
Review Article
Austin J Vet Sci & Anim Husb. 2024; 11(6): 1159.
Review on the Epidemiology, Diagnosis and Control Methods of Peste Des Petits Ruminants
Yalew Abiyu*
Ethiopian Institute of Agricultural Research, Pawe Agricultural Research Center, Ethiopia
*Corresponding author: Yalew Abiyu, Ethiopian Institute of Agricultural Research, Pawe Agricultural Research Center, P.O. Box 25, Pawe, Ethiopia. Email: yalew6vet@gmail.com
Received: July 29, 2024; Accepted: December 12, 2024 Published: December 19, 2024
Summary
Peste des Petits Ruminants (PPR) is a highly contagious and economically significant disease that affects sheep and goats. It is caused by the Morbilli virus, which belongs to the paramyxoviridae family. Initially reported in Cote d’Ivoire, PPR is currently prevalent in various regions across Africa, the Middle East, and Asia. Clinical symptoms of the disease include fever, conjunctivitis, nasal and ocular discharges, erosive stomatitis, diarrhea, and bronchopneumonia. The severity of PPR varies depending on the species, breed, and age of the animals. Factors such as poor nutrition, overcrowding, and concurrent infections can exacerbate the intensity of clinical manifestations. Animals susceptible to PPR can contract the disease through inhalation of droplets from infected animals, contact with contaminated objects, or ingestion of tainted food or water. Diagnosis of Peste des Petits Ruminants can be achieved through various methods. Isolating the virus and conducting virus neutralization tests are considered the gold standard for identifying the PPRV and detecting antibodies in serum, respectively. Prevention and control strategies for PPR involve managing animal movements, euthanasia, quarantine, and thorough cleaning and disinfection of infected areas. However, challenges such as illegal animal movements within and between countries, limited awareness of the disease’s epidemiology, and negligence in implementing control measures hinder efforts to contain the disease. The absence of a DIVA vaccine and the unclear role of wild animals in the epidemiology of PPR are also significant challenges. Therefore, implementing strict controls on animal movements, adhering to PPR control protocols, and developing heat-resistant DIVA vaccines are crucial steps in combating the disease. Indeed, clearly delineating the role of wild animals in the epidemiology of the disease is also necessary.
Keywords: Diagnosis; Control; Epidemiology; Goats; Morbilli virus; PPR; Sheep
Introduction
Sheep and goats are widely distributed ruminant animals that play a significant role in the rural economies of developing countries. They serve as a primary source of income for farmers, particularly during periods of drought and famine [42]. Due to their low production costs, high reproductive rates, and rapid growth, small ruminants are valuable assets for impoverished farmers, utilized for various purposes [23]. However, the production, productivity, and benefits derived by producers fall below anticipated levels due to various factors, including Peste des Petits Ruminants (PPR) disease, which impacts the production and productivity of small ruminants across a wide range of agro-climatic regions throughout the world [42].
Peste des petits ruminants is a highly contagious and economically significant transboundary viral disease affecting sheep and goats, characterized by high rates of morbidity and mortality. It is caused by the Peste des Petits Ruminants Virus (PPRV), which belongs to the genus Morbillivirus [38]. The disease manifests with clinical signs such as fever, conjunctivitis, oculo-nasal discharge, stomatitis, diarrhea, and bronchopneumonia. While a preliminary diagnosis can be made based on clinical signs and post-mortem examination, confirmation requires isolation through culture, as well as serological and molecular techniques [9].
A Peste des petits ruminants is transmitted through direct contact with infected animals introduced to a herd. Asymptomatic carriers can shed the virus for up to 12 weeks or even longer in recovered animals. Therefore, quarantine and testing are essential to reduce the risk of infection. Peste des petits ruminants is one of the most economically significant diseases affecting small ruminants, as it decreases animal productivity due to high morbidity and mortality rates. Mortality rates are generally lower in endemic areas and can vary between different species of infected animals [24]. The disease can be controlled and eradicated through measures such as quarantine, movement restrictions, euthanasia, disinfection of contaminated premises, and vaccination [12]. However, effective control and eradication require a thorough understanding of the disease's epidemiology and diagnostic methods. Hence, the objective of this review paper is to provide insights into the epidemiology, diagnostic techniques and control methods of peste des petits ruminants.
Insights into the Epidemiology of Peste des Petits Ruminats
Peste des Petits Ruminants
Peste des petits ruminants is a highly contagious disease affecting small ruminants that was initially mistaken for rinderpest due to similarities in their clinical signs. However, rinderpest was gradually ruled out when it was observed that it could not infect cattle exposed to infected small ruminants [41]. The severity of PPR can be classified as peracute, acute, subacute, or subclinical, depending on various predisposing factors and the virulence of the virus. The acute form of the disease is characterized by symptoms such as depression, high fever, anorexia, oculo-nasal discharge, erosive stomatitis, pneumonia, and severe diarrhea [28,39].
