
Research Article
Austin J Vet Sci & Anim Husb. 2025; 12(2): 1165.
In Vitro Assessment of Some Selected Herbs on the Eggs and Larvae Inhibitory Assays of Haemonchus Contortus
Ayankoso MT1,2*
¹Department of Animal Science, Faculty of Agriculture, Adekunle Ajasin University, Akungba Akoko, Ondo State, Nigeria
²Department of Animal Production and Health, Federal University of Agriculture, Abeokuta, Nigeria
*Corresponding author: Ayankoso MT, Department of Animal Science, Faculty of Agriculture, Adekunle Ajasin University, Akungba Akoko, Ondo State, Nigeria; Department of Animal Production and Health, Federal University of Agriculture, Abeokuta, Nigeria Email: taiwo.ayankoso@aaua.edu.ng
Received: February 25, 2025; Accepted: March 20, 2025; Published: March 24, 2025;
Abstract
The quest to explore natural alternatives as a replacement for conventional anthelmintics necessitates the investigation of some selected herbs (Allium sativum, Hibiscus sabdariffa, Telfairia occidentalis and Zingiber officinale) in treatment of Haemonchus contortus. The investigation of the inhibitory effect of herb extracts at different concentrations (25, 50, 75 and 100%) and volumes (150, 300 and 450 μl), respectively on eggs and larvae of H. contortus in an in vitro study in a Completely Randomized Design. Data collected were subjected to one-way Analysis of Variance. Results of this study showed that highest (P<0.05) egg inhibition (100.00 ± 0.00%) was obtained for A. sativum while Z. officinale had highest (P<0.05) inhibitory effect at 50, 75 and 100%, comparable to conventional albendazole. A. sativum extract at 150 μl had highest (P<0.05) larva inhibitory at 50 and 100% concentration, while H. sabdariffa was only highest (P<0.05) at 100% concentration. At 300 and 450 μl, highest larvae inhibition was at 50 and 75% concentration for A. sativum and H. sabdariffa, respectively. At 50-100% concentration, A. sativum and Z. officinale proved to be most effective and compete favourably with conventional albendazole. It could be concluded that all the selected plant types possess potential to control growth and proliferation of H. contortus at both egg and larva stages under in vitro study. Also, Allium sativum and Zingiber officinale proved to be effective and compete favourably with convectional albendazole on Haemonchus contortus egg inhibition assay at 50-100 % concentration. Considering different plant extracts at 150 μl on Haemonchus contortus larvae inhibitory assay, at 75 % concentration, Hibiscus Sabdariffa and Allium sativum were observed to be at par with conventional albendazole.
Keywords: Concentration; Anthelminths; Helminthiasis; Extract
Introduction
Helminthiasis has been extensively reported to be one of the major challenges confronting livestock all over the world [1-3]. They reduced feed intake and live-weight gains and increase death rate in goats and sheep farming systems [2,4,5] and make animals to be more vulnerable to other infections. Diverse species of helminths that cause production losses in the digestive tract and related organs of animals include Haemonchus, Cooperia, Bunostomum, Gaigeria, Fasciola, Oesphagostormum, Trichuris, Trichostronglus, etc [6]. Among these species Haemonchus contortus has been reported to be most populous all over the world and with numerous damages on ruminants. H. contortus has morphological structure which is difficult for animals to survive with [7,8].
The conventional means of controlling these worms have been through the use of chemotherapeutic approach [9], which had resulted in the development of resistance by the parasites to the active ingredients in the drugs [10,11]. This might be due to misapplication of drug in terms of rightful dosage and timing. Other alternative methods include the use of diets to improve the host resistance [4,12,13] and use of browse plants that are high above the ground level to reduce contact to the worms [14].
Before the advent of synthetic drugs, various parts of plants had been used in management of infirmities in both human medicine and animal veterinary with abundant proof of recovery [15]. In this present age, plants remain significant sources of medicines and most of the modern drugs are obtained from plant products [15]. But, the major challenge associated with use of plant parts as drugs is unstandardized methods of application in terms of volume and concentration requirements.
