
Research Article
Austin J Vet Sci & Anim Husb. 2025; 12(2): 1168.
Effects of Silver and Titanium Dioxide Nano Particles on Hatching Rates of Decapsulated and Non Decapsulated Artemia Franciscana Cysts
Tavana M, Kalbassi MR2*, Kenari AA3 and Johari SA4
1MSc. Graduated, Department of Aquaculture, Marine Sciences Faculty, Tarbiat Modares University, Mazandaran, Noor, Iran
2Professor, Department of Aquaculture, Marine Sciences Faculty, Tarbiat Modares University, Mazandaran, Noor, Iran
3Professor, Department of Aquaculture, Marine Sciences Faculty, Tarbiat Modares University, Mazandaran, Noor, Iran
4Assistant Professor, Fisheries Department, Natural Resources Faculty, University of Kurdistan, Iran
*Corresponding author: Mohammad Reza Kalbassi, Department of Aquaculture, Marine Sciences Faculty, Tarbiat Modares University, Mazandaran, Noor, Iran Tel: +98-911-2204336, Fax: +98-1226253499, Email: kalbassi_m@modares.ac.ir
Received: April 06, 2025 Accepted: April 18, 2025 Published: April 23, 2025
Abstract
Wastewater and effluent of nano technology products outputs the aquatic environment, agricultural lands, aquatic and terrestrial habitats and may pose a risk to the ecosystems. We evaluated the toxic effect of nano materials on the aquatic environment, the effect of colloidal Ag nanoparticles (in concentration of 0.1, 1, 10, 32 and 100 mg/L) and the suspension of TiO2 nanoparticles (in concentrations of 10, 32 and 100 mg/L) were examined on the hatching rate of Artemia franciscana cysts within 24 hours. The results showed that Ag and TiO2 nanoparticles significantly reduced the hatching rate of A. franciscana cysts than those in the control group (p < 0.05). The results also showed that hatching rate of cysts at AgNPs treatments were significantly less than TiO2NPs treatments and hatching rate of decapsulated cysts was significantly higher than undecapsulated cysts in AgNPs treatments (p<0.05). Deposited AgNPs especially on the alveolar layer could reduce significantly hatching rate of the undecapsulated cysts compared to decapsulated cysts. Also the most important reason of decrease in cyst hatching rate is probably unfavorable effects of nanoparticles on the process of water absorption by cysts. In fact nanoparticles impact on the glycogen secretion and hydrophilic process which consequently leads to hatching failure.
Keywords: Ecotoxicology; Silver; TiO2; Nanoparticles; Artemia fransiscana; Cyst; Hatching rate
Introduction
Nanotechnology is the ability of development and producing the novel materials, tools and systems in nano size and application of their new properties at the nano scale [1]. Today nanoparticles (NPs) have been widely used in various field of sciences such as biological science e.g. biosensor [2], therapeutics [3], medical sciences (e.g. drug delivery systems) [4], environmental science, energy and cosmetics [5]. Among the various nanomaterials, Ag and TiO2 Nanoparticles are two most widely used in both industry and daily life [6]. Estimated global annual production of Ag and TiO2 NPs are about 55 and 3,000 tons, respectively [7]. The presence of Ag and TiO2 NPs in the natural water was estimated from 0.03 to 0.32 and from 0.7 to 16 micrograms per liter (μg/L), respectively [8].
Due to its high nutritional value, Artemia (brine shrimp) has widely been considered as a live food for larval fish production and fish hatcherien. In addition, this organism could be used as a bioindicator to evaluate potential toxicity of various materials [9-11]. Possible use of Artemia in Ecotoxicological studies can be related to its highlight advantages characteristics and general physiological features such as adaptability to wide ranges of salinity (5-250 g L-1) and temperature (6- 35 °C), short life cycle, high adaptability to unfavorable environmental conditions, high fecundity, bisexual parthenogenetic reproduction strategy (by production of nauplii and or cysts)), small body size, and adaptability to divers nutrient resources as it is as a non-selective filter feeder [11].
