
Research Article
Austin J Vet Sci & Anim Husb. 2025; 12(2): 1169.
A Research Technique for the Oviductal Insemination of Chickens Using Laparoscopy
Liu B, Huang W, Guo Y, Xu J, Zhang A, Hou R, Chen Y, Huang Q and Yang S*
College of Veterinary Medicine, South China Agricultural University, Guangzhou, Guangdong, 510642, PR China
*Corresponding author: Shihua Yang, College of Veterinary Medicine, South China Agricultural University, Guangzhou, Guangdong, 510642, PR China Email: yangsh@scau.edu.cn
Received: April 26, 2025 Accepted: May 09, 2025 Published: May 13, 2025
Abstract
Laparoscopic oviductal artificial insemination (LOAI) is been wildly used in livestock and some feline wildlife’ which can bypass the physical barrier of the animal’s reproductive tract and allows semen to reach the insemination site directly. Here we try to innovate the existing technique of AI into the oviduct of poultry using hens. First, we establish the detailed parameters, such anesthesia, procedure of surgery, option of inflated air for hens. After established LOAI of hen, we compared the effects of traditional cloaca artificial insemination and laparoscopic insemination while using fresh sperm and frozen-thawed sperm. As a result, LOAI showed insemination possibility with low sperm. In conclusion, our data demonstrated that LOAI is a useful for oviductal artificial insemination for poultry.
Keywords: Chicken; Oviductal artificial insemination; Laparoscopy; Frozen sperm
Introduction
Artificial insemination (AI), often artificially transferring sperm into the cervix or uterine cavity of female animals, is a technique of the artificial assisted reproduction (ART) which has been wildly used in animals reproduction. The AI of mammals including cervix insemination and intrauterine insemination, aim to overcome the lack of mating ability or complete the donation of sperm fertility [1]. It makes in vivo fertilization and pregnancy come true without mating [2]. Therefore, the emergence of the AI technology has completely changed the cattle industry [3,4]. Moreover, AI has important implications for the expansion of rare birds and the expansion of the poultry industry [5-7].
Over exploitation and utilization of the natural resource has caused the habitat of birds to shrink, leading to more and more birds being listed as endangered species [8]. According to the Red List of Endangered Species at the International Union for Conservation of Nature and Resource in 2019 [9], more than 27,000 species are endangered, with birds accounting for 14 percent and an increase of 1% over 2018 [10]. That means 3,780 species of birds will be in danger of extinction and will rise year by year. Cage conservation has become the main reproduction method of rare birds, but the hatching rate of wild birds is very low. Artificial insemination of poultry is widely used in the breeding of non-domestic birds, especially endangered birds [11,12]. However, the existing artificial insemination technology can not meet the requirements, due that the oviduct cannot be everted, such as ducks and geese [13], or the birds with less ejaculation such as quail and guinea fowl [14]. So we need to innovate the existing AI technology.
Laparoscopic surgery is the main tool of microinvasive surgery, which contains a long-fiber cable system that refers to the visualization of abdomen or pelvis through a small incision with the help of a laparoscope. Due to the small incision, less bleeding and short recovery time, laparoscopic surgery has successfully replaced many open surgery [13,15]. Later, artificial insemination combined with laparoscopic surgery, defined laparoscopic oviductal artificial insemination (LOAI) as an advanced ART to overcome the physical barrier of the animal's reproductive tract and allow semen to reach the insemination site directly [16]. Experiments have shown that LOAI has a higher pregnancy rate than vaginal artificial insemination [16,17]. In addition, with the same amount of sperm, LOAI can make more females pregnancy [18]. But there is no report that LOAI was applied to bird artificial insemination to overcome the barrier of bird insemination. Therefore, this trial attempts to apply LOAI technology to preform oviductal artificial insemination of hens to find a new ART for endangered birds and valuable poultry.
Materials and Methods
Animals
Hy-line variety brown hens with 39-40 weeks old were brought from Liangtian Liangsha Chicken Farm. The hens were raised at the Animal Center of South China Agricultural University. Each hen was kept in a single cage and feeding freely eating and drinking. The study was approved by the Committee of the University on the Ethics of Animal Use in Experiments.
Media Preparation
All chemicals were obtained from Sigma Chemical Co. (St. Louis, MO, USA).
