
Review Article
Austin J Vet Sci & Anim Husb. 2025; 12(3): 1171.
Review on Epidemiology of Leptospirosis
Beshah A¹, Desa G² and Zenebe T³
¹Oromia Agriculture Bureau, Tulubolo, Ethiopia
²Animal Health Institute, Sebeta, Ethiopia
²Ethiopian Institute of Agricultural Research, Addis Ababa, Ethiopia
*Corresponding author: Abay Beshah, Oromia Agriculture Bureau, Tulubolo, Ethiopia Email: tilahun136@gmail.com
Received: June 03, 2025 Accepted: July 10, 2025 Published: July 14, 2025
Abstract
Leptospirosis is a zoonotic disease caused by infection with pathogenic species of Leptospira, which are spirochetes that can infect humans and animals. The bacteria can be found worldwide but is most commonly associated with tropical and subtropical regions. The disease has a high social and economic impact, especially in regions with environmental conditions that support the persistence of the bacteria. Therefore, the aim of this seminar paper is to understand the leptospirosis epidemiology, diagnosis, control and prevent the disease. Dogs play an essential role in transmitting the disease to humans through close contact. Prevention strategies for leptospirosis are based on education about the epidemiology and transmission mechanisms of the bacteria, particularly for occupationally exposed workers who come into contact with contaminated water or infected animals. The high disease burden and the limited coverage of effective vaccines highlight the lack of attention given to leptospirosis. Efforts to combat leptospirosis have been ongoing, with the World Health Organization establishing the Leptospirosis Burden Epidemiology Reference Group. Elimination of the carrier state, control of rodents in kennels, maintenance of environmental condition to discourage bacterial survival and isolation of infected animal need to be under taken in order to prevent the disease. Leptospirosis is highly transmitted to animal and human through contaminated food and water. Important control measures including control of livestock infection with good sanitation, immunization, and proper veterinary care should be taken.
Keyword: Epidemiology; Leptospirosis; Review
Introduction
Leptospirosis has been bacterial disease of human and numerous mammalian species resulting in morbidity and mortality. The disease has a global impact on human health and is considered to be burdening the world economy. More than 60,000 people die due to leptospirosis annually and almost one million are reported to be affected [1]. Leptospirosis is caused by a pathogenic spirochete bacterium of the genus Leptospira. Leptospira interrogans is a pathogenic species that cause leptospirosis while L. biflexa is nonpathogenic [2]. Leptospira have characteristic hooked ends and are tightly coiled with approximately 18 coils per cell [3]. Leptospires have a typical double membrane structure in which the cytoplasmic membrane and peptidoglycan cell wall are closely associated and are overlaid by an outer membrane [4].
When pathogenic Leptospira persist for up to many months under favorable conditions, they are expelled by the urine into the environment from the kidneys of natural hosts, primarily animals. Like, exposure to diseased animals, their urine, or an environment polluted by their pee (most commonly dirt and water) can cause infection. The mucous membranes and exposed skin are the contamination routes. A huge number of sylvatic and domestic animals serve as the reservoir of Leptospira, and leptospirosis is typically diagnosed in various animal types. There are more than 200 known L. interrogans human infections. Leptospirosis is caused by pathogenic serovars and is spread through a number of different mechanisms [5].
The central point on the epidemiology of leptospirosis is the state of the renal carrier, the animal that has its renal tubules colonized by leptospirae, which in turn are excreted in the urine infecting the environment [6]. Animal habitat sanitation, the availability of veterinary services for the quick detection and treatment of animal leptospirosis, and control programs for animal leptospirosis are just a few examples of the factors that can determine the source of infection in a given area [7]. At least one species of Leptospira seems to affect all mammals. Cattle, dogs, lambs, and pigs are reservoir hosts that can serve as temporary carriers for a period of time, whereas rodents typically serve as permanent carriers for the entirety of their lives. Therefore, it is believed that rodents are the main source of infection [8].
The main sources of the infection are urine of infected or carrier animals, contaminated surface water, mud, feed, soil, aborted fetuses and uterine discharges [9]. The disease can be directly transmitted through interaction with secretions, blood or urine of diseased animals, or indirectly through water polluted mainly with urine of reservoir animals. The core determinants of transmission of leptospiral infection are the presence of carrier animals, suitability of the environment for the survival of leptospirosis and its one health aspect [10].
Leptospirae penetrate intact mucous membrane of the oral cavity, nose, and eyes or abraded, scratched or water soft skin. They multiply rapidly after entering the vascular system, spread and further replicate in many tissues including kidney, liver, spleen, central nervous system, eye and genital tract. The incubation period of leptospirosis depends on dose, infectious strain and host but is averagely between 7-14 days [11]. Serology is the most frequently used diagnostic approach for leptospirosis [12].
Leptospirosis can be treated by antibiotics such as tetracycline, penicillin, doxycycline, streptomycin and erythromycin [13], while prevention is characterized by sanitary control and decrease in the risk of infection occurring due to contact with contaminated environments, infected wild animals and rodents [14]. Designing strategies to lower the likelihood of the disease's transmission requires an understanding of the epidemiological characteristics of leptospirosis [9]. Leptospirosis in domestic animals can be prevented by vaccination, prophylactic antibiotic treatment of exposed animals, quarantining newly introduced animals of any species for at least four weeks, rodent control, routine serological testing, improved environmental hygiene, separating young animals from adults, and safe artificial insemination [15].
Leptospirosis has been recognized as a re-emerging global public health problem due to the increased incidence in both developing and developed countries [16]. Leptospirosis affects risk groups that are exposed to animal reservoirs or contaminated environments, such as abattoir and sewage workers, salver workers, coal mines, plumbers, farm workers, veterinarians, pet shop owners, meat handlers, military personnel, slaughter house workers and workers in fishing industry [17]. Therefore, the aim of this seminar paper is to understand the leptospirosis causative agent, epidemiology, diagnosis, control and prevent the disease.
Literature Review
The Causative Agent
The taxonomic group of the disease Leptospira responsible for causing leptospirosis is classified under the order Spirochaetales, and is further sub-divided into three species namely saprophytic (like the Leptospira biflexa), highly pathogenic Leptospira interrogans and host interdependent Leptospira borgpetersenii [18,19]. The infection is biphasic, with a septicemic phase followed by an immune phase with antibody production and the urinary excretion of the organism [20].
Morphologically leptospires are corkscrew-shaped bacteria, which differ from other spirochaetes by the presence of end hooks. Leptospires have a typical double membrane structure in which the cytoplasmic membrane and peptidoglycan cell wall are closely associated and are overlaid by an outer membrane [4] (Figure 1).
Figure 1: Causative agent of Leptospirosis (Source: Levett et al., [9]).