Due to the large production and use of nanomaterials and their consequent entry into the aquatic environment as well as the limitations of available aquatic nanotoxicity data, the aim of the present work was identification and comparison the effects of Ag and TiO2 nanoparticles (as two widely used nanomaterials) on hatching rate of both decapsulated and non-decapsulated Artemia franciscana cysts.
Materials and Methods
Nanomaterials and Characterization
Colloidal silver nanoparticles (AgNPs) and powdered titanium dioxide nanoparticles (TiO2NPs) were used in the anatase form. The AgNPs, brand L (Nanocid) and TiO2NPs were purchased from Pars Nano Nasb Co (Tehran, Iran) and US Research Nano materials, Inc. (Houston, USA), respectively.
Prior to toxicity tests, to determine the geometry and size of nanomaterials, TEM analysis was performed using a transmission electron microscope (H-7100Fa, Hitachi-Japan). In order to calculate the average size of Ag and TiO2NPs, 700 particles were randomly selected on the images at 100,000 magnifications and measured using Axio Vision digital image processing software (Release 4.8.2.0, GmbH Carl Ziess Micro Imaging-German).
A stock suspension of 300 mg/L AgNPs was prepared by diluting of initial colloid (4000 mg/L) in double distilled water and this stock then were sonicated using a bath sonicator (U1250HD) for 30 min. In order to make a stock suspension of 300 mg/L TiO2NPs deionized water was added drop by drop into 0.3 g of TiO2NPs powder and mixed until a steady paste formed. Adding deionized water into this paste continued for 40 min when the mixture was reached to1000 ml. Mixture was placed into a jar and then was sonicated for 5 min using a bath sonication system (U1250HD) following 10 more minutes sonication using a probe sonicator (misonixs-400-01, 70 amplitude = 70-75 wat). Finally, the milky suspension of TiO2NPs was obtained. The suspension was placed in a dark place because the dilute suspension of TiO2NPs has high light sensitivity [12,13].
Hatching Conditions of Artemia Cysyts
The A. franciscana cysts (San Francisco Strain Brine shrimps- A.H.T brand- United States) were used in the present study. The cysts were incubated in conical tubes filled with the artificial seawater (ASW) adjusted at 3.5% salinity (35 g of Urmia lake salt was added to 1 L distilled water), and 30 °C (through the immersion of conical tubes in a water bath equipped with electric heater). The pH was adjusted at 8.0 ± 0.5 (by adding NaHCO3 solution) and range of light intensity values were about 1800 lux (provided by fluorescent lamps). Air was bubbled through the incubator via a glass tube extending to the bottom of the hatching vessel to keep all the cysts in continuous motion [14]. Average water hardness obtained by flame photometer was 145 mg/L CaCO3. In order to investigate the resistance effect resulting from Artemia cyst shell, against nanoparticles, cysts were hatched either decapsulated or non-decapsulated.
Toxicology Tests
Hatching rate of Artemia franciscana cysts in the presence of AgNPs (at the concentrations of 0.1, 1, 10, 32, and 100 mg/L) or TiO2NPs (at the concentration of 10, 32, and100 mg./L) were measured and compared to the control groups (without nanoparticles). In each treatment, after 24 hours of incubation (hatching time), 250 μl were sampled from each incubator in 6 replicate and hatching rates were calculated and compared to the controls. Samples were fixed using logol solution and then were examined under stereomicroscope and hatched napliies, umbrellas and unhatched cysts were counted. Hatching rates were calculated by the Van Stappen [14] formula:
(H (%) = N/(N+C) × 100).
In which, H is hatching rate, N is the number of hatched cysts, including the umbrella stage, and C is the number of total cysts (hatched and unhatched).