This necessitates the research into the use of plants such as Allium sativum, Hibiscus sabdariffa, Telfairia occidentalis and Zingiber officinale in controlling the growth and proliferation of Haemonchus contortus. These herbs have been used extensively in human nutrition as spices (A. sativum and Z. officinale), food drink and vegetable (H. sabdariffa) and T. occidentalis solely as vegetable with little or no attention to their medicinal purposes especially in livestock management. H. sabdariffa contains phytochemicals like alkaloids, saponin, tannin, phenols and sterols [16], Z. officinale comprises of constituents such as acids, shoagaols, gingerol, essential oils, fibre, amino acids and minerals [17], T. occidentalis possesses high level of phytochemicals such as alkaloids, phytic, tannins, flavonoids, saponin and vitamins A, C and riboflavin which work independently and collectively to prevent or cure ailments [18] and A. Sativum is rich in protein, minerals, vitamins, energy and contains highly rich sulphur compounds such as allicin, allin, diallyl sulfide, diallyl disulfide, ajoene and others which have tendency to restrict the growth and proliferation of microbial organisms and parasitic worms due to their antioxidant and antimicrobial properties [17,19].
Allium sativum possess phyto-compounds such saponin which has been documented to enhance inducement of defensive mucosal membrane, thereby perform curative role in the body of the host [20]. It is rich in tannins serve as a natural alternative to conventional anthelmintic which has been banned in some parts of the world [21,22]. The tannins could hinder major processes in worms such as oxygen exchange between the inside and outside of the egg [23].
The ability of tannins to bound to the surface of the eggshell, possibly through tannin-protein interactions, and either prevented the proteins that cause the actual hatching process [24] or the tannin form coat around the egg and disabled the penetration of the larvae through the eggshell [25]. Hibiscus sabdariffa at higher concentration (50 mg/ml) gave total elimination of nematodes through by the principle of agglutinin whereby clumps were formed round the eggs which prevent further metabolic activities [26] who observed extract of Hibiscus sabdariffa to paralyze Lumbricus terrestris. Telfaria occidentalis possesses phytochemicals such as alkaloids, phenol, and tannins which could inhibit the growth of H. contortus egg inhibitory assay [22].
Therefore, this study tends to investigate appropriate concentration and volume requirements of some selected herbs on the eggs and larvae inhibitory assays of Haemonchus contortus under in vitro study
Materials and Methods
Experimental Site
The study was carried out at the Veterinary Teaching Hospital, College of Veterinary Medicine and Post Graduate Research Laboratory of the Department of Animal Production and Health, College of Animal Science and Livestock Production, Federal University of Agriculture, Abeokuta, Ogun State, Nigeria. The experimental site is located on latitude 71’10o N and longitude 3’20 E [27]. The climate is tropical with mean annual rainfall of 1, 037m. The mean ambient temperature ranges from 28o C in December to 36o C in February with a yearly average of 34o C. The vegetation represents an interphase between the tropical rainforest and the derived savannah with a yearly average humidity of 83% [28].
Samples Collection
Dry calyces of roselle (Hibiscus sabdariffa), rhizomes of ginger (Zingiber officinale), cloves of garlic (Allium sativum) and fresh leaves of fluted pumpkin (Telfairia occidentalis) were purchased from open market in Abeokuta, Ogun State, Nigeria. Samples were carefully cleaned by removing pebbles and other debris materials. Samples of T. occidentalis leaves and rhizomes of Z. officinale were washed separately using sterile de-ionized water. All were bulked and kept until needed for use.
Extraction Processes
Extraction of Hibiscus sabdariffa: About 50g of well dried calyces of H. sabdariffa were measured out and soaked in 200 ml of water for a period of 24 hours to ensure thorough extraction by the process of maceration in accordance with the method reported by Handa et al. [29]. The soaked sample was thoroughly rubbed between handpalms and sieved using muslin cloth, the filtrate was gathered in a 500 ml beaker and the shaft (residue) was discarded.
Extraction of Telfairia occidentalis: About 50g of fresh leaf sample of T. occidentalis was measured out. This was thoroughly crushed and squeezed to ensure efficient removal of the extract using 200 ml of water which was sieved using muslin cloth. The filterate was collected in 500ml beaker and the residue was discarded.
Extraction of Allium sativum and Zingiber officinale: About 50g of cleaned A. sativum and 50g of fresh cleaned sample of Z. officinale were measured separately and milled completely with 200ml of water with the use of blender.
Extract reconstitution: The extracts obtained was refrigerated and later reconstituted for in vitro analysis for egg and larva inhibitory assays. Different concentrations were prepared by dilution of the stock solution to achieve concentrations in percentages of 25, 50, 75 and 100 % in line with Safi et al. [30].