Etiology
Peste des petits ruminants is caused by PPRV of the family Paramyxoviridae, subfamily Orthoparamyxovirinae and genus Morbilli virus [16]. The virus shares close antigenic relationships with rinderpest virus, canine distemper virus, measles virus, phocid distemper virus, and dolphin distemper virus [45]. Peste des petits ruminants virus is an enveloped, pleomorphic virus containing a nonsegmented single-stranded RNA genome. The genome of PPRV is the longest among the morbilli viruses, consisting of 15,948 nucleotides that encode two non-structural proteins (V and C proteins) and six structural proteins: nucleoprotein (N), phosphoprotein (P), matrix protein (M), fusion protein (F), hemagglutinin protein (H), and large polymerase protein (L). These proteins are arranged in the order of 3'-N-P(C/V)-M-F-H-L-5' within the viral genome [16,39] (Figure 1).
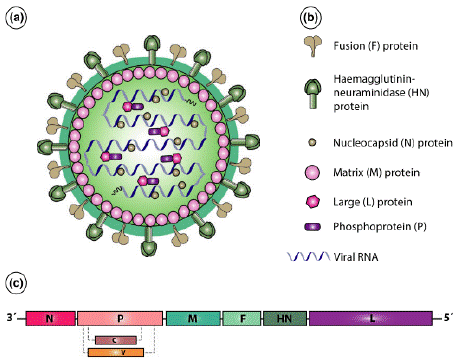
Figure 1: Structure and genome organization of the PPR virus. (a) Schematic
structure, (b) Structural components, and (c) Genome organization of PPRV
[29].
Lineage of the PPR Virus
Peste des petits ruminants virus has a single serotype and is divided into four lineages (lineage I, II, III, IV) classified based on phylogenetic analysis of the virus genes. Lineage I is commonly found in West Africa and has recently been identified in Central Africa. Lineage II isolates are prevalent in Western African countries, while Lineage III is reported in Eastern Africa and parts of the Middle East, including Arabian countries like Oman and Yemen. Lineage IV isolates are also present in the Arabian Peninsula, the Middle East, and South Asian countries [40]. The virulence of the PPR virus varies across these lineages. Studies on West African goats have shown that Lineage I cause per-acute to acute disease, Lineage II leads to mild to in apparent disease, Lineage III results in acute to mild infection, and Lineage IV causes acute PPR disease. Due to its mild nature, Lineage II is utilized in the initial attenuation process through multiple passages on Vero cells to produce the current PPR vaccine [11].
Origin and Geographic Distribution of PPR
The disease was first reported in Côte d'Ivoire, West Africa, in 1942 by Gargadennec and Lalanne. Currently, it is spreading across Sub-Saharan Africa, Morocco, the Arabian Peninsula, the Middle East, Turkey, Iran, Iraq, Pakistan, India, Bangladesh, Nepal, Tajikistan, Kazakhstan, Tibet, and China. With the notable exception of most southern African countries, including South Africa, Botswana, Namibia, Zimbabwe, Mozambique, and Malawi. Peste des petits ruminants is now recognized as endemic throughout Africa, the Middle East, and Central, East, and South Asia [13]. Approximately 59 countries, including Namibia and South Africa, have been declared free of the disease, with Namibia being the only country to hold zonal PPR-free status [33].
Host Range and Reservoirs
Peste des petits ruminants primarily impacts sheep and goats, with goats being more susceptible than sheep. Both goats and sheep exhibit typical clinical signs of the disease and can transmit the infection after being exposed to the PPRV, making them recognized reservoir hosts [46]. Cattle, pigs, buffalo, and camels are also susceptible to PPR, often experiencing subclinical infections. However, these animals do not contribute significantly to the epidemiology of the disease, as they are unable to shed the virus [27,34]. Additionally, PPR can infect wild ruminants, including wild goats, ibex, blue sheep, gazelles, springboks, saigas, buffalo, bushbucks, nilgai, kobs, waterbucks, oryx, duikers, hartebeests, and impalas. The severity of the disease in wild animals ranges from asymptomatic cases to those exhibiting severe clinical signs [36].
Host Determinants and Other Risk Factors
The morbidity of PPR can be influenced by various host factors including age, sex, breed, and species of the animals. Young sheep and goats are more vulnerable to PPRV infection due to their lower likelihood of having developed protective antibody titers [14]. Small ruminants aged between 3 to 18 months are more severely impacted compared to adults or unweaned young animals. Kids aged over four months and less than one year are particularly susceptible to the disease, with female small ruminants also showing a higher susceptibility. Goats are more severely affected than sheep, while the disease is non-pathogenic to cattle and African buffaloes [7]. Sahelian breeds of sheep and goats are considered more resistant compared to dwarf breeds in the humid and sub-humid zones of West Africa [34].