Statistical Analyses
All the measured data were incorporated in an excel database. All statistical tests were performed using SPSS v. 13.2. Normality test was done by Kolmogorov–Smirnov and Shapiro–Wilk depending on the type of data. If the data were non-parametric, Mann-Whitney U test was used for comparison of two independent groups and Kruskal- Wallis test was used for comparison of independent groups. For the analysis of normal data, The ANOVA were used for comparison of several independent groups and the Duncan test were used for means compare, while the Samples T-Test was used for compare of two independent groups. Also, one way ANOVA was used to compare several independent groups with control, and Dunnett's test was used to compare means. In the case of normal data with non homogeny variance, Games-Howell test was used to compare several independent groups.
Results
Properties of Nanomaterials
According to the TEM images, the shape of AgNPs was spherical and maximum diameter was about 129 nm (Figure 1). Only 2.28% of the particles were larger than 100 nm. Also 65.14% of the particles estimated to be in range of 1 to 13 nm and count median diameter (CMD) was equal to 6.47 nm (Figures 2 and 3). Geometric mean diameter (GMD) and Geometric standard deviation (GSD) of particles diameter was 12.65 nm and 1.46, respectively. The particle diameter arithmetic mean was 2.54± 1.14 nm.
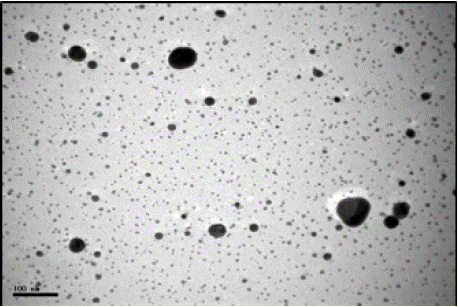
Figure 1: TEM image of silver nanoparticles (4000 mg/L).
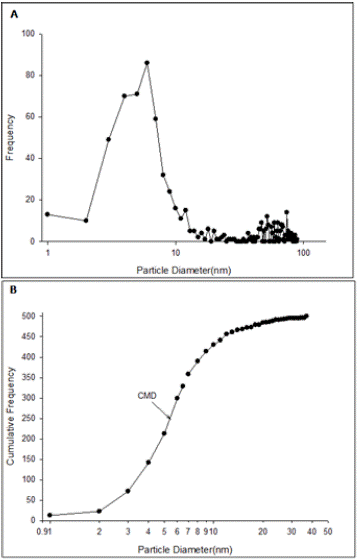
Figure 2: Size distribution of silver nano particles in undiluted suspension
(4000 mg/L) based on transmission electron microscope data. A: Number
Frequency, B: Cumulative Frequency. (CMD: Cumulative Median Diameter).
As per TEM images, TiO2NPs were needle or pyramidal shape (Figure 3). Also 86.23% of the particles estimated to be in range of 5 to 30 nm and 5.07% of the particles were larger than 50 nm and the maximum particle diameters was measured as 81 nm. Also median value of cumulative frequency particle size, GMD and GSD of TiO2NPs was calculated as 13.90 nm, 17.50 nm and 1.71, respectively. Also TEM images of suspended TiO2NPs showed that large clusters had formed in water; in which 28.47%, 52.55%, and 18.98% of the clusters had diameter between 15 to 100 nm, between100 to 500 nm and larger than 500 nm, respectively (Figure 4).
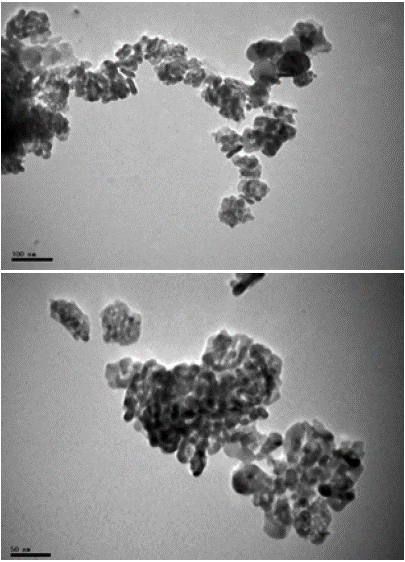
Figure 3: TEM images of titanium dioxide nanoparticles. (A): Dry TiO2
powder. (B): Suspended TiO2 powder (400 mg/L).Diameter).