There are 22 species of genus Leptospira among which 10 of them are regarded as pathogenic (Leptospira interrogans, L. kirschneri, L. noguchii, L. alexanderi, L. weilii, L. alstonii, L. borgpetersenii, L. santarosai, L. kmetyi, and L. mayottensis), 5 of them are of intermediate or unclear pathogenicity (L. inadai, L. fainei, L. broomii, L. licerasiae, and L wolffii), and the remaining 7 are nonpathogenic free-living organism species that do not contaminate animal hosts (L. biflexa, L. meyeri, L. wolbachii, L. vanthielii, L. terpstrae, L. yanagawae, and L. idonii) [21].
Physicochemical Properties of Leptospires
Moist environments with a neutral pH (7) deliver suitable conditions for survival of leptospires. Soil moisture, surface water temperature and humidity influence the life of leptospires in the environment [22,23]. They optimally survive for weeks in the environment situation. These organisms do not survive in freezing conditions. They are killed by dehydration or temperature in excess of 50°C. For disinfection purposes, leptospires are inactivated by 70% ethanol, glutaraldehyde, formaldehyde, detergents and acid. They are also destroyed by moist heat, at 121°C for 15 minutes and by pasteurization [24]. Leptospires are obligate aerobes with an optimum growth temperature of 28–30°C. They develop on basic media that has been supplemented with vitamins B1 and B12, long chain fatty acids, and ammonium salts.As the only carbon supply, longchain fatty acids are processed through – oxidation [6].
Leptospira has the general structural characteristics that distinguish spirochetes from other bacteria. The cell is encased in a threeto five-layer outer membrane. Beneath this outer membrane are the flexible, the helical peptidoglycan layer and that of the cytoplasmic membrane; these encompass the cytoplasmic contents of the cell. The structures surrounded by the outer membrane are collectively called the protoplasmic cylinder. An unusual feature of the spirochetes is the location of the flagella, which lie between the outer membrane and thepeptidoglycalayer. Leptospira specious differ significantly from other spirochete infection s like Treponema pallidum and Borrelia burgdorfer while sharing a number of general characteristics with other [2].
While other spirochetes' basal bodies resemble those of Grampositive bacteria, those of Leptospira periplasmic flagella are comparable to those of Gram-negative bacteria. Leptospira differs from other spirochetes in lacking glycolipids and having diaminopimelic acid rather than ornithine in its peptidoglycan. The periplasmic flagella are attached to the protoplasmic cylinder sub terminally at each end and extend toward the center of cell, but do not overlap as they do in other spirochetes. The number of periplasmic flagella per cell varies among the spirochetes. In liquid media, one or both ends are normally hooked. They are too thin to be visible under the ordinary microscope. Dark-field microscope is the most often in use to detect leptospires [5].
Epidemiology of Leptospirosis
Leptospirosis has a worldwide distribution. Many animals serve as reservoir of infections, widely distributed geographically and occurs mostly preferred area are tropical, subtropical and temperate zones [25]. Disease is recorded in both sexes with predominance in young adult males, and in urban and rural settings, it is an important occupational zoonosis of agriculture worker, sewer workers, mine workers, slaughterhouse employees, butchers, dairy farmers, veterinarians, animal handlers, kennel attendants, sanitary workers, construction workers, military personnel’s and fishermen [5].
The disease occurs in both temperate and tropical regions; the incidence in the tropics is approximately 10 times higher than in temperate regions [6]. This is attributed mainly to longer survival of leptospires in the warm and humid of environments. Leptospirosis is not limited to developing countries. Retrospective reviews of the disease epidemiology have been reported from the country Ireland, Denmark and Italy [20].
Wild and some domestic mammals like (cattle, pigs and dogs) are as well as reptiles and amphibians serve as a source of permanent maintenance hosts or reservoirs of the disease for more than 250 best-known serovars of the genus Leptospira. The organism infects a variety of both wild and domestic mammals, especially rodents, cattle, swine, dogs, horses, sheep, and goats. The disease rarely occurs in cats. Animals can be asymptomatic or develop clinical infection, which can be fatal. Infection in small rodents usually occurs during infancy, and, once infected, animals may shed the organism in their urine intermittently or continuously throughout life, resulting in contamination of the environment, particularly water [20].
Leptospires are universal spirochetes. The pathogenic leptospires shed in the urine of the carrier animals, contaminate the environment and cause human and animal infection. A seasonal pattern of disease has been identified, with peak incidences in warm climatic regions during the rainy seasons and summer or fall in temperate zones [9]. While the organism species is a usual contaminant of surface waters, pathogenic leptospires mainly colonize the proximal tubules of nephrons of their natural host animals (mainly in rodents and domestic mammals) and are excreted in urine [1,26]. Pathogenic leptospires do not multiply outside the host animals or in the environment [22].
The source of desease in an area is determined by factors such as rodent density, the population size of the farm and other domestic animals, the sanitation of animal habitats, availability of veterinary services for prompt detection and treatment of animal leptospirosis, and the control programs for animal leptospirosis etc. Several rodent species were related with the disease including Rattus rattus, R. norvegicus, Mus musculus, Bandicota bengalensis, Bandicota indica, and others [7,14].
Another animal like dogs although vaccinated against Leptospira can shed the organism in their urine and this may result in domestic transmission in the humans. In general, an infected animal can remain symptom-free and shed infectious organisms in the urine for its entire lifetime [27].
Infected animals transfer the leptospirosis infectious agent to their offspring either in-utero or during neonatal period. The disease is should be considered as a disease of the environment. In this regard, a lot of outbreaks have been related to heavy rain falls in various rural locations including India, Salvador, Nicaraguan, Philippines, Peru and Argentina [28,29]. In livestock, leptospires have been evidenced not only in the urine but also in semen and vaginal discharges, characterizing a reproductive disorder among animals [30].
Leptospires require special conditions for their development. Alkaline soil, muck, swamps, streams, rivers, animal organs, tissues, and diluted milk are among environments in which they can survive. The environment's PH, temperature, and the presence of an inhibition substance are all important for pathogenic leptospire survival. The microorganism survives in the environment if mean temperature remains at about 220C year round and the fluctuations are not more than 50C [31]. In general, they are sensitive to dryness, heat, acids and basic disinfectants [6]. Natural hosts are disseminating the agent in nature through their urine [32], because leptospires remain and multiply in the kidney tissue for long time and in some instances for the life of the host [20].
The Host
a. Maintenance Hosts
A maintenance or reservoir host is an animal that has been infected with a serovar of the pathogen that is suited to its host. Each serovar is adapted to a particular maintenance host, although they may cause disease in any mammalian species. A serovar behaves differently within its maintenance host species and incidental or accidental hosts [33]. The disease is maintained in nature by chronic infection of the renal tubules of these maintenance hosts [9].