Various risk factors such as mixed population flocks, introduction of new animals, return of unsold animals from markets, livestock trade, nomadic herding, and aggregation of susceptible animals play a significant role in the disease's spread. Stress factors like changes in diet, habitat, nutritional deficiencies, and intensification of farming practices also increase the risk of infection [7]. Disease outbreaks are typically minimized during the rainy season due to reduced animal movement as fodder availability improves. The increase in fodder availability during this time contributes to an enhancement in the animals' immune status [4].
Disease Pattern and Seasonal Occurrences
Significant variations exist in the epidemiological patterns of PPR across diverse agro-ecological systems and geographical regions. In regions characterized by high humidity, where the disease presents in an epizootic form, elevated morbidity and mortality rates are documented. Conversely, in arid and semi-arid regions, PPR tends to be nonfatal and typically manifests as subclinical infections [3]. Endemic regions display a seasonal trend with a higher frequency of outbreaks occurring at the onset of the cooler wet season [36].
The seasonal epidemiological patterns of the disease vary across different agro-ecological systems, influenced by the cultural and livelihood practices of smallholder farmers [10]. In regions with subtropical climates, PPR disease tends to be more prevalent during the winter and rainy seasons. The confinement and limited movement of animals in tropical countries during the rainy seasons can impact the animals' nutritional status, potentially increasing their susceptibility to PPRV infection [4].
Transmission of the Disease
Transmission of PPRV can occur through direct contact with infected animals, inhalation of aerosols, or contact with secretions from infected animals (such as ocular discharge, nasal discharge, saliva, and feces). The virus can be shed during subclinical cases or incubation periods [7]. Intra-herd transmission of PPRV happens between closely interacting animals, while inter-herd transmission occurs through direct contact in communal pastures, watering areas, and live animal markets [36]. Asymptomatically infected animals can shed the virus for up to 12 weeks, with shedding potentially lasting longer in recovered animals [12]. The shed virus can contaminate water, feed troughs, and bedding, serving as additional routes of transmission. Fortunately, the virus does not survive for extended periods outside the host, so most transmission occurs through contact during the febrile stage of the disease [4].
Morbidity and Mortality Rates
Morbidity and mortality in PPR disease can vary depending on the disease stage and the species and age of the affected animals. In severe cases, morbidity and mortality rates can reach as high as 100% and 90%, respectively [17]. Morbidity tends to be higher in goats compared to sheep, and small ruminants aged between 3 months and 2 years are more severely impacted than younger or older animals. In susceptible goat populations, mortality rates of 50-100% can also be expected. In endemic regions, there is a constant circulation of low levels of infection, with periodic outbreaks occurring when a naive population is introduced or expands. Such periodic outbreaks often result in nearly 100% mortality in both sheep and goats. High mortality rates have also been documented among captive animals [41].
Pathogenesis of PPR Disease
The Peste des petits ruminants virus shows a strong preference for lymphoid and epithelial tissues in the respiratory and gastrointestinal tracts. Upon entering through the respiratory system, the virus replicates in the lymph nodes and tonsils [11]. It then progresses to the retropharyngeal mucosa, spreading to local lymphatic tissues for further replication, leading to the onset of primary viremia within 2 to 3 days. This viremia enables the virus to disseminate to other lymphoid tissues and organs such as the spleen, bone marrow, and mucosae of the gastrointestinal and respiratory tracts, where it causes severe damage by replicating in endothelial, epithelial and monocyte cells [4,35]. The destruction of the host lymphoid tissues can results in lymphopenia and significant immunosuppression, rendering the animals more susceptible to secondary opportunistic infections by compromising their immune defenses [7,47].
Clinical Signs
The incubation period of the PPR virus typically ranges between 3 and 10 days. During the acute phase of PPR, infected animals commonly display symptoms such as fever (reaching up to 41°C) lasting for 3 to 5 days, along with depression, loss of appetite, and dryness of the muzzle [47]. Additional signs may include increased salivation, watery nasal and lachrymal discharges that progress to mucopurulent discharges.
Erosive lesions can appear in the oral cavity, potentially becoming necrotic as the disease advances. Subsequently, affected animals may develop diarrhea, coughing, and labored abdominal breathing in the later stages of the infection. The illness typically lasts for about 14 days, culminating in either recovery from the infection or death during the acute phase [30].
Diagnostic Methods of PPR
Differential Diagnosis
Peste des petits ruminants is often mistaken for other diseases affecting sheep and goats, such as rinderpest, Foot-and-Mouth Disease (FMD), bluetongue, contagious ecthyma (Orf), pneumonic pasteurellosis, Contagious Caprine Pleuropneumonia (CCPP), and gastrointestinal helminth infestations [34,37,38]. Foot-and-Mouth Disease (FMD) can be differentiated by the absence of respiratory issues and diarrhea, with marked lameness being a prominent symptom. Foul-smelling exudates from the mouth, commonly seen in PPR, are typically absent in cases of FMD. Bluetongue disease manifests with head region edema and bluish discoloration of the oral cavity. Conversely, CCPP does not present with mouth lesions or diarrhea; postmortem examinations of CCPP-infected animals often reveal lung adhesions to the chest cavity and fibrin deposits in the lungs [34]. Uncomplicated cases of contagious ecthyma (Orf) usually do not exhibit pneumonia, diarrhea, or significant oral lesions. Similarly, pneumonic pasteurellosis-infected animals do not typically display diarrhea or oral lesions [11,39].