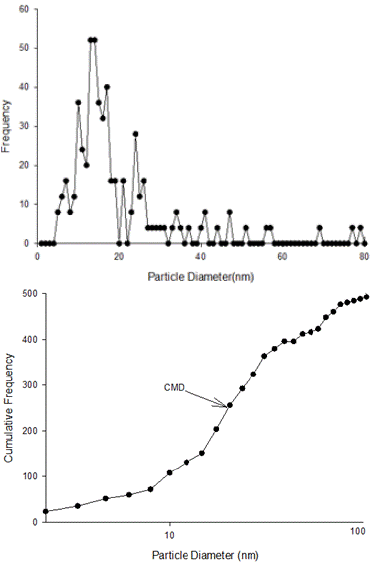
Figure 4: Size distribution of nano particles of titanium dioxide in
suspension (400 mg/L) based on transmission electron microscope data. A:
Number Frequency, B: Cumulative Frequency. (CMD: Cumulative Median
Diameter).
Effect of Agnps and Tio2nps on Hatching Rate of Artemia Franciscana Cyst
Hatching rate of the decapsulated cysts was not significantly different between concentrations of 0.1, 1, 10, and 32 mg/L AgNPs (p > 0.05). But exposure to 100 mg/L AgNPs significantly reduced the cysts hatching rate. In the case of undecapsulated cysts, all AgNPs concentrations were significantly different from each other (p < 0.05) and the concentrations of 0.1 and 100 mg/L AgNPs were showed maximum and minimum hatching rate, respectively. Moreover, hatching rate of both decapsulated and undecapsulated cysts in all AgNPs concentrations were significantly lower than the controls (p < 0.05). Hatching rate of decapsulated cysts was significantly higher than undecapsulated cysts in AgNPs treatments (Figure 5, A).
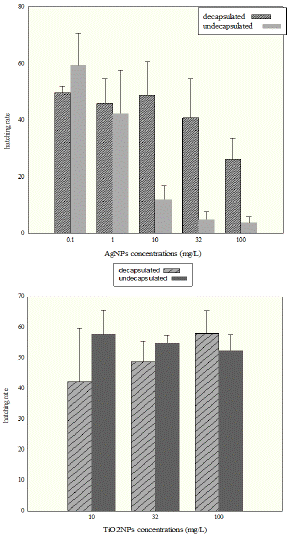
Figure 5 (A): Hatching rate of the decapsulated Artemia cysts at 100 mg/L
of AgNPs treatment was significantly lowered from 0.1, 1, 10 and 32 mg/L
treatments (B): hatching rate did not differed between decapsulated and
undecapsulated Artemia cysts and there were no significant differences
between different TiO2NPs concentrations (10, 32, and 100 mg.lit-1) (p >
0.05).
The results showed that hatching rates did not vary significantly between decapsulated and undecapsulated cysts. Also there were no significant differences between TiO2NP concentrations of 10, 32, and 100 mg/L (p > 0.05). Hatching rate of cysts in control groups was significantly higher than all TiO2NP treatments for both decapsulated and undecapsulated cysts (p < 0.05, Figure 5, B).
Statistical analysis showed that hatching rate of the decapsulated cysts of AgNPs and TiO2NPs treatments was not significantly different in 10 and 32 mg/L treatments (P>0.05); while there was a significant difference in hatching rate between 100 mg/L TiO2NPs and 100 mg/L AgNPs.
In the case of undecapsulated cysts, it was found that hatching rate of the cysts incubated with TiO2NPs was higher than those incubated with AgNPs in all equal concentrations (10, 32 and 100mg/L) (p < 0.05, Figure 6).