Maintenance host is characterized by high susceptibility to infection, endemic transmission within the host species, relatively low pathogenicity for its host, tendency to cause chronic rather than acute disease, producing insidious economic loss through reproductive losses, persistence of the serovar in the kidney and sometimes the genital tract, low antibody response to infection and low efficacy of vaccination in prevention of infection. Examples of this relationship are serovar Bratislava in swine and serovar hardjo bovis in cattle [33]. The majority of Leptospira serovars' primary reservoir hosts are wild animals, particularly rats andCattle, dogs, lambs, and pigs are reservoir hosts that can serve as temporary carriers for a period of time, whereas rodents typi-cally serve as permanent carriers for the entirety of their lives. Therefore, it is believed that rodents are the main source of infection [14].
b. Accidental (Incidental) Hosts
Accidental or incidental disease comes from exposing susceptible animals to non-host adapted serovars. The characteristics of an incidental host are a low susceptibility to infection, a high pathogenicity for the host, and a propensity for acute and severe illness rather than chronic disease. An illustration of this connection is the infection of cattle by the pig adapted serovar Pomona [33]. Accidental hosts for Leptospira species include people [34].
Risk Factors
a. Host and Management Risk Factors
Leptospirosis can affect animals of any age; however, it tends to affect young animals more frequently and with greater morbidity [9]. Leptospirosis affects practically all mammalian species; however, it seems to be less common in cats than in cattle, sheep, goats, dogs, horse and pigs. Cog razing or common grazing with infected animals, access to contaminated water sources like streams, rivers, floodwater, or drainage water, and buying or borrowing infected male animals for natural insemination are some management practices that increase the risk of infection [33] (Table 1, Figure 2).

Figure 2: Reservoir host contaminating the environment.
Source: (Miller et al., [73], Cárdenas et al., [74]).
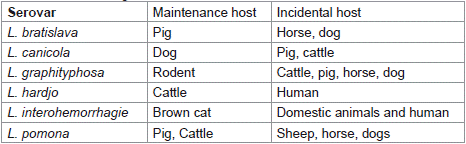
Table 1: Maintenance and incidental hosts for important
serovars of L. interrogans.
b. Pathogen Risk Factors
Virulent leptospirae resist the bactericidal action of complement and neutrophils in non-immune hosts but are rapidly killed by either mechanism in the presence of specific epithelial and endothelial antibody [35]. Leptospira's capacity to enter Vero cells and decrease macrophage apoptosis was connected with virulence; nonetheless, the organism has to break through host epithelial and endothelial cell barriers in order to disseminate hematogenously and localize in target organs such the liver and kidney [36]. A cytotoxic glycolipoprotein fraction is shown to inhibit hosts ATPase with the activity ascribed to the presence of long chain fatty acid L. pomona in cattle causes intravascular haemolysis due to hemolytic exotoxin [36].
Modes of Transmission
The existence of carrier animals, the suitability of the environment for leptospire survival, and interactions between people, animals, and the environment are the primary determinants of leptospiral infection transmission. Leptospires can survive in soil and spread the disease as a result of a number of environmental variables, including inadequate sanitation, stagnant water, climatic conditions, reservoirs, and rodent populations that are carriers of the disease [37]. The infecting agent is transmitted from one animal carrier to another via direct or indirect contact with urine or other body fluids that contain viable leptospires [38].
Direct transmission can happen through bite wounds, eating of contaminated tissues, venereal or placental transfer, and oronasal exposure to infected urine. Leptospire contact with mucous membranes and abraded skin can also be a method of transmission [39]. Indirect transmission, a very common form of transmission, occurs via handling infected animal tissues, exposure to contaminated sources of water, for example ponds, rivers and water catchment tanks, as well as soil, food [40]. The urine is the typical way that leptospires are spread. They can last in urine for up to six hours. They can survive for several weeks in moist soil, ponds, or slow-moving streams at a temperature of 22°C. Leptospirae may be present in the milk and semen of an infected bull, making it possible for the disease to spread through milk during natural breeding or artificial insemination, though this is not typical. Even while most attempts to isolate the leptospires from such aborted fetuses fail, they can be a source of infection from an animal that is known to be sick [35] (Figure 3).
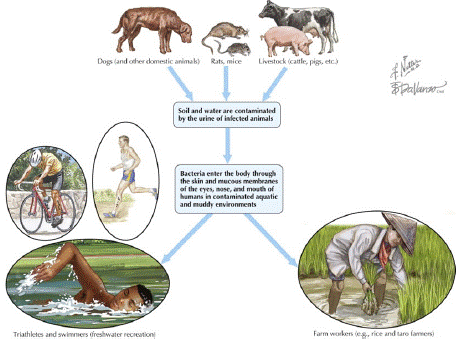
Figure 3: Ways of transmission of Leptospirosis.
Source: (Keystone et al., [75]).
Pathogenesis of Leptospirosis
The bacteria enter the body through penetrate intact mucous membrane of the mouth, nose or eyes or abraded, scratched or water softened skin. As soon as they enter the circulatory system, they multiply quickly and move to numerous tissues, including the kidney, liver, spleen, central nervous system, eye, and genital tract. The bacteria continue to release the pathogen in urine as they become established in the kidney's tortuous tubules. The period of shedding varies from a few weeks to many months [4,36]. Afterward the bacteria reach a higher concentration in blood and tissue, there is tissue damage due to endotoxins secreted by the pathogen, hemolysis is also secreted by the bacteria and leads to damage of blood cells. Damage to the endothelium causes ischemia and other problems. However, the humoral response has been seen to be active in the first week of infection, leading to phagocytosis by macrophages and neutrophils. The exact molecular basis of virulence is yet understood [4].
There are three possible pathways after the systemic circulation. The body will be free of leptospires and no clinical symptoms will be present if the animal has a high and sufficient antibody titer. A modest or brief leptospiremia can be present in an animal with a significant antibody, which is then followed by mild clinical symptoms. The animal will stop shedding leptospires after the leptospires have been removed by the kidneys. Leptospires will multiply in the bloodstream if the animal has a low or nonexistent antibody titer [41].
Injury to the endothelium can result in ischemia in a variety of organs, including the liver (hepatocellular injury), lungs, and kidneys (renal tubular necrosis). Neutrophils and thrombocytes are stimulated by lipopolysaccharides (LPS) in the outer membrane of the leptospires and this contributes to inflammation and coagulatory abnormalities. The LPS can contribute to the renal and hepatic damage. Meningitis can develop if the leptospires enter the nervous system or cerebral spinal fluid in the acute phase of the disease. If bacteria persist despite the antibody response, then immune-complex-mediated meningitis can occur. When this phenomenon occurs in the eyes it causes uveitis [6].
The incubation period of leptospirosis depends on dose, infectious strain and host but is averagely between 7-14 days [11]. According to Levett et al., [9] 5-7 days after infection, antibodies are evident. The proximal tubular cells and the tubular lumen in the kidneys must be reached by the leptospires after about two weeks. In the best-case scenario, leptospires will be removed from the blood and tissues by the antibodies. Leptospires won't be shed in the urine because the bacteria can also be removed from the kidneys. In some animals, the bacteria can continue to grow and remain in the renal tubular cells despite an elevated antibody titer. Leptospires may continue to shed in the urine for days, months, or even years as a result [42]. The invasive capacity of leprospirae may be related to their pathogenicity because nonpathogenic leptospirae do not penetrate cells as deadly as pathogenic leptospirae [36] (Figure 4).