TTE diluent contained Tris-HCl 12.69 mmol/L, TES 52.34 mmol/L, Glucose 111.11 mmol/L, Lactose 58.48 mmol/L, Raffinose 3.97 mmol/L , Penicillin G 0.19 mmol/L, Streptomycin 0.09 mmol/L, Egg yolk 20% (V/V) (taken from fresh eggs), all chemicals dissolved and mixed into pure water. The mixed solution was centrifuged at 7000 rpm for 1 h at 4°C and removed the precipitate. After adjusted the pH to 7.0 to 7.2, it was stored at -80°C, until warmed it up to 37°C before use.
TL-HEPES buffer contained PVA 0.1 mmol/mL, phenol red 0.01 mmol/mL, NaCl 127.0 mmol/mL, KCl 3.16 mmol/mL, CaCl2·2H2O 2.00 mmol/mL, MgCl2·6H2O 0.50 mmol/mL, Glucose 5.00 mmol/mL, NaH2PO4·H2O 0.35 mmol/mL, NaHCO3 2.00 mmol/mL, HEPES (Na salt) 5.00 mmol/mL, HEPES (acid form) 5.00 mmol/mL, Na-Lactate 10.00 mmol/mL.
The sperm detergent contained TL-HEPES 98 mL, pyruvate (100 mmol/L) 1 mL and bovine serum albumin 300 mg per 100 mL.
Sperm Collection and Diluent
Fresh Sperm Preparation: Semen was collected from adult rooster using traditional massage technique. Before sperm collection, the feathers around the cloacal cavity and tail of the cock should be cut, so that avoid of contamination of the feathers. Two technicians were involved in collecting semen. One technician held the cock's legs with his right hand to let it down on the table and massaged the cock's abdomen with his left hand. The other technician collected sperm when the cock “milk” come out.
Sperm motility and amount were calculated by microscopic examination as reported method [18-20]. After calculation, the corresponding amount of TL-HEPES buffer was added to make the sperm density reach about 2.0×107/mL. Then the diluted semen was kept in room temperature until use.
Frozen-Thawed Sperm Preparation: The semen was frozen and thawed with some modification [19,21]. Briefly, the collected cock semen was slowly added to TTE solution, mixed gently upside down, divided into each EP tubes 0.5 mL and stored in 4°C for 2 h. Then EP tubes were slowly added 0.5 mL TTE with 10% glycerol in 5 times, 100 uL each time, to end up with the glycerol concentration of 5%. Next the glycerin-mixed sperm dilution was absorbed into pre-cooled straws and sealed. Then the straws were kept on 5 cm above liquid nitrogen for 15 min and then directly stored in liquid nitrogen. After frozen more than one day in liquid nitrogen tank, the straws were removed and placed in the air for 10 s and put it into a 37°C water bath for 10 s. Cut the end of straw tube and let the liquid flow out into EP tubes naturally. Then add 2 mL the warmed TTE solution, centrifuge at 200×g for 5 min, discard the supernatant. Repeat the above steps twice. After washing, add 2 mL warmed TL-HEPES, sperm motility was counted under microscope. Appropriate TL-HEPES was added to dilute the sperm density to 2.0×107 /mL.
Artificial Insemination of Hen
Laparoscopic Oviductal Artificial Insemination: Laparoscopic oviductal artificial insemination of hen were performed as follows. After the chicken is completely anesthetized, the hen was kept lying on the right side, the right leg in front, and the left leg in the back, and then removed the feathers of the surgical area from the spine, down to the keel, left to the scapula, and right to the inner thigh, disinfected the surgical area with iodine, followed by alcohol deiodination.
An incision of 5 mm was made next to the distal xiphoid cartilage, the veress needle was inserted into abdomen through the small incision to air to fill the abdomen, and then the versee needle was removed and the trocar to open a pathway for the laparoscopy was inserted into the same incision. Staple was replace with laparoscopy. Another incision was made between the front of the hip bone and the last rib with a surgical blade and insert the specialized grasping forceps. Use the forceps to locate and clamp the funnel of the oviduct. The needle is inserted vertically above the fallopian tube and amount of sperm in 0.5 mL TL-HEPES buffer was injected into the funnel of oviduct. After surgery above, eggs by artificially inseminated hen were collected from day 2 to 22 (Day 1, the day of artificial insemination), then incubated for hatching.