Isolation of the Virus
Samples used for the successful isolation of PPRV must be collected during the hyperthermic phase and transported on ice. Blood, ocular, and nasal swabs collected in the early stages of the disease are the preferred samples [37]. The primary cell used for culturing has been the marmoset-B-lymphoblastoid (B95a) cell. Vero cells have long been the preferred cells for isolating and propagating PPRV. Recently, transformed monkey cells expressing sheep/goat signaling lymphocytic activation molecules (SLAM or CD150) have shown increased sensitivity for isolating the virus [7]. In culture and isolation techniques, monolayer cell cultures are inoculated with buffy coat, swab materials, or 10% tissue suspensions, and are examined daily for signs of Cytopathic Effect (CPE). The CPE caused by PPRV can develop within five days of inoculation, characterized by cell rounding and aggregation leading to syncytia formation. After five to six days, blind passages should always be performed, as CPE may take additional time to manifest [31].
Antigen Detection Methods
The antigen of PPRV can be detected using various methods. Immune capture ELISA is a rapid, virus-specific test that differentiates between PPRV and Rinderpest Virus (RPV) [10]. The agar gel immunodiffusion (AGID) test is used to diagnose PPR from swab and tissue samples. Compared to AGID, Counter Immunoelectrophoresis (CIEP) is quicker and more sensitive. However, both tests are less sensitive during the early stages of infection and in cases of mild disease. Hemagglutination is employed to confirm Cytopathic Effects (CPE) during virus infectivity titrations [32,37] and is also used to differentiate PPRV from RPV infection [26]. Sandwich ELISA detects the virus antigen using monoclonal antibodies (MAbs) raised against the N protein gene. This method can distinguish between PPR and RPV. Dot-ELISA is another technique used to detect PPRV antigen using anti-M protein and anti-N MAbs. Additionally, Cell-ELISA serves as a vaccine quality control tool [37]. Immunohistochemistry is utilized for PPR diagnosis when fresh or frozen tissues are unavailable and is also useful for the retrospective examination of preserved specimens [35].
Antibody Detection Methods
Different diagnostic methods are available for detecting antibodies against PPRV. The competitive ELISA (c-ELISA) and Virus Neutralization Tests (VNT) are the most important methods for virus antibody detection [10]. Currently, c-ELISA is the most commonly used diagnostic technique, especially for samples that have not been kept under ideal conditions [11]. Virus neutralization test is considered the gold standard test prescribed for international trade [37]. Blocking ELISA (b-ELISA) has also proven to be a highly sensitive and specific method for PPR antibody detection [25]. The Precipitinogen Inhibition Test detects antibodies based on their ability to inhibit the development of a precipitin line against hyperimmune serum. The Hemagglutination Inhibition Test detects antibodies produced against PPRV by adsorbing cross-reacting antibodies to rinderpest antigen from PPR serum, thereby leaving the specific antibodies to the PPR antigen. Additionally, Counter Immunoelectrophoresis (CIEP) is highly adaptable for titrating serum antibodies and can be used in sero-epidemiological and experimental studies on PPR [26].
Genome Detection Methods
Various molecular methods targeting the F, N, M, and H genes have been developed for PPR diagnosis. Among these, Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) and complementary DNA (cDNA) hybridization techniques stand out as the most sensitive diagnostic approaches [2,9]. Reverse transcriptase polymerase chain reaction is a precise, rapid, and reliable test for detecting and quantifying specific DNA molecules. It boasts sensitivity of the result approximately 1000 times higher than traditional virus titration on Vero cells [26]. Real-time RT-PCR is an exceptionally sensitive test for diagnosing PPR, being ten times more sensitive than conventional RT-PCR [31]. Additionally, N gene-based radioisotope 32P labeled cDNA probes are utilized to detect and differentiate PPRV from RPV without the necessity for culture and virus isolation. However, their routine diagnostic use is not recommended due to the short half-life of 32P and the requirement for specialized equipment to safeguard users from the hazardous nature of the isotope [37].
Socio-Economic Importance of the Disease
Peste des petits ruminants is considered a significant constraint for sheep and goat production, leading to substantial economic losses due to its highly contagious nature. Countries have reported annual economic losses ranging from tens to hundreds of millions of dollars [33]. Specifically, it has been documented that PPR results in yearly losses of $1.5 million in Nigeria, $39 million in India, $15 million in Kenya, and at least $1.5 million in Iran [34]. Globally, PPR is estimated to cause economic losses in the range of $1.45 to $2.1 billion each year, attributed to reduced production, animal mortality, and the expenses associated with caring for sick animals, including vaccination efforts. Approximately half of these losses occur in Africa, with around a quarter of the total loss in South Asia [18,33]. In Ethiopia, an estimated economic loss of 652,595 birr during drought periods and 1,683,120 birr during non-drought periods, with a total loss of $43,478.3 reported solely from the deaths of sheep and goat populations totaling 3,905 heads in the study farms [22].