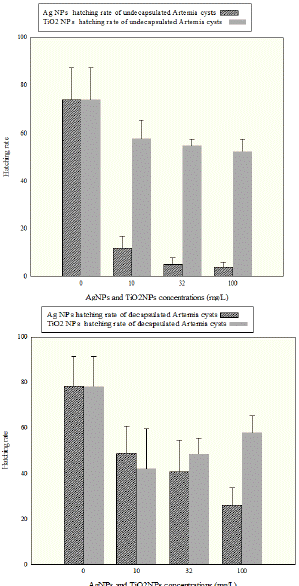
Figure 6: Comparison of toxicity effect silver nano particles with TiO2
nanoparticles on cyst hatching rate of Artemia franciscana. (A): hatching rate
of the undecapsulated Artemia cysts (B): hatching rate of the decapsulated
Artemia cysts.
Discussion
Artemia spp. is one of the most widespread saltwater organisms suitable for ecotoxicity testing. The most cited testing methods chronologically ordered belong to, Michael et al., Harwig and Scott, Sorgloos et al., Persoone and Vanhaecke, Persoone and Wells, Solis et al., Libralato et al., Manfra et al., Gambardella et al. [11,15-22] and also Artemia franciscana is one of the model organism in ecotoxicity testing [23], because it is widely used as a nutritious live food source to the larvae of a variety of marine organism [24] and is easy to maintain under laboratory conditions [10,25]. Regarding the toxic effects of Ag-NPs, some studies concerning rainbow trout sperm cells [26], Daphnia magna nauplii [27] Artemia salina nauplii, [22,28-32], in addition, toxic effects of TiO2 NPs were investigated on zebrafish [33,34], Daphnia magna [35], and Artemia salina nauplii [36,37].
Results of the present study revealed that exposure to AgNPs and TiO2NPs significantly reduce hatching rate of the Artemia franciscana cysts, also exposure to the AgNPs caused higher reduction of hatching rate when compared to TiO2NPs; Similarly Ozkan et al. [37] were found AgTiO2 to be more toxic to Artemia salina nauplii than TiO2; In previous study concerning the toxicity of nano silver on Artemia sp. cyst was found to be similar [38]; which this could be due to the effect from Ag+ ions that released from AgNPs on the ion regulatory of cysts. Similar to our study, Guadagnolo et al. [39] have been reported the effect of ion regulatory impairment on increased mortality in rainbow trout eggs.
When exposed tadpoles to double-wall carbon nano tubes Mouchet et al [40] found that aggregation of nano tubes had negative effect on oxygen uptake by embryos and also caused delayed hatching due to indirect effect of hypoxia. So the negative effects of silver ions on cysts, deposition of silver nano particles on the chorion could cause physical blockage and consequent hypoxia and fetal asphyxia. Deposited AgNPs especially on the alveolar layer could reduce significantly hatching rate of the undecapsulated cysts compared to decapsulated cysts.
In the present study, as shown in Figure 5 (A), minimum hatching rate of the Artemia franciscana cysts was observed in 100 mg/L AgNPs which same results was also reported by Arulvasu et al. [41] that revealed that as the concentration of the silver nanoparticles increases (from 2 nM to 10nM and 12nM) the effect of toxicity increases and the percentage of hatching of the Artemia cysts decreased from 56% to 31.6% and 21.6%. The effects on Artemia cysts were also consistent with those reported that silver nano particles at high concentrations of 50 to 100 mg/L could cause mortality and delayed hatching in zebrafish embryos [42].
Present study also revealed that TiO2NPs reduced the hatching rate in Artemia cysts (p < 0.05), however no significant difference was observed between all TiO2NP concentrations (p > 0.05). Because TiO2NPs induced the formation of free radicals which has been reported in Hund-Rinke and Simon [43]; the ROS production is especially relevant in the case of nanoparticles with photocatalytic properties such as TiO2. Therefore, there was ROS production potential of the suspensions of TiO2NPs in the present study and it likely could lead to significant reduction in the percentage of hatching in Artemia cysts. Aggregation of TiO2NPs in aqueous suspensions has been reported previously (e.g. Adams et al. [8]; Zhu et al. [10,44]). In present study, there was no significant differences between 100 mg/L TiO2NPs and control group for decapsulated Artemia cysts (Figure 5 and 6). Also, the result showed that as the concentration of TiO2NPs increased from 10 to 100 mg/L, hatching rate did not differ significantly.