Cloacal Artificial Insemination: Cloacal artificial insemination (CAI) of hen were performed as follows. The feathers around the cloaca were cut off before insemination. After obtaining diluted semen, two persons performed the AI of hen. One person grabbed the thighs of the hen with his right hand and then turn the hen to the right. Applies the proper pressure on the left side of the abdomen so that the hen venting, the hen was everted its vaginal orififice through the cloaca. The other person inserted the pipette containing amount of sperm in 0.5 mL TL-HEPES buffer into the cloaca 2-4 cm and then slowly injected the semen. After surgery above, eggs laid by artificially inseminated hen were collected from day 2 to 22 (Day 1, the day of artificial insemination), then incubated for hatching.
Egg Hatching
The laid eggs were collected from each cage and cage number and weight of each egg was noted. After washing and disinfecting, the eggs are transferred to the incubator (FT-KFC8, Shandong HSBC Incubation Equipment Factory) according to the principle of big head up and small head down. During the incubation period of 1-18 days, the temperature was maintained at 37.8°C, the humidity was 50%-60%, and the egg was turned every 2 hours for 90° each time. Stop turning eggs on the 19th day, keep the temperature 37.2°C and humidity 75%. On the 21st day, the dead embryo eggs and the infertile eggs were recorded and then removed while hatching.
Experimental Designs
Experiment 1: Parameters Examination of Laparoscopic Oviductal Artificial Insemination of Hen:
Some parameters of anesthesia, inflated air and the precise inserted position of laparoscopic instruments were examined in this study. As for anesthesia, twenty egg-laying hens were randomly divided into two groups, A and B, with 10 hens in each group. In order to anesthetize completely, group A were injected 0.1 mL with 10 times diluted Sumianxin Injection (Veterinary Research Institute, Munitions University) through the vein under the wings. While group B were given intramuscular injection of 0.1 mL 10 times diluted Sumianxin Injection. After that, all hen were observed the woke-up and recorded laying egg in following days. As for inflated air, cleaned carbon dioxide gas and atmosphere were tested for inflate abdominal cavity.
Experiment 2: Efficiency of Laparoscopic Oviductal Artificial Insemination Using Fresh Sperm in Hen: In order to exploring the effects of different amounts of fresh sperm and two methods of cloacal artificial insemination and laparoscopic oviductal artificial insemination on fertility rate and hatch ability of following eggs laid by hen indicated, twenty-eight laying hens with 39-40 weeks old were selected and randomly divided into 7 subgroups named CAI-fre 1, CAI-fre 10 and CAI-fre 100 for cloacal artificial insemination, and LOAI-fre 1, LOAI-fre 10 and LOAI-fre 100 for laparoscopic oviductal artificial insemination, and control, with 4 replicates in each group (Table 1). The total number of 0.2x106, 2x106, and 20x106 fresh motile sperm were used for insemination to CAI-fre 1, CAI-fre 10 and CAIfre 100 group by cloacal artificial insemination and to LOAI-fre 1, LOAI-fre 10 and LOAI-fre 100 by laparoscopic artificial oviductal insemination, respectively [22,23].
Insemination method
Sperm
Sperm number
Group
Laparoscopic oviductal artificial insemination, LOAI
Fresh sperm
0.2×106
2×106
LOAI-fre 10
20×106
LOAI-fre 100
Frozen sperm
0.2×106
2×106
LOAI-fro 10
20×106
LOAI-fro 100
Cloacal artificial insemination, CAI
Fresh sperm
0.2×106
2×106
CAI-fre 10
20×106
CAI-fre 100
Frozen sperm
0.2×106
2×106
CAI-fro 10
20×106
CAI-fro 100
Eggs collected from day 2 to 22 (the day of artificial insemination) after the LOAI or CAI.
Table 1: Sperm number of each group in insemination.
