et al. The 2019 report of the Lancet Countdown on health and climate change, ensuring that the health of a child born today is not defined by a changing climate. Lancet. 2019; 394: 1836-78.
- Vincent KE, et al. Cross-chapter box on Gender and Climate Change. Clim Change. Impacts, adaptation, and vulnerability. Part A. Global and sectoral aspects. Contribution of working Group II to the fifth assessment report of the Intergovernmental Panel on Climate Change Field CB, et al., editors. 2014; 105-107.
- Hurlbert M, et al. Chapter 7 Rodrigues R, Turner BL II, editors. Risk management and decision making in relation to sustainable development. IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems. 2019.
- Naswa P, Shrestha B. 2020.
- Gherasim A, Arhire LI, Nita O, Popa AD, Graur M, Mihalache L. The relationship between lifestyle components and dietary patterns. Proc Nutr Soc. 2020; 79: 311-23.
- Abbasalizad Farhangi M, Vajdi M, Nikniaz L, Nikniaz Z. The interaction between dietary inflammatory index and 6 P21 rs2010963 gene variants in metabolic syndrome. Eat Weight Disord. 2020; 25: 1049-60.
- Tavakolizadeh J, Goli F, Ebrahimi A, Hajivosough NS, Mohseni S. Effectiveness of a bioenergy economy-based psycho-education package on improvement of vegetative function, forgiveness, and quality of life of patients with coronary heart disease, A randomized clinical trial. Int J Body Mind Cult. 2021; 8: 36-50.
- Hyder KM, Mohan J, Varma V, Sivasankaran P, Raja D. Effects of muscle-specific exercises compared to existing interventions on insulin resistance among prediabetes population of South India. J Nat Sci Biol Med. 2021;12(2):230-6.
- Bazrafshani S, Randhawa H, Ghaedi Y, Khan S, Sharbatti S. The prevalence of overweight and obesity among health care providers in the emirate of Ajman, UAE. J Complement Med Res. 2020;11(3):40-50.
- Pathak G, Nichter M. Polycystic ovary syndrome in globalizing India, An ecosocial perspective on an emerging lifestyle disease. Soc Sci Med. 2015;146:21-8.
- Parker J, O’Brien C. Evolutionary and genetic antecedents to the pathogenesis of polycystic ovary syndrome [PCOS]. J ACNEM. 2021;40:12-20.
- Androulakis II, Kandaraki E, Christakou C, Karachalios A, Marinakis E, Paterakis T, et al. Visceral adiposity index (VAI) is related to the severity of anovulation and other clinical features in women with polycystic ovary syndrome. Clin Endocrinol (Oxf). 2014; 81: 426-31.
- Morley LC, Tang T, Yasmin E, Norman RJ, Balen AH. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev. 2017; 11: CD003053.
- Vryonidou A, Paschou SA, Muscogiuri G, Orio F, Goulis DG. MECHANISMS IN ENDOCRINOLOGY, Metabolic syndrome through the female life cycle. Eur J Endocrinol. 2015; 173: R153-63.
- Mitchell M, Armstrong DT, Robker RL, Norman RJ. Adipokines: implications for female fertility and obesity. Reproduction. 2005; 130: 583-97.
- Bruns CM, Kemnitz JW. Sex hormones, insulin sensitivity, and diabetes mellitus. ILAR J. 2004; 45: 160-9.
- Suliga E, Ciesla E, Rebak D, Koziel D, Gluszek S. Relationship between sitting time, physical activity, and metabolic syndrome among adults depending on Body Mass Index (BMI). Med Sci Monit. 2018; 24: 7633-45.
- Stefani KM, Kim HC, Kim J, Oh K, Suh I. The influence of sex and age on the relationship between sleep duration and metabolic syndrome in Korean adults. Diabetes Res Clin Pract. 2013; 102: 250-9.
- Rafati S, Isheh M, Azarbad A, Ghadiri Soufi F, Rahimi A, Kheirandish M. The association of sleep duration and metabolic syndrome in the Bandare-Kong cohort study, a cross-sectional survey (finding from Persian cohort study). Diabetol Metab Syndr. 2021; 13: 114.
- Tan X, Chapman CD, Cedernaes J, Benedict C. Association between long sleep duration and increased risk of obesity and type 2 diabetes, a review of possible mechanisms. Sleep Med Rev. 2018; 40: 127-34.
- Vandekerckhove M, Wang YL. Emotion, emotion regulation and sleep, An intimate relationship. AIMS Neurosci. 2018; 5: 1-17.
- Makris AP, Foster GD. Dietary approaches to the treatment of obesity. Psychiatr Clin North Am. 2005; 28: 117-39.
- Larsen TM, Dalskov SM, van Baak M, Jebb SA, Papadaki A, Pfeiffer AF, et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010; 363: 2102-13.
- Brown AJ, Setji TL, Sanders LL, Lowry KP, Otvos JD, Kraus WE, et al. Effects of exercise on lipoprotein particles in women with polycystic ovary syndrome. Med Sci Sports Exerc. 2009; 41: 497-504.