Prevention and Control
Peste des petits ruminants can be prevented, controlled, or eradicated through a combination of measures such as quarantine, movement control, euthanizing infected/exposed animals, vaccination, and cleaning and disinfection of infected premises. The rapid inactivation of PPRV in the environment is crucial for its control and eradication, as it typically remains viable for less than four days outside the host animal. Furthermore, PPRV can be effectively neutralized by various disinfectants such as sodium carbonate, sodium hydroxide, sodium hypochlorite, phenolic compounds, citric acid, alcohols, and iodophors [39].
In endemic areas, PPR is controlled through vaccination. Animals that recover from infection develop strong immunity, which can last for at least four years and potentially for life. To prevent infections in susceptible wildlife and captive wild animals like gazelles, they should be kept from contact with sheep and goats. Vaccination may also be feasible for these species [4,12].
Furthermore, carcasses of deceased animals and contact fomites should be either buried or burned. Barns, tools, and other items that have been in contact with sick animals must be disinfected. Vaccination should be administered before the onset of the rainy season and annually in endemic regions [10]. However, currently, there is no vaccine available with the ability to Differentiate Infected from Vaccinated Animals (DIVA), despite various trials that have been conducted [37].
Mass immunization is the most effective method to control Peste des Petits Ruminants (PPR). Highly efficient commercial vaccines, developed in the 1980s through the attenuation of the Nigeria 75/1 strain on Vero cells, offer lifelong immunity with a single dose. Despite its global use, this vaccine has low thermal stability, lasting 2-6 hours at 37°C post-reconstitution. To extend its preservation time, a cryoprotectant mixture containing trehalose is added, enabling storage for 5-14 days at 45°C in lyophilized form and for 21 hours at 37°C post-reconstitution. These thermal stabilizers facilitate vaccine transportation to remote regions without requiring a cold chain. Additionally, alternative heat-resistant PPR-recombinant pox virus vaccines have been developed [48].
Current PPRV attenuated vaccines are thermolabile, necessitating strict maintenance of the cold chain until administration to animals to prevent thermal inactivation. Commercially available freezedried vaccines remain stable for a minimum of two years at 2°C to 8°C and for several years at -20°C. Once reconstituted, the vaccine should be used promptly, within 30 minutes of dilution. Various cell culture attenuated vaccine strains like PPRV-Nigeria75/1 and PPRV-Sungri/96 are employed for PPR control in small ruminants, with Nigeria75/1 widely utilized in Africa, the Middle East, and parts of Asia. Despite trials, a vaccine with the capacity to Differentiate Infected from Vaccinated Animals (DIVA) is not currently available [37].
Purpose
Method
population freedom from infection
Individual freedom from infection
eradication policies
Confirmation of
CasesPrevalence
and
surveillanceTo check Immune status post vaccination
RT-PCR
-
++
++
+++
+
-
Real time RT-PCR
-
++
+++
+++
+
-
Isolation from culture
-
-
-
++
-
-
Ic- ELISA
+
++
+++
+
-
LFD
-
-
++
++
-
-
AGID
-
-
+
+
-
-
CIEP
-
-
-
+
-
-
VNT
+++
+++
-
++
++
++
C- ELISA
+++
+++
+++
+
+++
+++
AGID
-
-
+
+
-
+
CIEP
-
-
-
+
-
-
Key: +++= recommended, validated; ++= suitable method and need further validation; += may be used in some situations, but cost, reliability or other factors severely limits its application; – = not appropriate. Source: [31].
Table 1: Diagnostic tests available for diagnosis of PPR and their purpose.
Status of the Disease in Ethiopia
Peste des petits ruminants was clinically suspected for the first time in Ethiopia in 1977. However, clinical and serological evidence confirming its presence was not documented until 1991 [1,43]. The virus was subsequently isolated in 1996 as part of lineage III. Its full genome was sequenced in 2014 [16]. Currently, there are five full genome sequences of PPRVs available in GenBank, with accession numbers KJ867540, KJ867541, MK991798, MK991799, and MK991800 from Ethiopia [16]. A national sero-surveillance of PPR conducted in Ethiopia in 1999 revealed an overall seroprevalence of 6.4% in goats and sheep [19]. Peste des petits ruminants is endemic in Ethiopia, and the National Veterinary Institute (NVI) is producing a live attenuated vaccine using the PPR75/1 (LK6 Vero74) strain to combat the disease [10]. Various serological studies conducted across different regions of the country have reported seroprevalence rates ranging from 0.7% to 75.7% in small ruminants (Table 2).