Experiment 3: Efficiency of Laparoscopic Oviductal Artificial Insemination Using Frozen-Thawed Sperm in Hen: In order to exploring the effects of different amounts of frozen-thawed sperm and two methods of artificial cloacal insemination and laparoscopic oviductal artificial insemination on fertility rate and hatch ability of following eggs laid by hen indicated, twenty-eight laying hens with 39-40 weeks old were selected and randomly divided into 7 subgroups named CAI-fro 1, CAI-fro 10 and CAI-fro 100 for artificial cloacal insemination, and LOAI-fro 1, LOAI-fro 10 and LOAI-fro 100 for laparoscopic artificial oviductal insemination, and control, with 4 replicates in each group (Table 1). The The total number of 0.2x106, 2x106, and 20x106 motile sperm from frozen-thawed semen were inseminated to CAI-fro 1, CAI-fro 10 and CAI-fro 100 group respectively, and LOAI-fro 1, LOAI-fro 10 and LOAI-fro 100 were also inseminated with the responding amount sperm through laparoscopic artificial insemination respectively.
Statistical Analysis
In this experiment, Excel2016 was used to preliminarily sort out the data, and IBM SPSS Statistics 24 statistical software was used for relevant statistical analysis. Each repeat was used as the test unit, and the factors were analyzed by one-way analysis of variance. And Duncan method was used to make pairwise comparison between the mean pairs of each treatment group in different indexes. 0.05 was used as the criterion for judging the significance of difference in statistical results of various data in this study. The results were expressed as Mean ± SED. And the photos were made by GraphPad Prism 8.
Results
Parameter Examination of Laparoscopic Oviductal Artificial Insemination of Hen
First, the effect of muscle anesthesia and intravenous anesthesia on laying rate of hens was studied. If soft shell eggs were found on the first day after anesthesia, no counting was done. The egg production of 10 chickens in each group after anesthesia is shown in the Table 2. As shown, there was no significant difference in the number of eggs picked up per day between the two groups (P > 0.05), it was almost the same on the fourth day. However, during the operation, after the intravenous injection, the hens almost woke up and could stand up immediately in 15 min. However, the hens did not wake up after the intramuscular injection and were in a coma state after the operation. Therefore, the anesthesia was given by intravenous injection under the wing for LOAI.
Anesthesia methods
Day1
Number of eggsDay2
Number of eggsDay3
Number of eggsDay4
Number of eggsDay5
Number of eggsDay6
Number of eggsintramuscular injection (n=10)
3.00±2.16
4.00±1.7
7.00±0.8
9.40±0.69
9.80±0.42
10.00
intravenous injection (n=10)
5.00±1.63
6.00±1.5
8.00±0.8
9.70±0.67
10.00
10.00
Values are means ± SED. The number of hens is 10 for each group.
Table 2: Effects of different anesthesia methods on laying rate of hens.
In order to slow down the stress caused by operation, The operation area of the recipient hens were unhaired a few days before operation. The operation area was routinely disinfected. The skin was cut about 5 mm length at the last edge of xiphoid process with a scalpel. The pneumoperitoneum needle was inserted into the incision. After the abdomen was inflated (Pressure 8 mmHg), the pneumoperitoneum needle was pulled out. The trocar (for 5 mm laparoscope) was inserted from the pinhole, and then the laparoscope (Diameter 5 mm) was inserted through the trocar. After the egg yolk and oviduct could be clearly seen on the screen, the laparoscope was fixed. In the front of the bound leg, touch the most prominent hip bone, and cut the skin about 5 mm length between the front side of hip bone and the last rib with a scalpel. Insert the grasping forceps (Diameter 5 mm) at the incision until the grasping forceps can be seen on the screen. Turn over the grasping forceps to find out the funnel of fallopian tube, grasp it gently, and insert the injection needle close to the hip bone and almost parallel to the grasping forceps. Under laparoscopic observation, insert the injection needle into the funnel of fallopian tube, and inject the sperm diluent into the funnel. The injection needle, grasping forceps and laparoscope were taken out in turn, and the skin incision was sutured routinely. The whole procedure is no more than ten minutes.
As for Inflated air option, first, hens were inflated with cleaned carbon dioxide gas at room temperature, all the chickens (five hens indicated) died during the operation. However, after being changed to cleaned atmosphere, the chickens survived the operation. So all data of the experiment using atmosphere for LOAI was recorded (Figure 1).