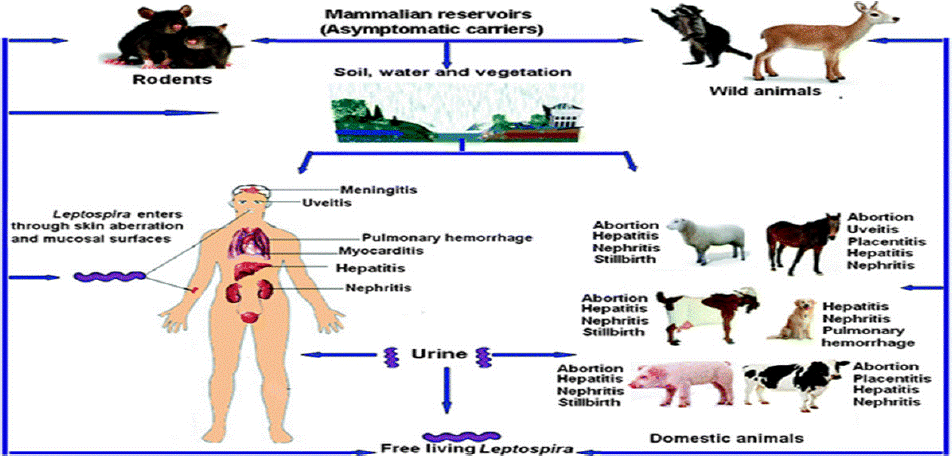
Figure 4: Leptospira invasion, pathogenesis and persistence.
Source: (Yan et al., [76]).
Clinical Signs of The Disease in Animals
With the exception of the fact that infection with L. interohaemorragiae typically results in severe septicemia, leptospirosis clinical signs are comparable in all animal species. The disease is both highly variable and non-specific, depending on both; host and pathogen factors that important proportion of infections are; asymptomatic or subclinical, and when symptoms do occur, onset is typically 2 to 30- days after exposure, with average incubation time of 7 to 12-days [43].
In humans the disease is characterized by variety of symptoms, including high fever, vomiting, jaundice (yellow skin and eyes), red eyes, headache, chills, muscle aches, abdominal pain, diarrhea, rash etc. Many of these symptoms can be mistaken for those of other illnesses, unknown. Some infected individuals may not exhibit any symptoms at all. Leptospirosis clinical symptoms in animals are frequently linked to kidney illness, liver disease, or reproductive issues [43].
The clinical signs of leptospirosis in cattle can be acute, sub-acute, or chronic, with only minimal differences between the species distressed. The leptospiremic phase is marked by septicemia, high fever, anorexia, petechiation of the mucosa, depression, acute hemolytic anemia with hemoglobinuria, jaundice, and pallor of the mucosa, as well as other clinical symptoms of acute ness subacute disease. The sub-acute form of lentospirosis differs from the acute form only in degree. Fever is mild and hemoglobinuria is common but jaundice may or may not be present [6,44].
The majority of the time, clinical symptoms of chronic infections in livestock is linked to reproductive losses due to abortion, stillbirth, infertility, mastitis, and milk drop syndrome. Typically, abortions take place in the final trimester of pregnancy. Only pregnant or nursing cows experience infertility and milk loss because, Leptospira germs prefer to multiply in these organs [33]. The herd's milk yield may decrease suddenly and by up to 50% of the cows at once. The decline may last up to 8 weeks, however individual cows' milk production will return to normal in 114 days [35].
Goats and sheep are susceptible to both severe and subclinical infections, which can cause reproductive issues such infertility, abortions, and stillbirth [45]. In various studies anorexia, lethargy and vomiting were the three most common clinical signs in dogs with leptospirosis. Weight loss, polyuria, diarrhea, abdominal or lumbar pain, musculoskeletal pain and dehydration were also common [46].
Equine leptospirosis has clinical signs that are substantially the same as those seen in other animals, such cattle, with low grade fever, lethargy, and anorexia being the most typical manifestations in milder disease. A variety of common symptoms, such as conjunctival suffusion, jaundice, anemia, petechial hemorrhages on the mucosa, and general depression, may manifest in more severe versions of the disease. Additionally, particularly in foals, renal failure can happen. Placentitis, abortion, and stillbirth are all possible outcomes of infection in pregnant mares [10] (Figure 5).

Figure 5: Abortion in dairy cow due to Leptospira.
Source: (Anwar et al., [77]).
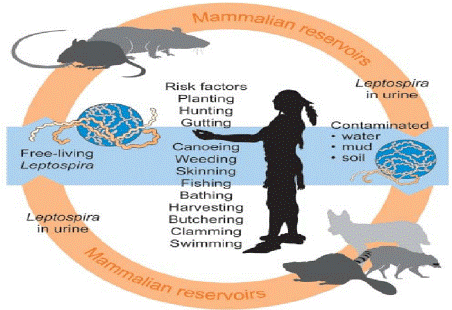
Figure 6: Risk factors for human.
Source: (Van et al., [78]).
Necropsy Findings
Vasculitis, endothelial damage, and inflammatory infiltrates made up of monocytes, plasma cells, histiocytes, and neutrophils are the hallmarks of leptospirosis. On physical examination, petechial hemorrhages are frequent, and the degree of icterus frequently results in discolored organs [9]. The liver, kidneys, heart, and lungs show the most obvious histopathology, although other organs may also be affected depending on how severe the individual infection is. In the kidneys, interstitial nephritis is the main finding along with a significant cellular infiltration made up of neutrophils and monocytes. The overall structure of the liver is not significantly altered, but intrahepatic cholestasis, hypertrophy, and hyperplasia of Kupffer cells are evident. The renal tubules include leptospires [33].
The anatomical basis for proteinuria in leptospirosis may be inferred from electron microscopy findings that the tubular cell brush boundaries are denuded, the tubular basement membrane is thickened, and the tubular cells demonstrate mitochondrial depletion. Minor glomerular alterations are also observed. Interstitial myocarditis with a predominance of lymphocytes and plasma cells infiltration, petechial hemorrhages (especially in the epicardium), and mononuclear infiltration in the epicardium, pericardial effusions, and coronary arteritis are among the pathological findings in the heart. While perivascular cuffing and vascular lesions in the meninges are seen in the brain, pulmonary congestion and hemorrhage are frequently seen in the lungs [9].