Study regions/areas in Ethiopia
Number tested by c-ELISA, species of animal (prevalence)
Reference
Afar (Awash Fentale)
238 Sheep and Goats (36.6%)
[15]
Gambella
779 Sheep and Goats (27.2%)
Afar
384 Sheep and Goats (38.3%)
[28]
Amhara
672 Sheep and Goats (18.3%)
[17]
Amhara (North Shewa)
1065 Sheep (11%), 1325 Goats (9.6%))
[6]
Benishangul
Asosa
321 Sheep and Goats (75.7%)
[44]
Metekel
452 Sheep and Goats (73.45%)
[43]
Oromia
700 Sheep and Goats (48.43%)
[19]
806 Sheep and goats (27.42%)
[21]
SNNPR
Siltie Zone
160 Sheep and Goat (24.2%)
[23]
Gurage zone
221 Sheep and Goats (33%)
Bench Maji
429 Sheep and Goats (3.7%)
[20]
Kafa zone
539 Sheep and Goats (0.7%)
Somali
472 Sheep and Goats (41%)
[14]
Tigray
240 Goats (47.5%)
[5]
Table 2: Seroprevalence studies of PPR in different hosts and regions of Ethiopia.
Conclusion and Recommendations
Peste des petits ruminants is a highly contagious transboundary disease that affects both domestic and wild ruminants, with sheep and goats being the most severely impacted reservoir hosts. However, the role of other domestic and wild animals in the disease's epidemiology remains unclear. Close contact is a significant factor in the transmission of the disease. Several diagnostic tests, including serological and molecular methods, are used to diagnose PPR by detecting the virus, its antigens, and the antibodies produced against PPRV. The most commonly recommended diagnostic assays are IC-ELISA, RT-PCR, Virus Neutralization Tests (VNT), and Competitive ELISA (c-ELISA). However, these assays are rarely available in developing countries. Of the various prevention and control measures for PPR, vaccination is the most important in endemic areas. Nevertheless, challenges such as a lack of appropriate vaccine storage equipment, illegal animal movements across borders, absence of effective DIVA vaccine and inadequate ground-level disease control practices hinder effective prevention and control of the disease. Therefore, it is recommended that comprehensive control and prevention strategies, such as robust vaccination programs, are rigorously enforced to combat the disease effectively. Prioritizing research and development efforts toward the creation of a DIVA vaccine, which offers substantial benefits in managing and curtailing PPR outbreaks, is also crucial. Furthermore, there is a critical need to clearly delineate the roles of both domestic and wild animals in order to enhance our understanding of disease transmission dynamics and improve preventive measures.
References
- Abiyu Y, Jibril Y, Sibhatu D. Sero-epidemiology of Peste des petits Ruminants in Pawe District of Metekel Zone, Northwest Ethiopia. In Fekede Feyissa, Zewdie Wondatir, Lamma Fita and Temesgen Jembere (eds.). Livestock Research Proceedings. Results of Livestock Research Completed during 2022, Ethiopian Institute of Agricultural Research (EIAR), Addis Ababa, Ethiopia. 2023a: 541-553.
- Abiyu Y, Urge Tadele M, Aliye A. Molecular Detection of Peste des Petits Ruminants in Selected Districts of Awi Zone, Northwest Ethiopia. In Fekede Feyissa, Zewdie Wondatir, Lamma Fita and Temesgen Jembere (eds.). Livestock Research Proceedings. Results of Livestock Research Completed during 2022, Ethiopian Institute of Agricultural Research (EIAR), Addis Ababa, Ethiopia. 2023b: 677-687.
- Abraham G. Epidemiology of peste des petits ruminants virus in ethiopia and molecular studies on virulence. PhD Thesis, Institut National Polytechnique de Toulouse, France. 2005.
- Abubakar M, Irfan M, Manzoor S. Peste des petits ruminants in Pakistan; past, present and future perspectives. J Anim Sci Technol. 2015; 57: 32.
- Afera B, Hussien D, Amsalu K. Seroprevalence of peste des petits ruminants in goats of southern parts of Tigray region. Glob Vet. 2014; 12: 512-516.
- Agga GE, Raboisson D, Walch L, Alemayehu F. Epidemiological Survey of Peste des Petits Ruminants in Ethiopia: Cattle as Potential Sentinel for Surveillance. Front Vet Sci. 2019; 6: 302.
- Alemu B. Epidemiology and identification of peste des petits ruminants (ppr) virus circulating in small ruminants of eastern amhara region bordering afar, ethiopia. MSc Thesis, Addis Ababa University, College of Veterinary Medicine and Agriculture, Bishoftu, Ethiopia. 2014.
- Alemu B, Gari G, Libeau G, Kwiatek O, Kidane M, Belayneh R, et al. Molecular detection and phylogenetic analysis of Peste des petits ruminants virus circulating in small ruminants in eastern Amhara region, Ethiopia. BMC Vet Res. 2019; 15: 84.