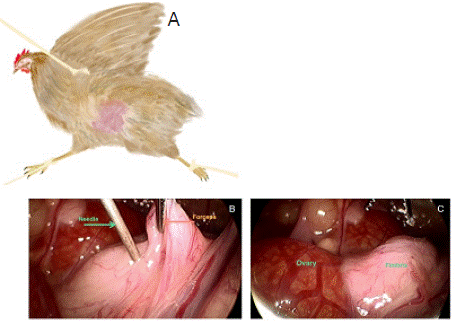
Figure 1: Fixed method of hen and transfer sperm into oviduct under
laparoscopic view of the abdomen. (A) fixed model of hen. Yellow circle for
pneumoperitoneum needle and trocar; red circle for forceps, and blue circle
for injection needle. (B) view of the abdomen under laparoscopy. (C) Transfer
semen into oviduct under laparoscopy.
Efficiency of Laparoscopic Oviductal Artificial Insemination Using Fresh Sperm in Hen
Fresh sperm was collected on the day of the experiment. The motility of fresh sperm was 84.75% ± 1.75% just before AI. Semem was diluted in different concentrations (Table 1) and then performed LOAI and CAI, respectively. The eggs collected after insemination were hatched and its fertility and hatchability were compared between the two insemination groups (Figure 2). As a result, fertility of LOAI fre 1, LOAI-fre 10 and LOAI-fre 100 were increased following the increasing sperm transferred by LOAI. Moreover, LOAI-fre 100 fertility was greater than LOAI-fre 1. But the number sperm of CAIfre 1 and CAI-fre 10 as the same number of LOAI-fre 1 and LOAI-fre 10 did not fertilize the egg while using cloacal artificial insemination. As for hatchability, LOAI-fre 10 and LOAI-fre 100 were greater than LOAI-fre 1. But in cloacal artificial insemination, only CAI-fre 100 had hatchability due that CAI-fre 1 and CAI-fre 10 had not got the fertilized eggs. In addition, hatchability of LOAI-fre 10 and LOAI-fre 10 and LOAI-fre 100 were no significantly different.
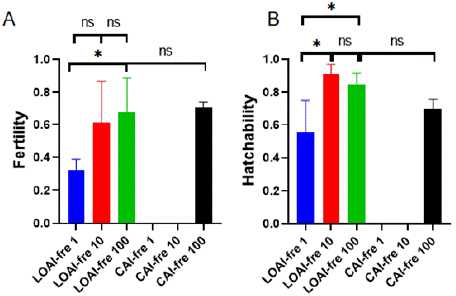
Figure 2: Fresh sperm fertility and hatchability of eggs fertilized with
laparoscopic oviductal artificial insemination (LOAI-fre 1 and LOAI-fre 10 and
LOAI-fre 100) and cloacal artificial insemination (CAI-fre 1 and CAI-fre 10
and CAI-fre 100).
Efficiency of Laparoscopic Oviductal Artificial Insemination Using Frozen Sperm in Hen
The motility of frozen sperm is 41.00% ± 2.00% just before AI. Semem was diluted as Table 1. Semem was diluted in different concentrations and then fertilized eggs to compare its fertility and hatchability with laparoscopic oviductal artificial insemination and cloacal artificial insemination. Fertility and hatchability of collected eggs from both artificial insemination with different number of fresh sperm were compared (Figure 3). There was only LOAI-fro 100 and CAI-fro 100 with the same fertilization rates. Also hatchability of LOAI-fro 100 and CAI-fro 100 were similar. But LOAI-fro 1 and LOAI-fro 10, and CAI-fro 1 and CAI-fro 10 had not fertilized and hatched.
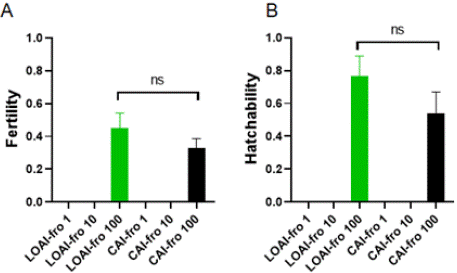
Figure 3: Frozen sperm fertility and hatchability of eggs fertilized with
laparoscopic oviductal artificial insemination (LOAI-fro 1 and LOAI-fro 10 and
LOAI-fro 100) and cloacal artificial insemination (CAI-fro 1 and CAI-fro 10
and CAI-fro 100).
Discussion
The present study was apparently the first to establish a method of laparoscopic oviductal artificial insemination in hen. The results explored that fertility and hatchability of eggs fertilized with laparoscopic oviductal artificial insemination were successful with relative less sperm, compared with cloacal artificial insemination.