Collection Of Haemonchus Contortus and Infestation of Lambs
Haemonchus contortus was obtained from a popular Sheep and Goats Abattior, Akinyele, Oyo State in line with the technique reported by Sonibare et al. [31]. About twenty abomasa of goats which were heavily loaded with H. contortus infestation were identified and gathered from the processing slab and Haemonchus were pulled for from abomasa contents. The abamasa worms collected were identified in accordance to the techniques postulated by Zajac et al. [32]. The Haemonchus obtained were carefully washed in a nutritive medium prepared in line with the procedure of Hubert and Kerbeouf [33] and Dryden et al. [34]. The medium contained 5 μl of Amphotericine B which was used to prevent the growth of fungi that might attempt to feed on the eggs of the worms; 10 mg yeast was added to provide nutrition for the hatched larvae and two drops of Epsom salt solution was also added for electrolyte maintenance.
Two lambs (9 months old) with average weight of about 7.5 + 0.22 kg mean were obtained from Small Ruminant Unit, Directorate of University Farms, Federal University of Agriculture, Abeokuta, Ogun State. These lambs were orally treated for worm infestation using albendazole at 10ml per 10 kg body weight. About two hundred to two hundred and fifty (200-250) H. contortus each were transferred to the abomasa of the lambs through a left sided celiotomy. After the operation, the lambs were housed in secluded pens. The animals were fed with grasses that were cut during sunny period to avoid contamination of worms inoculated.
Egg Hatch Inhibitory Assay (EHIA)
The procedures used by Coles et al. [35] with adjustments was adopted to perform egg hatching inhibitory assay, the research development on determination of egg hatch assessment in response to anthelmintic resistance measure is of vital importance in combatting worm activities.
Fresh parasite eggs were obtained from donor lambs that have been previously infected with Haemonchus contortus. Faecal samples collected were ground with pestle and mortar, which was washed repeatedly with saline solution to obtain eggs suspension. The eggs suspension obtained was distributed using micro pipette into 60 microtitre multiwell plates with approximately 100 eggs in 200 μl of the egg. In the test wells, each herb extracts in percentage concentrations of 25, 50, 75 and 100 % were used and albendanzole was used as a positive control at 10 mg/ml which were replicated thrice. The prepared plates were nurtured in a cool and humid surrounding at about 27 oC temperature for 2 days after which a drip of Lugol’s iodine solution was put to each well to prevent further process of hatching, and all the unhatched eggs and L1 larvae in each well were counted modified MacMaster technique described by Fakae et al. [36]. The eggs rise to the top, so that they were all in focus against the upper slide when mounted under the light microscope. Every egg found within the squares on each of the chambers were counted at ×10 objective. The number of eggs within the two chambers multiplied by 50, represents the number per gram in the original sample. The number of eggs per gram (epg) was expressed as follows:
EPG = N × 50, where N = Total number of eggs counted in the two chambers.
The percent inhibition of egg hatching was calculated as follows:
Percentage inhibition = 100 (1- Ptest /Pcontrol) according to Coles et al. [35],
where: Ptest is the number of eggs hatched and P control is the respective numbers in water control.
Larva Development Inhibitory Assay (LDIA)
Larva development inhibitory assay was carried out by the method suggested by Ademola et al. [37]. The test was carried out using 180 pieces of 5 ml test tubes. Nourishing solution (200 μl) was put to 200 μl eggs suspension containing approximately 100 eggs. The tube was covered and put in an incubator at 27°C for 48 hours for first-stage larva formation. The extracts were added at different concentrations of 25, 50, 75 and 100 % at different volumes of 150, 300 and 450 μl and albendazole at 10 mg/ml concentration was used as positive control. Each treatment was replicated thrice. On the 7th day, the worms (third stage larva) were counted by separating the larvae into two groups; living (L3) and dead larvae. The percentage larva inhibition was calculated as follows according to Coles et al. [35]:
Larva inhibition (%) = 100 (1- Ptest/Pcontrol)
where: Ptest is the number of larvae form (L3) or the number of hatched larvae that developed into infective larvae (L3) in test ingredients, and Pcontrol is the respective numbers in water control.
Results
Influence of Different Plant Aqueous Extracts on Haemonchus Contortus Egg Inhibition Assay
The result of egg inhibition assay is presented in Table 1. Amongst the extracts of four (4) plant types tested in this study. Similar egg inhibitory trend was obtained for T. occidentalis and H. sabdariffa. Highest (P<0.05) value (100.00 ± 0.00 %) was obtained for T. occidentalis extract at 100 % concentration, followed by similar inhibitory efficacy for albendazole, and 50 and 75 % of the T. occidentalis with the least (55.56 ±12.83 %) obtained at 25 % concentration of the extract. H. sabdariffa had the highest percentage inhibitory (100.00± 0.00 %) at 100 % concentration of the extract while the least percentage inhibition (72.22± 3.20 %) was obtained at 25 % concentration of the extract. A. sativum extract had the highest similar percentage inhibition values (100.00±0.00 %) at 50, 70, 100 and albendazole (94.44 ± 3.21 %) except at 25 % concentration which gave the least value (88.88 %). Z. officinale had the highest percentage inhibition (88.89 ± 6.41 and 83.33 ± 3.20 %) at 100 % concentrations of the extract and albendazole, respectively while, the least (71.11 ± 9.62 %) was obtained at 25 % concentration.