- World Health Organization. Obes Prev Manag Glob Epidemic. 1998.
- National Institutes of Health – National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Obes Res. 1998; 6: 51S-210S.
- Dorostghoal M, Erfani Majd N, Nooraei P. Maternal caffeine consumption has irreversible effects on reproductive parameters and fertility in male offspring rats. Clin Exp Reprod Med. 2012; 39: 144-52.
- Braga DP, Halpern G, Figueira R, Setti AS, Iaconelli A Jr, Borges E Jr. Food intake and social habits in male patients and its relationship to intracytoplasic sperm injection. Fertil Steril. 2012; 97: 53-9.
- Attaman JA, Toth TL, Furtado J, Campos H, Hauser R, Chavarro JE. Dietary fat and semen quality among men attending a fertility clinic. Hum Reprod. 2012; 27: 1466-74.
- Afeiche MC, Gaskins AJ, Williams PL, Toth TL, Wright DL, Tanrikut C, et al. Processed meat intake is unfavorably and fish intake favorably associated with semen quality indicators among men attending a Fertility Clinic. J Nutr. 2014; 144: 1091-8.
- Ricci E, Al-Beitawi S, Cipriani S, Alteri A, Chiaffarino F, Candiani M, et al. Dietary habits and semen parameters, a systematic narrative review Andrology. Andrology. 2018; 6: 104-16.
- Santos M, Rodríguez-González GL, Ibáñez C, Vega CC, Nathanielsz PW, Zambrano E. Adult exercise effects on oxidative stress and reproductive programming in male offspring of obese rats. Am J Physiol Regul Integr Comp Physiol. 2015; 308: R219-25.
- Rato L, Alves MG, Cavaco JE, Oliveira PF. High-energy diets, a threat for male fertility? Obes Rev. 2014; 15: 996-1007.
- Hjollund NH, Storgaard L, Ernst E, Bonde JP, Olsen J. The relation between daily activities and scrotal temperature. Reprod Toxicol. 2002; 16: 209-14.
- Mastaloudis A, Leonard SW, Traber MG. Oxidative stress in athletes during extreme endurance exercise. Free Radic Biol Med. 2001; 31: 911-22.
- Saleh RA, Agarwal A. Oxidative stress and male infertility, from research bench to clinical practice. J Androl. 2002; 23: 737-52.
- Safarinejad MR, Azma K, Kolahi AA. The effects of intensive, long-term treadmill running on reproductive hormones, hypothalamus-pituitary-testis axis, and semen quality, a randomized controlled study. J Endocrinol. 2009; 200: 259-71.
- Lalinde-Acevedo PC, BJM M-T, Agarwal A, du Plessis SS, Ahmad G, Cadavid ÁP, et al. Physically Active Men Show Better Semen Parameters than Their Sedentary Counterparts. Int J Fertil Steril. 2017; 11: 156–65.
- Gaskins AJ, Mendiola J, Afeiche M, Jørgensen N, Swan SH, Chavarro JE. Physical activity and television watching in relation to semen quality in young men. Br J Sports Med. 2015; 49: 265-70.
- La Vignera S, Condorelli RA, Vicari E, D’Agata R, Calogero AE. Effects of the exposure to mobile phones on male reproduction, a review of the literature. J Androl. 2012; 33: 350-6.
- Lee HJ, Pack JK, Kim TH, Kim N, Choi SY, Lee JS, et al. The lack of histological changes of CDMA cellular phone-based radio frequency on rat testis. Bioelectromagnetics. 2010; 31: 528-34.
- Figà-Talamanca I, Cini C, Varricchio GC, Dondero F, Gandini L, Lenzi A, et al. Effects of prolonged autovehicle driving on male reproductive function, a study among taxi drivers. Am J Ind Med. 1996; 30: 750-8.
- Yamagata K. Do coffee polyphenols have a preventive action on metabolic syndrome associated endothelial dysfunctions? An assessment of the current evidence. Antioxidants (Basel). 2018; 7: 26.
- Wong THT, Wong CH, Zhang X, Zhou Y, Xu J, Yuen KC, et al. Louie JCY. The association between coffee consumption and metabolic syndrome in adults, a systematic review and meta-analysis. Adv Nutr. 2021; 12: 708-21.
- Feng T, Elson CO. Adaptive immunity in the host-microbiota dialog. Mucosal Immunol. 2011; 4: 15-21.
- Luck H, Khan S, Kim JH, Copeland JK, Revelo XS, Tsai S, et al. Gut-associated IgA+ immune cells regulate obesity-related insulin resistance. Nat Commun. 2019; 10: 3650.
- Petersen C, Bell R, Klag KA, Lee SH, Soto R, Ghazaryan A, et al. T cell-mediated regulation of the microbiota protects against obesity. Science. 2019; 365: eaat9351.
- Tilg H, Zmora N, Adolph TE, Elinav E. The intestinal microbiota fuelling metabolic inflammation. Nat Rev Immunol. 2020; 20: 40-54.