Since the 1950s, AI has been used in large-scale farming of chickens [13]. The application of AI greatly reduces the cost of production, and the number of cocks that used for breeding is greatly reduced. Male to female ratio changes from 1:10 to 1:100 [13]. By now we first set up the laparoscopic oviductal artificial insemination in hen. During the experiment, the average time spent on each chicken from anesthesia to surgery was less than 10 minutes, similar to other animals such as sheep [18]. In this study, anesthesia is essential, because surgery stress may affect eggs production of hens. In order to reduce the stress caused by anesthesia on chickens, we found Sumianxin II be better than other anaesthetic (data not shown), and muscle anesthetized chicken took a few minutes to fall asleep while intravenous anesthetized chickens can fall asleep in less than 1 minute and waked up soon after the surgery. It not only can reduce the time of surgery but also can avoid over anesthesia.
Carbon dioxide gas always employed for blow-up abdominal cavity in laparoscopic surgery, but in this study in hen, it was fatal. Another key factor affecting the success of laparoscopic surgery is the entry position of the instrument. Inaccurate entry position could cause puncturing of yolk or egg, or bulging of air sac, or even puncturing of blood vessel or other tissue structure.
Some articles point out that only 1% of sperm injected through the fallopian tube can reach SSTs (sperm storage tubules) [10]. Here it stored last for a few days or few weeks. When the hen ovulates, the sperm is released from the SST and transferred to the oviduct fimbria [13]. LOAI, however, injected sperm directly into the funnel of the fallopian tube, bypassing the SSTs, allowing more sperm to be stored in the sperm nets. This is why we inject sperm directly into fimbriae of uterine tube. Therefore, it is possible to make some birds with less sperm or weaker sperm to complete the insemination work, and also improve the utilization rate of the sperm, and even frozen sperm. LOAI can bypass the physical reproductive barrier, which is also reflected in cats and sheep [18].
It indicated that In the case of poor sperm quality or low sperm motility, LOAI can obtain higher fertilization rate and hatching rate. The sperm with low sperm motility may be blocked during the transfer of fallopian tube. Mohan indicated that each AI injection of 89.10×106 sperm reached the highest fertility, 91.07%. However, 29.70×106 sperm can only reach 77% fertilization rate [13]. Aurore's test shows that 200× 106 fresh sperm AI can reach 97.1% fertility [24]. S. Tabatabaei indicated that inseminated with 50, 75, 100 and 150 million spermatozoa, fertility rates were 52.85%, 72.37%, 87.64% and 89.12%, respectively [3]. But all the experiments above were inseminated weekly, which is differ from our experiment that inseminated for once and collected eggs for 21 days. It also explained why fertility in this experiment is lower than others. Moreover, 0.2×106 fresh sperm transferred into fimbria tubal also can accomplish fertility of hen, which could be milli of sperm number as the reports [1,25].
However, LOAI also has some disadvantages. The umbrella of the fallopian tube is very fragile and can be torn easily. The egg yolk in abdominal cavity is easily broken by experienced operators, which may cause peritonitis or Intraperitoneal adhesions. Moreover, anesthesia and surgery affect laying egg the next days.
In conclusion, the present findings demonstrated setup of laparoscopic oviductal artificial insemination in hen, which should be usefulness for bird reproduction and biological protection.
Funding
This work was supported by grants from the Guangdong Special Support Program (2019BT02Y276), the National Natural Science Foundation of China (81941006, 81771579).
References
- Magyar LA. History of artificial insemination. Ther Hung. 1991; 39: 151-153.
- Navarro-Rubio S, Guell F. Understanding the correlation between artificial insemination and offspring health outcomes. Birth Defects Research. 2020; 112: 7-18.
- Van Eetvelde M, Heras S, Leroy J, Van Soom A, Opsomer G. The importance of the periconception period: Immediate effects in cattle breeding and in assisted reproduction such as artificial insemination and embryo transfer. Advances in Experimental Medicine and Biology. 2017; 1014: 41-68.
- Costa NP, Gonella-Diaza A, Pugliesi G, Cordeiro MM, Scollari SC, Mello BP, et al. Effects of recombinant bovine somatotropin on pregnancy per artificial insemination, corpus luteum cellular composition and endometrial gland morphometry in beef cattle. Theriogenology. 2020; 141: 180-185.