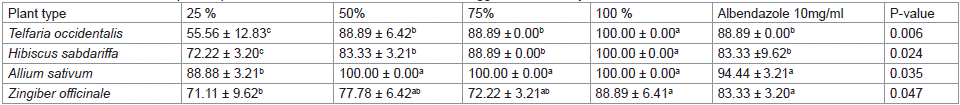
Table 1: Influence of different plant aqueous extracts on Haemonchus contortus egg inhibition assay.
Effect of Different Plant Extracts at Concentration of 150 μl on Larvae Development Inhibitory Assay of Haemonchus Contortus
The influence of different plant extracts at 150 μl on Haemonchus contortus larvae inhibitory assay is presented on Table 2. The values obtained for various plant types were significant (P<0.05) except for Zingiber officinale. Value (88.89 ± 0.00 %) obtained for Telfairia occidentalis group was highest in control (albendazole), followed by 76.00 ± 0.00 % obtained at 100 % concentration of Telfairia occidentalis, while least similar values were obtained for other concentrations. Percentage inhibition of larvae development assay increased with increasing percentage extract of Hibiscus sabdariffa with the highest value observed in groups administered 100% concentration (100.00 ±0.00) but similar to albendazole (94.00 ± 3.11 %) while least similar values (63.33 ±3.11, 64.36 ±3.12 and 71.22 ±4.20 %) were obtained for 50, 25 and 75 % concentration, respectively. Highest similar inhibition percentages (100.00 ± 0.00, 100.00 ± 0.00 and 91.06 ±6.23 %) were obtained in Allium sativum for 100 and 75 % concentration of the extract, and albendazole, respectively.
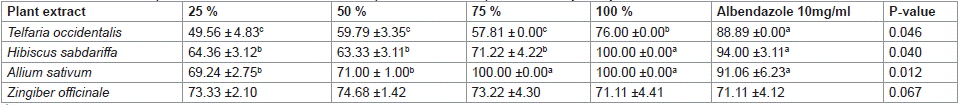
Table 2: Effect of different plant extracts at concentration of 150 μl on larvae development inhibitory assay of Haemonchus contortus.
Effect of Different Plant Extracts at Concentration of 300 μl on Larvae Development Inhibitory Assay of Haemonchus Contortus
The impact of different plant extracts at 300 μl on Haemonchus contortus larvae inhibitory assay is presented in Table 3. The results obtained for different plant extracts at different concentrations were significant (P<0.05) on inhibition of H. contortus larvae development. Extracts of Telfaria occidentalis gave highest similar values (84.89 ±0.00, 81.00 ±0.00 and 79.89 ± 0.00%) for albendazole and, 100 and 75 % of its extract, respectively. The results obtained for Hibiscus sabdariffa followed similar trend with that of T. occidentalis. Similar highest inhibitory values were obtained in A. sativum at different concentrations and albendazole, except at the 25 % concentration which had the least value (66.44 ±3.15%). Zingiber officinale had the highest inhibitory value (81.23 ±4.41%) at 100 % concentration the extracts whereas the least similar values were obtained for other concentrations and those administered albendazole.
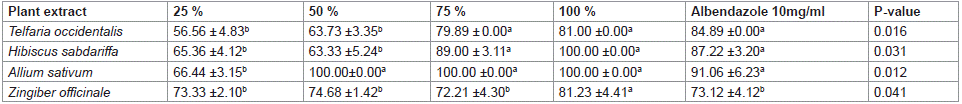
Table 3: Effect of different plant extracts at concentration of 300 μl on larvae development inhibitory assay of Haemonchus contortus.
Effect of Different Plant Extracts at Concentration of 450 μl on Larvae Development Inhibitory Assay of Haemonchus Contortus
Table 4 shows effect of different plant extracts at concentration of 450 μl on larvae development inhibitory assay of Haemonchus contortus. All the results obtained for different plant extracts at different concentrations were significantly (P<0.05) influenced by different extracts administered except for Zingiber officinale.