- Balamurugan V, Hemadri D, Gajendragad MR, Singh RK, Rahman H. Diagnosis and control of peste des petits ruminants: a comprehensive review. Virus Dis. 2014; 25: 39-56.
- Bedore B, Mustefa M, Tamire M, Geinoro T. Current Status of Occurrence and Socio-Economic Impacts of Peste Des Petits Ruminants Virus (PPRV) on Small Ruminant Population in Ethiopia. Am J Epidemiol Public Health. 2019; 3: 012-016.
- Bello MB. Serological studies on peste des petits ruminants (ppr) in sheep, goats and camels in sokoto state, nigeria. MSc Thesis, Ahmadu Bello University, Faculty of Veterinary Medicine, Zaria, Nigeria. 2013.
- CFSPH. Peste des Petits Ruminants. The Center for Food security and Public Health, Institute for International Coorporationin Animal Biologics, Ames, Iowa. 2008.
- Clarke BD, Islam MR, Yusuf MA, Mahapatra M, Parida S. Molecular detection, isolation and characterization of Peste des petits ruminants virus from goat milk from outbreaks in Bangladesh and its implication for eradication strategy. Transbound Emerg Dis. 2018; 65: 1597-1604.
- Dejene W. Sero-epidemiology and spatial distribution of peste des petits ruminants virus antibodies in some selected pastoral areas of Somali regional state, Ethiopia. MSc Thesis, Addis Ababa University, College of Veterinary Medicine and Agriculture, Bishoftu, Ethiopia. 2016.
- Delil F, Asfaw Y, Gebreegziabher B. Prevalence of antibodies to peste des petits ruminants virus before and during outbreaks of the disease in Awash Fentale district, Afar, Ethiopia. Trop Anim Health Prod. 2012; 44: 1329-1330.
- Dundon WG, Diallo A, Cattoli G. Peste des petits ruminants in Africa: a review of currently available molecular epidemiological data. Arch Virol. 2020; 165: 2147-2163.
- Fentie T, Teshome Y, Ayele B, Molla W, Fenta N, Nigatu S, et al. Sero epidemiological study of peste des petits ruminants in small ruminants in Amahara region, Ethiopia. Comp Clin Path. 2018; 27: 1029-1036.
- Fine AE, Pruvot M, Benfield CTO, Caron A, Cattoli G, Chardonnet P, et al. Eradication of Peste des Petits Ruminants Virus and the Wildlife-Livestock Interface. Front Vet Sci. 2020; 7: 50.
- Gari G, Serda B, Negesa D, Lemma F, Asgedom H. Serological Investigation of Peste Des Petits Ruminants in East Shewa and Arsi Zones, Oromia Region, Ethiopia. Vet Med Int. 2017.
- Gebre T, Deneke Y, Begna F. Seroprevalence and Associated Risk Factors of Peste Des Petits Ruminants (PPR) in Sheep and Goats in Four Districts of Bench Maji and Kafa Zones, South West Ethiopia. Glob Vet. 2018; 20: 260-270.
- Gelana M, Gebremedhin EZ, Gizaw D. Seroepidemiology of Peste des Petits Ruminants in Sheep and Goats in the selected District of Horu Guduru Zone, Western Ethiopia. Res Vet Sci. 2020; 132: 527-534.
- Gizaw F, Merera O, Zeru F, Bedada H, Gebru M, Abdi RD. Sero Prevalence and Socioeconomic Impacts of Peste Des Petits Ruminants in Small Ruminants of Selected Districts of Afar, Ethiopia. J Vet Sci Technol. 2018; 9: 513.
- Hailegebreal G. Seroprevalence of Peste Des Petits Ruminants in Selected Districts of Siltie and Gurage Zones, South Region, Ethiopia. J Vet Sci Technol. 2018; 9: 529.
- Ishag OM, Saeed IK, Ali YH. Peste des petits ruminants outbreaks in White Nile. Onderstepoort J Vet Res. 2015; 82: 897-900.
- Kamel M, El-sayed A. Toward peste des petits virus (PPRV) eradication: Diagnostic approaches, novel vaccines, and control strategies. Virus Res. 2019; 274: 274.
- Kihu SM. Risk factors and socioeconomic effects associated with spread of peste des petits ruminants (PPR) in Turkana County, Kenya. PhD Thesis, University of Nairobi, Department of Veterinary Pathology, Microbiology and Parasitology, Nairobi, Kenya. 2014.
- Luna AN. Preparation of purified peste des petits ruminants virus antigen. MSc Thesis, Bangladesh Agricultural University, Department of Pathology, Mymensingh, Bangladesh. 2012.
- Megersa B, Biffa D, Belina T, Debela E, Regassa A, Abunna F, et al. Serological investigation of Peste des Petits Ruminants (PPR) in small ruminants managed under pastoral and agro-pastoral systems in Ethiopia. Small Rumin Res. 2011; 97: 134-138.