Diagnosis
Leptospirosis is diagnosed by combining epidemiological data, clinical findings, serology, and cultures [47]. Serologic tests include the macroscopic slide test, complement fixation test (CFT), micro agglutination test (MAT) and enzyme linked immune sorbent assay (ELISA). The CFT, macroscopic slide test and ELISA are genus specific and are useful for screening sera and detecting IgM levels. It can be complex and involves direct and indirect diagnostic tests. Indirect tests detect anti-leptospiral antibodies while direct ones investigate antigens or nucleic acids of leprospiraein animal tissues or body fluid. The choice of test is determined on the type of animal (herd or individual test) and the technique offered in the area [32].
In the acute phase, during the febrile period, leprospirae can be found in blood, lymph, urine, semen, milk and cerebrospinal, thoracic and peritoneal fluid, as well as in fragments of organs collected during necropsy (liver, kidney and lung) and in miscarriage products, such as the fetus and placenta [48,49].
Direct Examination: Dark field microscopy, immunofluorescent stain and silver impregnation of fixed tissues are methods of direct examination. Method of direct examination by using dark field microscopy is limited to urine because other body fluids contain artifacts similar to Leptospira organisms, therefore, low speed centrifugation clears the entering particles but will not sediment. Methods using formalinized urine have been described but they destroy motility, which aids in identification of Leptospira. However, negative result of Leptospira under direct examination does not rule out Leptospirosis [50].
Animal Inoculation: A sensitive technique for the isolation of Leptospira consists of the intraperitoneal inoculation of young guinea pig with fresh plasma or urine, within few days’ spirochetes become demonstrated in the peritoneal cavity. The animals should be inspected twice daily, and from the third to the seventh day, a drop of peritoneal fluid can be evaluated for active leprospirae using dark field microscopy [51]. On the death of the animal haemorrhagic lesions with spirochetes are found in many organs [35].
Serology: Leprospirae in serum is detected by the ELISA tech-nique, complement fixation testing, macroscopic and microscopic agglutination assays, and other methods. The macroscopic agglutination examination is a screening test and uses dead Ag but suffers from specificity. The microscopic agglutination test (MAT) is the most commonly used serological test for the diagnosis of lepsopirosis. In animals which survive infection, Leptospira can be readily diagnosed on the bases of demonstrating rising antibody titer in acute or convalescent sera [52].
MAT is particularly useful in diagnosis of disease associated with incidental host adapted serovars, or acute disease associated with host adapted serovars. It’s less useful in the diagnosis of chronic infection or may persist from sub-clinical infection [44]. The ELISA test is much more accurate than other tests and has much advantage from point of view of laboratory practices. It has excellent diagnostic specificity and sensitivity, convenient technical feature including automation and can be used efficiently as serenity test for large number of serum samples [53].
Molecular Method of Diagnosis: DNA amplification using PCR and DNA primers have become an excellent diagnostic tool for detecting the presence of Leptospira in animal tissues and fluids and it can be applied to blood, urine, CSF and tissue samples at ante or post mortem. Several primary pairs for PCR detection of Leptospira have been described; some are based on specific gene targets such as 16s to 23s ribosomal genes or repetitive elements while others have been constructed from genomic libraries [54].
Culture: It is possible to extract Leptospira germs from bodily fluids, primarily urine. However, if the target tissue is not autolyzed, tissue from dead animals offers a larger chance of a successful isolation. The kidney, liver, lungs, and brain are examples of such target tissue. If the agent is suspect for abortions, isolation could be attempted from non-autolyzed abortion materials or tissue samples from a freshly aborted fetus. Isolation of the microorganism from fetal tissue (kidney, liver, lungs) confirms maternal infection [6]. Isolation requires expensive and properly prepared and kept culture media. Inoculated media are incubated at 28-30 °C for several weeks or months. Cultures are incubated in dark and quite environment. Time of incubation depends on the serovar. Serovars such as Pomona and Grippotyphosa require the least time incubation up to 10 days. Regardless of time required for isolation, the inoculated culture media must be protected from contamination, thus require the addition of antimicrobial agents selected to inhibit growth of contaminants [34].
Treatment
As soon as leptospirosis is diagnosed, treatment with potent antibiotics should begin ideally before the fifth day following the commencement of sickness. Serological tests do not become positive until about a week after the onset of sickness, thus clinicians should never delay the use of antibiotics while awaiting the results of laboratories. Antibiotics to treat leptospirosis include penicillin G and Doxycycline. In more severe cases cefotaxime or ceftriaxone should be preferred [55]. Antibiotics are used to reduce fever and bacteria within the bloodstream thus rapidly reduce fatal complications of infection such as liver or kidney failure. Glucose and salt solution infusions may be administered [56].
Control and Prevention
Important control measures include control of livestock infection with good sanitation, immunization and proper veterinary care. Leptospirosis prevention includes controlling rodents in kennels, eradicating the carrier state, maintaining environmental conditions to prevent bacterial survival, and isolating infected animals. It is characterized by sanitary control and a reduction in the risk of infection from contact with contaminated environments, infected wild animals, as well as synanthropic animals and rodents [14].
Limiting rodent and wild animal interaction with cattle and their feed and water is frequently difficult to do but lowers the risk for leptospirosis transmission. Static water that hasn't been drained or fenced off may help prevent transmission. The major risk for control is introduction of carrier animals of any species or reintroduction by rodents, or by other wild life. It is because of this risk that most programs aim at containment rather than eradication. The first step in control is to identify the source of original infection [16].
Vaccines are available for use in cattle, dogs, and pigs. These vaccines offer serovar-specific protection from leptospirosis on a shortterm basis, approximately 1 year [57]. There are numerous vaccines available for livestock against different serovars, including those that are most frequently detected in each nation. For instance, a pentavalent vaccine is offered in Canada and the United States for the serovars Pomona, Grippotyphosa, Canicola, Icterohaemorrhagiae, and Hardjo [58]. Icterohaemorrhagiae and Canicola serovars are present in one dog vaccine, whereas Icterohaemorrhagiae, Canicola, Grippotyphosa, and Pomona serovars are present in the other [11].
To date various types of vaccines have been experimentally considered as good candidates for effectively preventing infection, or at least clinical disease [59]. Recombinant, lipopolysaccharide, DNA and inactivated - attenuated vaccines have been experimentally tested with various results on effectiveness and safety among animals. Those widely investigated for use in animals are attenuated and inactivated vaccines, but the protection conferred by them is partial, due to lack of cross immunity among serovars [60]. Leptospirosis is in domestic animals can be controlled through vaccination with inactivated whole cells or an outer membrane preparation [61].
Public Health Significance of Leptospirosis
Leptospirosis continues to be an important health hazard with related economic burden, especially among rural dwellers in tropical regions of the world [62]. Risk groups for the disease include those who work in abattoirs and sewage facilities, salvers, coal mines, plumbers, farm workers, veterinarians, pet store owners, meat handlers, military personnel, slaughter house workers, and those in the fishing industry [17]. Although large herbivores are also considered to be significant sources of infection, small mammals like rodents make up the majority of the key leptospirosis reservoirs [63]. The majority of reservoir hosts don't show any symptoms of the disease clinically. These reservoirs can act as a source of infection to humans and other domestic animals that may in turn transmit the infection to humans and other susceptible animals [64].