Similar to the present study, Ates et al. [45] and Ozkan et al. [37] were found that the aggregate sizes increased with respect to TiO2 concentrations. Barelds et al. [46] were exposed the Artemia salina nauplii to four different concentrations of TiO2NPs (0.01, 0.1, 1 and 10 mg/L) and illustrated that the aggregation increased sharply when the TiO2NPs concentration increased from 0.01 to 10 mg/L. Their study also demonstrated that bioavailability of TiO2NPs for Artemia salina nauplii were reduced due to their aggregation and consequently out of reach. In another study acute exposure of Artemia nauplii and adults was conducted in sea water in a concentration range from 10 to 100 mg/L TiO2NPs for 24 and 96 h [45,47]. These results suggested that suspensions of the TiO2NPs were nontoxic to Artemia, most likely due to the formation of benign TiO2 aggregates in water. This is consistent with previous findings which suggest it can be caused by possible aggregation of nano-TiO2 of different sizes ranged from 100 to 500 nm (Figure 3).
Conclusion
The present work demonstrated that exposure of both decapsulated and undecapsulated Artemia cysts to either AgNPs or TiO2NPs was significantly reduced the hatching rate than controls and the toxicity of AgNPs were higher than TiO2NP because TiO2NPs rapidly aggregate. Regarding to the increasingly growing use of nanomaterials and future advances in medical sciences and technologies, many of them will release into the marine environment. Hence it can be concluded that the uncontrolled release of these nanoparticles, could impose a large negative consequence on the hatching percentage of organisms like artemia which lives in deltaic places and salt marshes area which is prone to entering the nanomaterial infected sewages. Accordingly, this study would provide the useful baseline information to researchers for future studies.
Acknowledgement
The authors gratefully acknowledge the support of the TarbiatModares University of Iran which funded this research through a M.Sc. thesis project. Also the authors wish to thank Prof. Il Je Yu and Dr. Ji Hyun Lee whom prepared the TEM micrographs.
References
- Schmid K, Riediker M. Use of nano particles in Swiss industry a targeted survey. Environmental Science & Technology. 2008; 42: 2253-2256.
- Prow T, Grebe R, Merges C, Smith J, McLeod S, Leary J, Lutty G. Nano particle tethered biosensors for auto regulated gene therapy in hyperoxicendothelium. Nanomedicine. 2006; 2: 276.
- Czupryna J, Tsourkas A. Suicide gene delivery by calcium phosphate nano particles: a novel method of targeted therapy for gastric cancer. Cancer Biology and Therapy. 2006; 5: 1691-1692.
- Jin S, Ye K. Nano particle-mediated drug delivery and gene therapy. Biotechnology Progress. 2007; 23: 32-41.
- Perugini P, Simeoni S, Scalia S, Genta I, Modena T, Conti B, Pavanetto F. Effect of nano particle encapsulation on the photo stability of the sunscreen agent, 2-ethylhexyl-p-methoxycinnamate. International Journal of Pharmaceutics. 2002; 246: 37-45.
- Adams LK, Lyon DY, Alvarez PJJ. Comparative eco-toxicity of nano scale TiO2, SiO2, and ZnO water suspensions. Water Research. 2006; 40: 3527– 3532.
- Piccinno F, Gottschalk F, Seeger S, Nowack B. Industrial production quantities and uses of ten engineered nano materials for Europe and the world. Journal of Nano particle Research. 2012; 14: 1109–1120.
- Batley GE, Kirby JK, McLaughlin MJ. Fate and risks of Nano materialsin aquatic and terrestrial environments. Accounts of Chemical Research. 2013; 46: 854–862.
- Kerster HW, Schaeffer DJ. Brine shrimp (Artemia salina) nauplii as a teratogen test system. Eco toxicology Environment Safety. 1983; 7: 342-349.