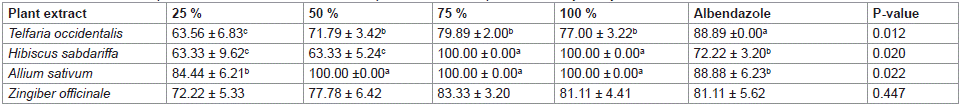
Table 4: Effect of different plant extracts at concentration of 450 μl on larvae development inhibitory assay of Haemonchus contortus.
Highest value (88.89 ±0.00) was obtained for albendazole while the least value (63.56 ± 6.83) was obtained at 25 % for Telfaria occidentalis. Hibiscus sabdariffa gave the highest similar larvae development inhibitory values (100.00 ± 0.00, 100.00 ± 0.00 and 88.88 ± 6.23 %) at 100, 75 % concentrations of the extract and albendazole, respectively. Meanwhile, least similar (P>0.05) values (63.33 ±5.24 and 63.33 ±9.62) were obtained at 25 and 50 % concentrations of the H. sabdariffa extract, respectively. Allium sativum had the highest inhibition rate (100.00 ± 0.00) at 50, 75 and 100 % concentrations whereas the least similar (P>0.05) values (88.88 ±6.23 and 84.44 ±6.21) were obtained for albendazole and 25% concentration of the extract, respectively.
Discussion
The efficacy of plant extracts in worm control could not be overemphasized as it provides a perfect alternative to the use of conventional anthelmintics which has been reported to have residual impacts on animals as well as the consumers [1]. Exploring anthelmintic potential of plant extracts could be promising, affordable and available without any deleterious effects on animals [15,38] which may be associated to the available phytochemicals and other bioactive compounds present in these plants such alkaloids, tannins, phenols and many others [39-41].
The least influence of Telfaria occidentalis on H. contortus egg inhibitory assay at lower concentrations might be due to relatively low level of phytochemicals such as alkaloids, phenol, and tannins in it which agrees with the work of Yusuf et al. [22] who reported that plants with low levels of the aforementioned phytochemicals had reduced impact in combating worms. The highest egg inhibitory value obtained at highest concentration of T. occidentalis corroborates Kushi et al. [18] who observed that T. occidentalis possesses phytochemicals capable of working independently and/or collectively to prevent proliferation of micro-organisms. Complete eggs destruction observed at higher concentration might be due to hypertonic effect of T. occidentalis on H. contortus eggs.
Hibiscus sabdariffa can be said to have worked by the principle of agglutinin through the observed clumps formed round the eggs which prevent further metabolic activities, this could be related to the work of Khalid and Mohan [26] who observed extract of Hibiscus sabdariffa to paralyze Lumbricus terrestris through similar procedure. Best egg inhibition obtained at higher concentration could imply better clump formation at such higher concentration which gave superior performance over the conventional albendazole. Khalid and Mohan [26] also reported better efficiency of aqueous extract of H. Sabdariffa at higher concentration (50mg/ml) which is in consonance with this present study. Many sulphur-rich compounds found in biochemical screening of Allium sativum could be associated to best significant reduction of H. contortus. This phenol, in conjunction with other bioactive compounds present such as 2,5-Furandicarboxaldehyde, 2,5-Furandione, 3-methyl-, 1H-Imidazole, 2(5H)-Furanone and (E)- 2,5-Furandione, 3-methyl-, and 1-(3,3-dimethyloxiranyl) Homopiperazine might be responsible for the restriction in growth and survival of the eggs due to their antioxidant, anthelminthic and antimicrobial properties by the principle of dehydration [17,19,42]. Outcome of this research was also similar to the report of Gaafar [43] in which garlic completely eradicate oocyctes of Cryptospridium (a protozoan parasite) in mice. Indiscriminate and misapplication of chemotherapeutic procedures had resulted in building of resistance by pathogen and/or parasite with residual effects on animals [1,44] which had occasioned in placing ban on conventional drugs [7,9]. The results obtained from this study predicted appropriate volume and concentration needed in application of the selected plant extracts in treating H. contortus at larvae stage as opined by Ikyume et al. [45]. Findings of this study revealed that at lower volume (150 μl), best larva growth restriction could only be achieved at higher concentration and this followed similar pattern for all the extracts. This corroborates the study conducted by Zenebe et al. [46] who reported less effectiveness at lower volume in an in vitro study conducted on H. contortus adulticidal inhibitiory assay using Cissus quadrangularis and Schinus molle. The current study revealed that Hibiscus sabdariffa and Allium sativum could favourably compete with conventional albendazole in an in vitro assay.