- Dubey RA, Johari DC, Singh RP, Singh BP. A study of artificial insemination in relation to fertility and hatchability in white Leghorn birds. Indian Vet J. 1977; 54: 116-118.
- Nakamura Y. Avian biotechnology. Advances in Experimental Medicine and Biology. 2017; 1001: 187-214.
- Rakha BA, Ansari MS, Akhter S, Akhter A, Blesbois E. Intravaginal insemination depth influences fertility outcomes in Indian red jungle fowl (Gallus gallus murghi). Animal Biotechnology. 2021; 32.
- Moorman CE, Plush CJ, Orr DB, Reberg-Horton C. Beneficial insect borders provide northern bobwhite brood habitat. PLoS One. 2013; 8: e83815.
- The IUCN Red List of Threatened Species. 2019
- Kawai Y, Hata T, Suzuki O, Matsuda J. The relationship between sperm morphology and in vitro fertilization ability in mice. J Reprod Dev. 2006; 52: 561-568.
- Ingram M. The birds and the bees and the plants. World Conserv. 1998; 9.
- Du J, Luo J, Huang J, Wang C, Li M, Wang B, et al. Emergence of genetic diversity and Multi-Drug resistant campylobacter jejuni from wild birds in beijing, china. Frontiers in Microbiology. 2019; 10: 2433.
- Mohan J, Sharma SK, Kolluri G, Dhama K. History of artificial insemination in poultry, its components and significance. World’s Poultry ence Journal. 2018; 74: 1-14.
- Shit N, Singh RP, Sastry KV, Agarwal R, Singh R, Pandey NK, et al. Effect of dietary l-ascorbic acid (L-AA) on production performance, egg quality traits and fertility in japanese quail (Coturnix japonica) at low ambient temperature. Asian-Australas J Anim Sci. 2012; 25: 1009-1014.
- Soper. Mastery of Endoscopic and Laparoscopic Surgery. Surg Laparo Endo Per. 2005; 19: E161.
- Anakkul N, Suwimonteerabutr J, Tharasanit T, Khunmanee S, Diloksumpan P, Berg D K, et al. Sperm distribution and fertilization after unilateral and bilateral laparoscopic artificial insemination with frozen-thawed goat semen. Theriogenology. 2014; 82: 1137-1144.
- Mccappin N, Murray RD. Some factors affecting pregnancy rate in ewes following laparoscopic artificial insemination. Veterinary Record. 2011; 168: 99.
- Sathe SR. Laparoscopic artificial insemination technique in small Ruminants-A procedure review. Front Vet Sci. 2018a; 5: 266.
- Ping S, Wang F, Zhang Y, Wu C, Tang W, Luo Y, Yang S. Cryopreservation of epididymal sperm in tree shrews (Tupaia belangeri). Theriogenology. 2011; 76: 39-46.
- Lachance C, Goupil S, Tremblay RR, Leclerc P. The immobilization of human spermatozoa by STAT3 inhibitory compound V results from an excessive intracellular amount of reactive oxygen species. Andrology. 2016; 4: 133-142.
- Qi XL, Xing K, Huang Z, Chen Y, Wang L, Zhang LC, et al. Comparative transcriptome analysis digs out genes related to antifreeze between fresh and frozen-thawed rooster sperm. Poult Sci. 2020; 99: 2841-2851.
- Kumar S, Pande DN. Effect of external stimuli on egg production in laying hens. Indian Veterinary Medical Journal. 1981.
- Brown JE, Harris GC, Hobbs TD. Effect of Intraperitoneal Insemination on Egg Production and Fertilizing Capacity of Fresh and Frozen Chicken Sperm. ScienceDirect. Poultry Sci. 1963; 42: 810-815.
- Rothlisberger R, Aurore V, Boemke S, Bangerter H, Bergmann M, Thalmann GN, et al. The anatomy of the male inferior hypogastric plexus: What should we know for nerve sparing surgery. Clinical Anatomy. 2018a; 31: 788-796.
- Tabatabaei S. The Effect of Spermatozoa Number on Fertility Rate of Chicken in Artificial Insemination Programs[J]. journal of animal and veterinary advances. 2010; 9: 1717-1719.