- Nunes BS, Carvalho FD, Guilhermino LM, Van Stappen G. Use of the genus Artemia in ecotoxicity testing. Environmental Pollution. 2006; 144: 453-462.
- Persoone G, Wells PG. Artemia in aquatic toxicology: A review. In: Sorgellos P., Bengtson D. A., Decleir W., Jaspers E. (Eds.), Artemia Research and its Applications. Morphology, Genetics, Strain Characterization, Toxicology. vol. I. Universa Press, Wetteren, Belgium. 1987.
- Jiang J, Oberdo¨rster G, Biswas P. Characterization of size, surface charge, and agglomeration state of nano particle dispersions for toxicological studies. Nano particle Research. 2008; 11: 77–89.
- OECD WPMN. Environment, Health and Safety Publications, Series on the Safety of Manufactured Nano materials. 2010; P: 67.
- Van Stappen G. Introduction, biology and ecology of Artemia. Live food culture book. 1976; 79-136.
- Michael AS, Thompson CG, Abramovitz M. Artemia salina as a test organism for bioassay. Science. 1956; 123: 464.
- Harwig J, Scott PM. Brine shrimp (Artemia salina L.) larvae as a screening system for fungal toxins. Applied Microbiology. 1971; 21: 1011-1016.
- Paul Vanhaecke, Patrick Sorgeloos. International study on Artemia. XVIII. The hatching rate of Artemia cysts – A comparative study. 1982; 1: 263-273.
- Paul Vanhaecke, Guido Persoone, Christine Claus, Patrick Sorgeloos. Proposal for a short-term toxicity test with Artemia nauplii.1981; 5: 382-387.
- PN Solis, CW Wright, MM Anderson, MP Gupta, JD Phillipson. A microwell cytotoxicity assay using Artemia salina (brine shrimp). Planta Med. 1993; 59: 250-252.
- G Libralato, C Losso, A Volpi Ghirardini. Toxicity of untreated wood leachates towards two saltwater organisms (Crassostrea gigas and Artemia franciscana). 2007; 144: 590-593.
- Manfra, et al. Long-term Lethal Toxicity Test with the Crustacean Artemia franciscana. Journal of Visualized Experiments. 2012.
- Gambardella, et al. On the management of open innovation. Research Policy. 2014; 43: 903–913.
- L Manfra, A Tornambè, F Savorelli, A Rotini, S Canepa, M Mannozzi, et al. Ecotoxicity of diethylene glycol and risk assessment for marine environment. Journal of Hazardous Materials. 2015; 284: 130-135.
- Radhika Rajasree SR and Ganesh k. Studies On The Toxicological Effects Of Engineered Nanoparticles In Environment - A Review. International Journal on Applied Bio-Engineering. 2010; 4: 44-53.
- MV Barahona, S Sánchez-Fortún. Toxicity of carbamates to the brine shrimp Artemia salina and the effect of atropine, BW284c51, iso-OMPA and 2-PAM on carbaryl toxicity. Environmental Pollution. 1997; 104: 469-476.
- Hasan Mohamed Elarabi1, Fuadah Johari. The Determinant Factors Effecting the Job Satisfaction and Performance in Libyan Government Hospital. Asian Social Science. 2014; 10: 8.
- Asghari S, Johari SA, Lee J Hyun, Kim YS, Jeon YB, Choi HJ, et al. Toxicity of various silver nanoparticles compared to silver ions in Daphnia magna. Journal of Nanobiotechnology. 2012; 10: 1-14.
- Garaventa, et al. Improved survival of children with neuroblastoma between 1979 and 2005: a report of the Italian Neuroblastoma Registry. J Clin Oncol. 2010; 28: 2331-2338.
- Gaiser EE, McCormick PV, Hagerthey SE & Gottlieb AD. Landscape Patterns of Periphyton in the Florida Everglades. Critical Reviews in Environmental Science and Technology. 2011; 41: 92–120.