The increased H. contortus eggs inhibition reduction as A. sativum concentration increased, might be due to the increase in phytochemicals such as alkaloid and phenol being most abundant in it. It could also be due to the presence of bioactive compounds such as furan derivaties: (2,5-Furandicarboxaldehyde, 2,5-Furandione, 3-methyl-, 1H-Imidazole, 2(5H)-Furanone and (E)- 2,5-Furandione, 3-methyl-, and 1-(3,3-dimethyloxiranyl) and Homopiperazine (used in synthesis of conventional anthelminthic) identified to possess the ability to destroy the cuticle (outer layer) of the eggs and thereby prevent hatching and further growth into larvae [21]. At highest volume of different plant types used, superior performance was observed which might be attributed to the increase in the volume of the secondary plant metabolites present to arrest or destroy the worm and also supports the report of Alawa et al. and Molan et al. [47,48] who opined that plant extracts gave promising outcomes in treatment of Haemonchus with lowered motility, destruction and death of parasites up to 100 %. The outcome of this study at 300 μl volume is closely related to the results obtained at 450 μl. This indicated that extending the volume used to this extent might result to the wastage of the extract since similar outcome is obtained.
Conclusion
The following conclusions could be drawn from this study: All the selected plant types considered in this study (Allium sativum, Hibiscus sabdariffa, Telfaria occidentalis and Zingiber officinale) possess the potential to control the growth and proliferation of Haemonchus contortus at both egg and larva stages under in vitro study. Allium sativum and Zingiber officinale at 50-100 % concentration proved to be effective and compete favourably with conventional albendazole on Haemonchus contortus egg inhibition assay. Also, Allium sativum and Hibiscus sabdariffa at 150 μl concentration on H. contortus larvae development inhibitory assay were observed to be at par with conventional albendazole at 75 % concentration. Meanwhile, at 300 and 450 μl volume of different plant extracts on H. contortus larvae development inhibitory assay, A. sativum was most efficient from 50- 100 % concentration and observed to work perfectly like albendazole. Thus, the anthelmintic efficacy displayed by different plant types used could be traced to the abundance of the phytochemicals present most especially tannin and its derivatives.
References
- Kaplan RM and Vidyashankar AN. An inconvenient truth: Global worming and anthelmintic resistance. Veterinary Parasitology. 2012; 186: 70–78.
- Arsenopoulos KV, Fthenakis GC, Katsarou EI. and Papadpoulos E. Haemonchosis: A challenging parasitic infection of sheep and goats. Animals. 2021; 11: 363.
- Ayankoso MT. Influence of varying protein-energy levels in the population of helminths in naturally infected West African Dwarf sheep. Nigerian Journal of Animal Science and Technology. 2022; 5: 22-28.
- Ayankoso MT, Sowande OS, Iposu SO, Sonibare OA, Oni AO, Sowande OK, et al. Effects of diet containing different protein-energy densities on the performance of semi-intensively raised West African Dwarf (WAD) sheep. Journal of Applied Agricultural Research. 2021; 4: 53-59.
- Roeber F, Jex AR and Gasser RB. Impact of gastrointestinal parasitic nematodes of sheep, and the role of advanced molecular tools for exploring epidemology and drug resistance -Australian perspective. Parasite Vectors. 2013; 6: 153.
- Fajimi K, Taiwo AA, Ayodeji I0, Adebowale EA and Ogundola FI. A therapeutic on gastro- intestinal helminth parasites of goats using pawpaw seeds as a drench. Proceedings of the international conference on sustainable crop and Livestock production for improved livelihoods and natural resources management, West African held at the International Livestock Research Institute (ILRI) in partnership with International Institute of Tropical Agriculture, between November. 2021; 9-23: 485-491.
- Saddiqi HA, Jabbar A, Sarwar M and Iqbal Z. Small ruminant resistance against gastrointestinal nematodes: A case study of Haemonchus contortus. Parasitology Research. 2011; 109: 1483-1500.
- Torres-Fajardo RA, Gonzalez-Pech PG, Sandoval-Castro CA and Torres- Acosta JF. Small ruminant production based on rangeland to optimize animal nutrition and health: Building an interdisciplinary approach to evaluate nutraceutical plants. MDPI Journal. 2020; 10: 1799.
- Prichard RK. Anthelmintic resistance. Veterinary Parastolog. 1994; 54: 259- 268.
- Haile A, Amindo OD, Tembely S, Mukasa-Mugerwa E, Tibbo M, Yami A, Baker LR and Rege JIO. Effects of dietary protein supplementation and infection with gastrointestinal nematode parasites on some nutrition and metabolic parameters in Ethopian Menz and Horro sheep. Livestock Production Sciences. 2004; 91: 183 – 195.