Most people agree that rodents are the main means of leptospirosis transmission [6]. Two of the rat species that predominate dwelling in close proximity to humans are the Rattus and Rattus norvegicus. Both of them have been described as the main reservoir of pathogenic Leptospira species [65]. Additionally, other species such as dogs, cattle, and pigs have all been implicated as maintenance hosts of the bacteria, whereby they contract the sickness, recover from it, but the germs continue to live and spread continually in their kidneys [66]. Occupational and recreational exposure to contaminated water bodies, contact with diverse animal species, and contact with animal tissue, animal urine, and damp environments have all been linked to risk factors [33].
Leptospirosis has been recognized as a re-emerging global public health problem due to the increased incidence in both developing and developed countries [16]. The prevalence of the disease has significantly decreased in comfortable countries, and the majority of cases are now linked to recreational exposure to the contaminated water In contrast, it appears that the incidence is rising in developing nations [67]. According to Pavli and Maltezou, [68] Men are more frequently diagnosed with leptospirosis compared with women and this has been traditionally attributed to the over representation of men in high-risk occupations.
Status of Leptospirosis in Ethiopia
Leptospirosis is a largely unknown disease in Ethiopia, despite the fact that domestic animals have been known to contract it. The prevalence and spread of leptosprosis, however, are significantly influenced by climatological, socioeconomic, and cultural factors. Due to ignorance, leptospirosis is currently underreported in Ethiopia, although the real frequency is anticipated to be significant. It is a disease of tropical countries and often is endemic, although in Ethiopia its prevalence in humans is totally unknown and there is no documented evidence on its occurrence [69].
The tropical climate, frequent contact of the populace with mud and stagnant water, particularly with the water used to irrigate the sugarcane plantation, a lack of hygiene, and unsanitary living conditions may all contribute to Wonji’s high prevalence of leptospirosis (Annual Report, 2002/2003). Leptospirosis was found in 47.46% of the 59 fever patients who visited the outpatient department of Wonji Hospital [70]. Males were more likely than females to contract the condition. Leptospiral antibodies were discovered in domestic animals in Ethiopia forty years ago, with incidences of 91.2% in horses, 70.7% in cows, 57.1% in pigs, 47.3% in goats, 43.4% in sheep, 15.4% in camels, and 8.3% in chickens [69]. However; there is little recent information about animal leptospirosis. According to Tsegaye et al [71], a total of 184 out of 418 horses had antibody titres of at least one of 16 serovars, demonstrating the presence of 16 serovars of Leptospira species in central and southern Ethiopian horses. This indicates that at least one serovar was present in 44% of the horses in the sample. Wonji Hospital in central Ethiopia reported the first case of human leptospirosis there; there, 47.5% of feverish patients (n = 59) tested positive for leptospiral infection [72,79].
Conclusion and Recommendations
Leptospirosis has been known to affect both man and numerous mammalian species worldwide resulting in morbidity and mortality. Infection in domesticated animals and wild animals can result in financial loss and provide a risk of spreading to nearby communities. Direct transmission of the illness is possible through contact with the secretions, blood, or urine of infected animals; whereas indirect transmission occurs when contaminated water is used as a reservoir for the disease. In tropical areas where people and animals live in close contact, and warm and humid conditions favor environmental survival and transmission of the pathogen. Leptospirosis is seriously contaminated urine is highly infectious for people and for susceptible animal. When handling infected animals or dealing with areas of contamination, protective gear should be worn such as gloves, eye protection and face masks. Although, easy to treat with most antibiotics, Leptospirosis is easy to diagnosis by microscopic, culture and serology.
Based on the above conclusion, the following points are forwarded as recommendations:
• Contact with urine on mucous membranes or skin abrasions should be avoided.
• Important control measures including control of livestock infection with good sanitation, immunization, and proper veterinary care should be taken.
• Societies and professional at risk must wear protective glove when exposed to animal reservoirs.
• Proper control measures and public awareness should be made in endemic areas.
References
- Mwachui MA, Crump L, Hartskeerl R, Zinsstag J, Hattendorf J. Environmental and behavioural determinants of leptospirosis transmission: a systematic review. PLoS neglected tropical dise. 2015; 9: 3843.
- Robi DT. Epidemiology and zoonotic implication of leptospirosis in domestic animals in Ethiopia. Academic Journal of Animal Diseases. 2020; 9: 19-32.
- Doern GV. Detection of selected fastidious bacteria. Clinical infectious dise. 2000; 30: 166-173.
- Mohammed H, Nozha C, Hakim K, Abdelaziz F, Rekia B. Leptospira: morphology, classification and pathogenesis. J.of Bacteriol Parasitology. 2011; 2: 66.
- Pal M, Hadush A. Leptospirosis. An infectious emerging waterborne zoonosis of global significance. Air Water Borne Dis. 2017; 6: 133.
- Adler B and Moctezuma A. Leptospira and leptospirosis. J.Vet. micro. 2010; 140: 287-296.
- Matthias MA and Levett PN. Leptospiral carriage by mice and mongooses on the island of Barbados. The West Indian Medical J. 2002; 51: 10-13.
- Yadeta W, Bashahun GM and Abdela N. Leptospirosis in Animal and its Public Health Implications: A Review. World Applied Science J. 2016; 34: 845-853.
- Levett PN, Morey RE, Galloway R, Steigerwalt AG and Ellis WA. Reclassification of Leptospira parva Hovind-Hougen et al. 1982 as Turneriella parva gen. nov., comb. nov. Inter. j. of systematic and evolutionary microbiology. 2005; 55: 1497-1499.
- Verma A, Stevenson B, Adler B. Leptospirosis in horses. Vet. microbiology. 2013; 167: 61-66.
- Sykes JE, Hartmann K, Lunn KF, Moore GE, Stoddard RA and Goldstein RE. Small animal consensus statement on leptospirosis: diagnosis, epidemiology, treatment, and prevention. J.of vet. Inter. Medicine. 2011; 25: 1-13.
- Toyokawa T, Ohnishi M, Koizumi N. Diagnosis of acute leptospirosis.Expert review of anti-infective therapy. 2011; 9: 111-121.
- Katz AR, Buchholz AE, Hinson K, Park SY, Effler PV. Leptospirosis in Hawaii, USA, 1999–2008. Emerging Infectious Dis. 2011; 17: 221.
- Tilahun Z, Reta D, Simenew K. Global epidemiological overview of leptospirosis.Int J Microbiol Res. 2013; 4: 9-15.
- Himani D, Suman MK and Mane BG. Epidemiology of leptospirosis: an Indian perspective. J.of Foodborne Zoonotic Dis. 2013; 1: 6-13.