- Paragh G, Seres I, Harangi M, Fülöp P. Dynamic interplay between metabolic syndrome and immunity. Adv Exp Med Biol. 2014; 824: 171-90.
- Luan YY, Zhang L, Peng YQ, Li YY, Liu RX, Yin CH. Immune regulation in polycystic ovary syndrome. Clin Chim Acta. 2022; 531: 265-72.
- National Heart Lung and Blood Institute (NHLBI) and the North American Association for the Study of Obesity (NAASO). The practical guide, identification, evaluation, and treatment of overweight and obesity in adults. Bethesda: National Institutes of Health; 2000.
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002; 346: 393-403.
- Kakoly NS, Khomami MB, Joham AE, Cooray SD, Misso ML, Norman RJ, et al. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: a systematic review and meta-regression. Hum Reprod Update. 2018; 24: 455-67.
- Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2012; 18: 618-37.
- Poehlman ET, Dvorak RV, DeNino WF, Brochu M, Ades PA. Effects of resistance training and endurance training on insulin sensitivity in nonobese, young women: a controlled randomized trial. J Clin Endocrinol Metab. 2000; 85: 2463-8.
- Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, Balise RR, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women, the A TO Z Weight Loss Study, a randomized trial. JAMA. 2007; 297: 969-77.
- Iqbal N, Vetter ML, Moore RH, Chittams JL, Dalton-Bakes CV, Dowd M, et al. Effects of a low-intensity intervention that prescribed a low-carbohydrate vs a low-fat diet in obese, diabetic participants. Obesity (Silver Spring). 2010; 18: 1733-8.
- Fabricatore AN, Wadden TA, Ebbeling CB, Thomas JG, Stallings VA, Schwartz S, et al. Targeting dietary fat or glycemic load in the treatment of obesity and type 2 diabetes, a randomized controlled trial. Diabetes Res Clin Pract. 2011; 92: 37-45.
- Rolls BJ, Roe LS, Beach AM, Kris-Etherton PM. Provision of foods differing in energy density affects long-term weight loss. Obes Res. 2005; 13: 1052-60.
- Ello-Martin JA, Roe LS, Ledikwe JH, Beach AM, Rolls BJ. Dietary energy density in the treatment of obesity, a year-long trial comparing 2 weight-loss diets. Am J Clin Nutr. 2007; 85: 1465-77.
- Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008; 359: 229-41.
- Gudzune K. Dietary and behavioral approaches in the management of obesity. Gastroenterol Clin North Am. 2016; 45: 653-61.
- Lee K, Kim H, Rebholz CM, Kim J. Association between different types of plant-based diets and risk of dyslipidemia, A prospective cohort study. Nutrients. 2021; 13: 220.
- Cullmann M, Hilding A, östenson CG. Alcohol consumption and risk of pre-diabetes and type 2 diabetes development in a Swedish population. Diabet Med. 2012; 29: 441-52.
- Guasch-Ferré M, Zong G, Willett WC, Zock PL, Wanders AJ, Hu FB, et al. Associations of monounsaturated fatty acids from plant and animal sources with total and cause-specific mortality in two US prospective cohort studies. Circ Res. 2019; 124: 1266-75.
- Yaribeygi H, Atkin SL, Simental-Mendía LE, Sahebkar A. Molecular mechanisms by which aerobic exercise induces insulin sensitivity. J Cell Physiol. 2019; 234: 12385-92.
- He X, Wu D, Hu C, Xu T, Liu Y, Liu C, et al. Role of metformin in the treatment of patients with thyroid nodules and insulin resistance, A systematic review and meta-analysis. Thyroid. 2019; 29: 359-67.
- Chang HC, Hsieh CF, Tantoh DM, Ko PC, Kung YY, Lin MC, et al. HDL and associated factors stratified by sex and menopausal status, results from a community-based survey in Taiwan. Oncotarget. 2018; 9: 16354-67.
- Rasaei B, Karim NA, Talib RA, Noor IM, Karandish M. The effect of simultaneous consumption of coffee caffeine and sleep deprivation on plasma ghrelin and leptin levels. Int J Nutr Sci. 2019; 4: 88-96.
- Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr. 2010; 91: 1550-9.
- Wu Y, Zhai L, Zhang D. Sleep duration and obesity among adults, a meta-analysis of prospective studies. Sleep Med. 2014; 15: 1456-62.
- Naderpoor N, Shorakae S, de Courten B, Misso ML, Moran LJ, Teede HJ. Metformin and lifestyle modification in polycystic ovary syndrome, systematic review and meta-analysis. Hum Reprod Update. 2015; 21: 560-74.
- Teede HJ, Hutchison SK, Zoungas S. The management of insulin resistance in polycystic ovary syndrome. Trends Endocrinol Metab. 2007; 18: 273-9.
- Clark AM, Thornley B, Tomlinson L, Galletley C, Norman RJ. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod. 1998; 13: 1502-5.