- Waller PJ. Changes in anthelmintic resistance status of Haemonchus contortus and Trichostrongylus colubriformis exposed to different anthelmintic selection pressures in grazing sheep. International Journal of Parasitology. 2006; 19: 99-110.
- Sonibare AO, Sowande OS, Iposu SO, Luka J, Ayankoso M and Egbetade AO. Performance and Parasitology of Semi-Intensively Managed West African Dwarf Sheep Exposed to Gastrointestinal Helminth Infected Padocks and Varied Protein-Energy Feeds. Iran Journal Parasitology. 2016; 11: 550- 567.
- Kearney PE, Murray PJ, Hoy JM, Hohenhaus M and Kotze A. The ‘toolbox’ of stragegies for managing Haemonchus contortus in goats: What’s in and what’s out. Veterinary parasitology. 2016; 220: 93-107.
- Butter NL, Dawson JM, Walklin D and Buttery PJ. Effects of dietary condensed tannins on gastrointestinal nematodes. Journal of Agricultural Science. 2000; 137: 461-469.
- Singh E, Sharma S, Dwivedi J and Sharma S. Diversified potentials of Ocimum sanctum Linn exhaustive survey. Journal of National Production of Plant Resource. 2012; 2: 39-48.
- Builder PF, Ezeobi CR, Tanfa FD, Buildes MI. Assessment of the intrinsic and stability properties of the freeze-dried and formulated extract of Hibiscus sabdariffa Linn. (Malvaceae). African Journal Pharmarceutical and Pharmacology. 2010; 4: 304-313.
- Kolapo AL, Popoola TOS, Sanni MO and Afolabi RO. Preservation of soybean daddawa condiment with dichloromethane extract of ginger. Research Journal of Microbiology. 2007; 6: 13-18.
- Kushi LH, Byers T and Doyle C. American Cancer Society Guidelines on Nutrition and Physical Activity for Cancer Prevention, Reducing the risk of Cancer with Healthy Food Choice and Physical Activity. CA Cancer Journal Clinical. 2006; 56: 254-281.
- Shang AO, Shi-Yu CAO, Xiao-Yu XU, Ren-You GAN, Guo-Yi T, Harold C, Mavumengwana V And Hua-Bin LI. Bioactive compounds and biological functions of garlic (Allium sativum L). Foods. 2019; 8: 246.
- Njoku UO, Umeh CG and Ogugofor MO. Phytochemical profiling and GCMS analysis of aqueous methanol fraction of Hibiscus asper leaves. Future Journal of Pharmaceutical Sciences. 2021; 7: 59.
- Hoste H, De-Montellano CM, Manolaraki F, Brunet S, Ojedarobertos N, Fourquaux I, et al. Direct and indirect effect of bioactive tannin- rich tropical and temperate legume against nematode infection. Veterinary Parasitology. 2012; 186: 18-27.
- Yusuf AO, Surajudeen MA, BT. Common Plant Bioactive Components Adopted in Combating Gastrointestinal Nematodes in Small Ruminant – A Review. Agricultura Scientia. 2023; 20: 61-73.
- Athanasiadou S, Kyriazakis I, Jackson F and Coop RL. Direct anthelmintic effects of condensed tannins towards different gastrointestinal nematodes of sheep: In vitro and in vivo Studies. Veterinary Parasitology. 2001; 3: 205-219.
- Molan AL and Faraj AM. The effects of condensed tannins extracted from different plants species on egg hatching and larva development of Teldorsagia circumcinta (Nematoda: Trichostrongylidae). Folia Parasitology. 2010; 57: 62-68.
- Engstrom MT, Karonen M, Ahern JR, Baert N, Payre B, Hoste H, etal. Chemical structures of plants hydrolysable tannins reveal their in vitro activity against egg hatching and motility of Haemonchus contortus nematodes. Journal of Agricultural and Food Chemistry. 2016; 64: 840-851.
- Khalid M and Mohan A. Antihelmintic Activity of water extracts of Hibiscus Sabdariffa using Lumbricus terrestris as a test worm. American Journal of Life Science Researches. 2021; 7: 12-16.
- Google Earth Map. 2024.
- Accuweather. 2024.