- Vijayachari P, Sugunan AP and Shriram AN. Leptospirosis: an emerging global public health problem. J.of biosciences. 2008; 33: 557-569.
- Monahan AM, Miller IS and Nally JE. Leptospirosis: risks during recreational activities. J.of applied microbiology. 2009; 107: 707-716.
- McBride AJ, Athanazio DA, Reis MG and Ko AI. Leptospirosis. Current Opinion. Infectious Dis. 2005; 18: 376-386.
- Lehmann JS, Matthias MA, Vinetz JM and Fouts DE. Leptospiral pathogenomics. Pathogens. 2014; 3: 280-308.
- Zavitsanou A and Babatsikou F. Leptospirosis: epidemiology and preventive measures. Health Science J. 2008; 2: 166-219.
- Marquez A, Djelouadji Z, Lattard V and Kodjo A. Overview of laboratory methods to diagnose Leptospirosis and to identify and to type leptospires. Int Microbiol. 2017; 20: 184-193.
- Spickler AR and Leedom Larson KR. Leptospirosis (Factsheet). Iowa State University. Animal infectious dise. 2013; 16: 628-688.
- Joshi YP, Kim EH and Cheong HK. The influence of climatic factors on the development of hemorrhagic fever with renal syndrome and leptospirosis during the peak season in Korea: an ecologic study. BMC infectious dis. 2017; 17: 406.
- Prevention (US) and National Association of State Public Health Veterinarians (US). Compendium of measures to prevent disease associated with animals in public settings, 2005 (Vol. 54). US Dept. of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention. 2005.
- Costa F, Hagen JE, Calgano J. Morbidity and mortality of leptospirosis: A systematic review. PLoS Negl Trop Dis. 2015; 9: 10-33.
- Lau CL, Smythe LD, Craig SB, Weinstein P. Climate change, flooding, urbanisation and leptospirosis: fuelling the fire?.Transactions of the Royal Society of Tropical Medicine and Hygiene. 2010; 104: 631-638.
- Freitas JCD, Silva FGD, Oliveira RCD, Delbem áCB, Müller EE, Alves LA and Teles PS. Isolation of Leptospira spp from dogs, bovine and swine naturally infected. Ciência rural. 2004; 34: 853-856.
- Ma J. Smith h, Joseph P, Gilman Rh, Bautista CT, Campos KJ, et al. Environmental exposure and leptospirosis, Peru. Emerging Infectious Dise. 2004; 10: 1016-1022.
- Vanasco NB, Schmeling MF, Lottersberger J, Costa F, Ko AI and Tarabla HD. Clinical characteristics and risk factors of human leptospirosis in Argentina (1999–2005). Acta Tropica. 2008; 107: 255-258.
- Lilenbaum W, Varges R, Brandão FZ, Cortez A, De Souza SO, Brandão PE, Richtzenhain LJ and Vasconcellos SA. Detection of Leptospira spp. in semen and vaginal fluids of goats and sheep by polymerase chain reaction. Theriogenology. 2008; 69: 837-842.
- Melo LDSS, de Castro MB, Leite RC, Moreira ÉC and de Melo CB. Principais aspectos da infecção por Leptospira sp em ovinos. Ciência Rural. 2010; 40: 1235-1241.
- Lucheis SB and Ferreira Jr RS. Ovine leptospirosis in Brazil.Journal of Venomous Animals and Toxins including Tropical Dis. 2011; 17: 394-405.
- Radostits OM, Gay CC, Hinchcliff KW and Constable PD. Veterinary Medicine E-Book: A textbook of the diseases of cattle, horses, sheep, pigs and goats. Elsevier Health Sciences. 2006.
- Ko A, Goarant C, Picardeau M. Leptospira, the dawn of the molecular genetics era for an emerging zoonotic pathogen. Journal of Nature Reviews Microbiology. 2009; 7: 736-747.
- Fentahun T and Alemayehu M. Leptospirosis and its public health significance: a review. European J. of Applied Sciences. 2012; 4: 238-244.
- Craig E, Greene J, Sykes A, Cathy K, Hartmann. Infectious disease of the dog and cat. 3rd edition, Canada, Saunders. 2006; 402-417.
- Nally JE, Arent Z, Bayles DO, Hornsby RL, Gilmore C, Regan S, et al. Emerging infectious disease implications of invasive mammalian species: the greater white-toothed shrew (Crocidura russula) is associated with a novel serovar of pathogenic Leptospira in Ireland. PLoS neglected tropical diseases. 2016; 10: 5174.
- Bryan HM, Darimont CT, Paquet PC, Ellis JA, Goji N, Gouix M and Smits JE. Exposure to infectious agents in dogs in remote coastal British Columbia: Possible sentinels of diseases in wildlife and humans. Canadian j.of vet. Research. 2011; 75: 11-17.
- Thayaparan S, Robertson I, Fairuz A, Suut L, Abdullah M. Leptospirosis, an emerging zoonotic disease in Malaysia.Malaysian J. of Pathology. 2013; 35: 123-132.
- Colegrove KM, Lowenstine LJ, Gulland FM. Leptospirosis in northern elephant seals (Mirounga angustirostris) stranded along the California coast. J. of Wildlife Dis. 2005; 41: 426-430.
- Goldstein RE. Canine leptospirosis. Veterinary Clinics: Small Animal Practice. 2010; 40: 1091-1101.
- Azócar-Aedo L, Smits HL, Monti G. Leptospirosis in dogs and cats: epidemiology, clinical disease, zoonotic implications and prevention. Archivos de medicina vet. 2014; 46: 337-348.
- Haake DA and Levett PN. Leptospirosis in humans. Curr Top Microbio Immunol. 2015; 387: 65-97.
- Radostits OM, CC Gay, KW Hinclcliff and PO Constable. Veterinary medicine: A text book of thedisease of cattle, sheep, pigs, goat and horses, 10 ed. London, Saunders. 2007; 1094-1110.
- Zacarias F, Vasconcellos S, Anzai E, Giraldi N, Freitas J & Hartskeerl R. Isolation of leptospira serovars Canicola and Copenhagen from cattle urine in the state of Parana, Brazil. Brazilian, J.of Microbiology. 2008; 39: 744-748.
- Greenlee JJ, Bolin CA, Alt DP, Cheville NF and Andreasen CB. Clinical and pathologic comparison of acute leptospirosis in dogs caused by two strains of Leptospira kirschneri serovar grippotyphosa. American j. of vet. Research. 2004; 65: 1100-1107.
- Schreier S, Doungchawee G, Chadsuthi S, Triampo D, Triampo W. Leptospirosis: Current situation and trends of specific laboratory tests. Expert Rev. Clinical Immunology. 2013; 9: 263-280.
- Segura ER, Ganoza CA, Campos K, Ricaldi JN, Torres S, Silva H, et al. Clinical spectrum of pulmonary involvement in leptospirosis in a region of endemicity, with quantification of leptospiral burden. Clinical infectious dise. 2005; 40: 343-351.