- Kumar DS, Prasad RMV, Kishore KR, Rao ER. Effect of Azolla (Azolla pinnata) based concentrate mixture on nutrient utilization in buffalo bulls. Indian J. Anim. Res. 2012; 46: 268-271.
- Falugi F, Kim HK, Missiakas DM, Schneewind O. Role of Protein A in the Evasion of Host Adaptive Immune Responses by Staphylococcus aureus. mBio. 2013; 4. 10.1128.
- Giovanni Libralato. The case of Artemia spp. in nanoecotoxicology. Marine Environmental Research. 2014; 101: 38-43.
- Xiong J, Onal M, Jilka R, et al. Matrix-embedded cells control osteoclast formation. Nat Med.2011; 17: 1235–1241.
- Yan S, et al. Optimization of Seed Germination and Detection of Germination Inhibitors in Gardenia Fruits. Journal of Southern Agriculture. 2014; 45: 376- 382.
- Zhu X, Chang Y, Chen Y. Toxicity and bio accumulation of TiO2nano particle aggregates in Daphnia magna. Chemosphere. 2010; 78: 209–215.
- Clemente FM, Wong DP, Martins FML & Mendes RS. Acute Effects of the Number of Players and Scoring Method on Physiological, Physical, and Technical Performance in Small-sided Soccer Games. Research in Sports Medicine. 2014; 22: 380–397.
- Göya C, Ece A & Hamidi C. Reply to Ozkan et al. regarding ‘Acoustic radiation force impulse elastography for detection of renal damage in children’. Pediatr Radiol. 2015; 45: 462.
- Jeong, Sangyun, et al. The Evolution of Gene Regulation Underlies a Morphological Difference between Two Drosophila Sister Species. Cell. 2008; 132: 783-793.
- Guadagnolo CM, Brauner CJ, Wood CM. Effects of an acute silver challenge on survival, silver distribution and ionoregulation within developing rainbow trout eggs (Oncorhynchus mykiss). Aquatic Toxicology. 2000; 51: 195–211.
- Mouchet F, Landois P, Sarremejean E, Bernard G, Puech P, Pinelli E. Characterisation and in vivo eco toxicity evaluation of double-wall carbon nanotubes in larvae of the amphibian Xenopus laevis. Aquatic Toxicology. 2008; 87: 127–137.
- Arulvasu, et al. Effect of dietary administration of Zingiber officinale on growth, survival and immune response of Indian major carp, Catla catla (Ham.). International Journal of Pharmacy and Pharmaceutical Sciences. 2013; 5: 108-115.
- Asharani PV, Wu YL, Gong Z, Valiyaveettil S. Toxicity of silver nano particles in zebra fish models. Nanotechnology. 2008; 19: 1–8.
- Hund-Rinke K, Simon M. Ecotoxic effect of photocatalytic active nano particles (TiO2) on algae and daphnids. Environmental Science and Pollution Research. 2006; 13: 225-232.
- Zhu X, Zhu L, Li Y, Qi R, Duan Z, Lang YP. Comparative toxicity of several metal oxide nano-particle aqueous suspensions to zebra fish (Danio rerio) early developmental stage. Journal of Environmental Science and Health, Part A. 2008; 43: 278–284.
- Ates M, Daniels J, Arslan Z, Farah IO. Effects of aqueous suspensions of titanium dioxide nano particles on Artemia salina: assessment of nano particle aggregation, accumulation, and toxicity. Environmental Monitoring and Assessment. 2012.
- Barelds H, Ropstad E, Jonker FH. The uptake and effects on survival of nano silver and nano titanium dioxide in brine shrimp (Artemia nauplii). Norwegian School of Veterinary Science Utrecht University. 2010; 1-26.
- Chinnasamy A, Samoumichael J, Durai P, Devakumar C. Toxicity effect of silver nano particles in brine shrimp Artemia. Indian Journal of Clinical Biochemistry. 2013; 1: 29-34.