- Handa SS, Khanuja SPS, Longo G and Rakesh DD. Extraction Technologies for Medicinal and Aromatic Plants, (1st edition), no. 66. Italy: United Nations Industrial Development Organization and the International Centre for Science and High Technology. 2008; 130-147
- Safi AR, Khattak B, Hussain M and Attaullah M. Biological control of Haemonchus contortus by fungal antagonists in small ruminants. Applied Ecology and Environmental Research. 2018; 16: 5825-5835.
- Sonibare AO, Kumshe HA, Okewole AO, Talabi AO. Survival and infectivity studies of in vitro cultivated larvae of H. contortus in sheep and goat in Nigeria. Journal of Veterinary World. 2011; 4: 347-359.
- Zajac AM and Conboy GA. Veterinary Clinical Parasitology, 8th Edition, Wiley Blackwell, USA. 2012.
- Hubert J and Kerboeuf D. A microlarval development Assay for the detection of anthelmintic resistance in sheep namatode. Veterinary Records. 1992; 130: 442-446.
- Dryden MW, Payne PA, Ridley R and Smith V. Comparion of common faecal floation techniques for the recovery of parasite eggs and oocystes. Veterinary Therapeutics. 2005; 6: 15-28.
- Coles GC, Bauer C, Borgsteede FHM, Geerts S, Klei TR. Taylor MA, et al. World associstion for the advancement of veterinary methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Veterinary Parasitology. 1992; 44: 35-44.
- Fakae BB, Harrison LJ, Ross CA and Sewell MM. Heligmosomoides polygyrus and Trypanosoma congolense infections in mice: A laboratory model for concurrent gastrointestinal nematode and trypanosome infections. Parasitology. 1994; 108: 61–68.
- Ademola IO, Akanbi AI and Idowu SO. Comparative nematicidal activity of chromatographic fraction of Leucaena leucocephala seed against gastrointestinal sheep nematodes. Pharmaceutical Biology. 2005; 43: 599- 604.
- Singh R and Singh K. Zingiber officinale: A spice with multipl roles. Research Journal of Life Scicences, Bioinformatics, Pharmaceutical and Chemical Sciences. 2019; 5: 113-125.
- Haruna AH, Mairo M. Phytochemical analysis of Telfairia occidentalis and Occimum gratissimum samples collected from Gwarimpa Abuja, Nigeria. Journal of Diseases and Medicinal Plants. 2019; 5: 17-21.
- Nazir I and Chauhan SK. Qualitative phytochemical analysis of Allium sativum (Garlic) and Curcuma longa (Tumeric). Journal of Entomolgy and Zoology Studies. 2019; 7: 545-547.
- Muhammad A and Idris SA. Phytochemicals screening and proximate analysis of garlic (Allium sativum). Archives of organic and inorganic chemical sciences. 2019; 4: 1.
- Prohp TP and Onoagbe IO. Acute toxicity and dose response studies of aqueous and ethanol extracts of triplochton scleroxylon, K. Schum (sterculiaceae). International Jouirnal of Apllied Biology and Pharmaceutical Technology. 2012; 3: 400-409.
- Gaafar MR. Efficacy of Allium sativum (Garlic) against Experimental Cryptosporidiosis. Alexandria Journal of Medicine. 2012; 48: 59-66.
- Prichard R. Drug resistance in nematodes. In Antimicrobial Drug Resistance: Mechanisms of Drug Resistance; Mc Gill University: Sainte-Anne-de- Bellevue, QC, Canada. 2009; 621–628.
- Ikyume TT, Sowande OS, Dele PA, Yusuf AO, Monday S, Egunjobi OK, et al. Effect of varying levels of garlic (Allium sativum) powder on growth, apparent nutrient digestibility, rumen ecology, blood profile and cost analysis of feeding West African Dwarf goats. Malaysian Society of Animal Production. 2017; 20: 61-74.
- Zenebe S, Feyera T and Assefa S. In vitro anthelmintic of crude extract of aerial part of Cissus quadrangularis L. amd leaves of Schimus molle L. against Haemonchus contortus. BioMed Research International. 2017; 17: 1-6.
- Alawa CBI, Adamu AM, Gefu OJ, Ajanusi JO, Abdu PA and Chiezey NP. In vivo efficacy of vernonia amygdalina (Compositae) against natural helminth infection in Bunaji (Bos indicus) calves. Pakistan Veterinary Journal. 2010; 30: 215-218.
- Molan AL, Alexander R, Bbookes IM and Mcnabb WC. Effects of Sulla condensed tannins on the degradation of ribulose-1,5bisphosphate carboxylase/oxygenase (rubisco) and on the viability of three sheep gastrointestinal nematodes in vitro. Journal of Animal Veterinary Advances. 2003; 3: 165-174.