- Aguiar DM, Gennari SM, Cavalcante GT, Labruna MB, Vasconcellos SA, Rodrigues AA, et al. Seroprevalence of Leptospira spp in cattle from Monte Negro municipality, western Amazon. Pesq.Vet. Brasileira. 2006; 26: 102- 104.
- Regmi L, Pandey K, Malla M, Khanal S and Pandey BD. Sero-epidemiology study of leptospirosis in febrile patients from Terai region of Nepal. BMC infectious dise. 2017; 17: 628.
- Sharma M and A Yadav. Leptospirosis epidemiology, diagnosis and control, Journal of Infectious Disease and Antimicrobial Agents. 2008; 25: 93-103.
- Subharat S, Wilson PR, Heuer C, Collins-Emerson JM, Smythe LD, Dohnt MF, et al. Serosurvey of leptospirosis and investigation of a possible novel serovar Arborea in farmed deer in New Zealand. New Zealand vet. J. 2011; 59: 139-142.
- Picardeau M, Bertherat E, Jancloes M, Skouloudis AN, DurskiK, Hartskeerl RA. Rapid tests for diagnosis of leptospirosis: current tools and emerging technologies. Diagn Microbiol Infect Dis. 2014; 78: 1-8.
- Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, et al. Leptospirosis: a zoonotic disease of global importance. The Lancet infectious dise. 2003; 3: 757-771.
- Ressner RA, Griffith ME, Beckius ML, Pimentel G, Miller RS, Mende K, et al. Antimicrobial susceptibilities of geographically diverse clinical human isolates of Leptospira. Antimicrobial agents and chemotherapy. 2008; 52: 2750-2754.
- Devishree R. Management of leptospirosis: A short review. J. of Pharmaceutical Sciences and Research. 2005; 7: 759.
- Hartskeerl R, Collares-Pereira M, Ellis W. Emergence, control and reemerging leptospirosis: dynamics of infection in the changing world. Clinical Micr. and Infection. 2011; 17: 494-501.
- Divers T. Leptospirosis in ruminants. In: Merck Veterinary Manual. 2016.
- Wang Z, Jin L, Wegrzyn A. Leptospirosis vaccines. Microbial cell factories. 2007; 6: 39.
- Eslabão MR, Dellagostin OA and Cerqueira GM. LepBank: A Leptospira sequence repository and a portal for phylogenetic studies. Infection, Genetics and Evolution. 2010; 10: 586-590.
- Palaniappan RU, Ramanujam S, Chang YF. Leptospirosis: pathogenesis, immunity, and diagnosis. Current opinion in infec.dise. 2007; 20: 284-292.
- Wasinski B and Dutkiewicz J. Leptospirosis-current risk factors connected with human activity and the environment. Annals of Agricultural and Environmental Medicine. 2013; 20: 11-23.
- Ellis WA. Animal leptospirosis. In Leptospira and leptospirosis. 2015; 99-137.
- Agampodi S, Kodituwakku M, Palihawadana P, Ginige S, Jayasinghe W, Sivasothy A, Wijesinghe R. Risk Factors and Reservoir Species for Leptospirosis in Sri Lanka Principal Investigators. J. of Foodborne Zoonoti Dise. 2013; 1: 6-13.
- Loan HK, Van Cuong N, Takhampunya R, Kiet BT, Campbell J, Them LN, et al. How important are rats as vectors of leptospirosis in the Mekong Delta of Vietnam? Vector-Borne and Zoonotic Dis. 2015; 15: 56-64.
- Goarant C. Leptospirosis: risk factors and management challenges in developing countries. Research and reports in tropical medi. 2016; 7: 49.
- Tangkanakul W, Smits HL, Jatanasen S and Ashford DA. Leptospirosis: an emerging health problem in Thailand. Southeast Asian J.of Tropical Medicine & Public Health. 2005; 36: 281-288.
- Pavli A and Maltezou HC. Travel-acquired leptospirosis.J. of travel medicine. 2008; 15: 447-453.
- Martins G and Lilenbaum W. Leptospirosis in sheep and goats under tropical conditions.Tropical animal health and production. 2014; 46: 11-17.
- Eshetu Y, K Simone, M Tsehaynesh, W Dawit, N Bethlehem, G Neway, et al. Human leptospirosis, in Ethiopia: A pilot study in Wonji Hospital, Ethiopian J. of Health Development. 2004; 18: 48-51.
- Tsegaye K, Potts A, Akililu N, Lötter C and Gummow B. Circulating serovars of Leptospira in cart horses of central and southern Ethiopia and associated risk factors. J. of Preventive Vet. Medicine. 2016; 125: 106-115.
- Yimer E, Koopman S, Messele T, Wolday D, Newayeselassie B, Gessese N, et al. Human leptospirosis in Ethiopia: a pilot study in Wonji. Ethiopian j.of Health Development. 2004; 18: 48-51.
- Miller MD, Annis KM, Lappin MR, Lunn KF. Variability in results of the microscopic agglutination test in dogs with clinical leptospirosis and dogs vaccinated against leptospirosis. J.of veterinary inter. Medicine. 2011; 25: 426-432.
- Cárdenas NC, Infante GP, Pacheco DAR, Diaz JPD, Wagner DCM, Dias RA, et al. Seroprevalence of Leptospira spp infection and its risk factors among domestic dogs in Bogotá, Colombia. Vet. And Animal Scie. 2018; 6: 64-68.
- Keystone JS, Freedman DO, Kozarsky PE, Connor BA, Nothdurft HD. Travel Medicine E-Book: Expert Consult-Online and Print. Elsevier Health ci.Clinical Micr.and Infection. 2012; 17: 49-50.
- Yan W, Faisal SM, McDonough SP, Divers TJ, Barr SC, Chang CF, et al. Immunogenicity and protective efficacy of recombinant Leptospira immunoglobulin-like protein B (rLigB) in a hamster challenge model. Microbes and infection. 2009; 11: 230-237.
- Anwar K, Naveed Khan N, Mujtaba M. Seroprevalence of leptospirosis in aborted dairy cattle in Peshawar district suburb, Khyber Pakhtunkhwa Pakistan. Int. J. of Current Microbiology and Applied Sci. 2013; 2: 73-78.
- Van CD, Doungchawee G, Suttiprapa S, Arimatsu Y, Kaewkes S and Sripa B. Association between Opisthorchis viverrini and Leptospira spp. infection in endemic Northeast Thailand. Parasitology inter. 2017; 66: 503-509.
- Petrakovsky J, Bianchi A, Fisun H, Najera P, Pereira M. Animal Leptospirosis in Latin America and the Caribbean Countries, Reported Outbreaks and Literature Review (2002-2014), Int. J.of Environmental Research and Public Health. 2014; 11: 10770-10789.