- Munir M. Role of Wild Small Ruminants in the Epidemiology of Peste Des Petits Ruminants. Transbound Emerg Dis. 2014; 61: 411-424.
- Muniraju MB. Establishment of reverse genetics system for PPR virus to develop recombinant vaccines. PhD Thesis, University of Warwick, School of Life Sciences and Pirbright Institute, England. 2015.
- OIE. Peste des petits ruminants (infection with peste des petits ruminants virus). OIE Terrestrial manual, World Organization for Animal Health, Paris, France. 2019: 1-16.
- OIE. PESTE DES PETITS RUMINANTS: Aetiology Aetiology Epidemiology Diagnosis Prevention and Control References. OIE Technical Disease Cards, World Organisation for Animal health. 2020: 1-16.
- OIE and FAO. Global control and eradication of peste des petits ruminants: Investing in veterinary systems, food security and poverty alleviation. World Organiation for Animal Health and Food Agriculture Organiationof the United Nations. 2015: 1-23.
- Robi DT. Epidemiology of Peste Des Petits Ruminants in sheep and goats in Ethiopia. Acad Res J Agri Sci Res. 2019; 7: 503-512.
- Rudra PG. Prevalence and molecular characterization of Peste Des Petits Ruminants (PPR) in Goat Pran Gopal Rudra. MSc Thesis, Chattogram Veterinary and Animal Sciences University, Department of Medicine and Surgery, Chittagong, Bangladesh. 2019.
- Ruget AS, Tran A, Waret-Szkuta A, Moutroifi YO, Charafouddine O, Cardinale E, et al. Spatial Multicriteria Evaluation for Mapping the Risk of Occurrence of Peste des Petits Ruminants in Eastern Africa and the Union of the. Front Vet Sci. 2019; 6: 455.
- Santhamani R, Singh RP, Njeumi F. Peste des petits ruminants diagnosis and diagnostic tools at a glance: perspectives on global control and eradication. Arch Virol. 2016; 161: 2953-2967.
- Senbeto YA, Sibhatu D, Jibril Y. Seroprevalence and associated risk factors of Peste des petits ruminants in selected districts of Awi zone, Northwest Ethiopia, Heliyon. 2024; 10: e38882.
- Senbeto YA. Epidemiology of peste des petits ruminants, isolation and molecular detection of the virus in selected distrits of Awi and Metekel zones, Northwest Ethiopia. MVSc Thesis. Addis Ababa University College of Veterinary Medicine and Agriculture, Bishoftu, Ethiopia. 2022.
- Shahriari R, Khodakaram-tafti A, Mohammadi A. Molecular characterization of Peste des Petits ruminants virus isolated from four outbreaks occurred in southern Iran. BMC Vet Res. 2019; 15: 177.
- SOP. Peste des petits ruminants standard operating procedures: Overview of etiology and ecology. Standard Operating Procedures Manual, Foreign Animal Disease Preparedness and Rresponse Plan, Washington, DC. 2013.
- Swai ES, Kapaga A, Kivaria F, Tinuga D, Joshua G, Sanka P. Prevalence and distribution of Peste des petits ruminants virus antibodies in various districts of Tanzania. Vet Res Commun. 2009; 33: 927-936.
- Woldemichael G, Aki A, Gurmessa K. Study on Sero-prevalence and Risk factor of Peste des Petits ruminant disease in Small Ruminant at Metekel zone of selected District in Benishangul Gumuz Regional State, Western Ethiopia. Rep Opinion. 2018; 10: 56-65.
- Yalew S, Woldemichal G, Mamo M. Seroprevalence of Peste Des Petits Ruminant’s Virus Antibody inin Assosa Zone, Benishangulgumuz Region, Ethiopia. ARC Journal of Animal and Veterinary Sciences. 2019; 5: 29-33.
- Khan HA, Siddique M, Abubakar M, Arshad MJ, Hussain M. Prevalence and distribution of PPRV infection in small ruminants. Small Ruminants Research. 2008; 79: 152-157.
- Schulz C, Fast C, Wernery U, Kinne J, Joseph S, Schlottau K, et al. Camelids and Cattle Are Dead-End Hosts for Peste-des-Petits-Ruminants Virus. Viruses. 2019; 11: 1133.
- Ebissa T. Antigen and Molecular Detection of Peste Des Petits Ruminants Virus from Disease Outbreak Cases in Sheep and Goats in Asossa Zone, Benishangul-Gumuz Region, Ethiopia. MSc Thesis, Addis Ababa University, College of Veterinary Medicine and Agriculture, Bishoftu, Ethiopia. 2020.
- Albina E, Kwiatek O, Minet C, Lancelot R, Servan de Almeida R, Libeau G. Peste des petits ruminants, the next eradicated animal disease? Veterinary Microbiology. 2013; 165: 38-44.