
Research Article
Annals Thyroid Res. 2025; 11(1): 1093.
Rapidly Growing Thyroid Masses in Adults: Urgent Diagnostic Challenges and Effective Management – Single - Centre Experience and Literature Review
Miguélez-GonzÁlez M¹*, FernÁndez-SÁnchez A¹, Álvarez Álvarez MB², Quiceno-Arias HD² and Osorio- Silla I³
¹Endocrinology Service, Fundación Jiménez Díaz Hospital, Madrid, Spain
²Anatomical Pathology Service Fundación Jiménez Díaz Hospital, Madrid, Spain
³General Surgery Service, Fundación Jiménez Díaz Hospital, Madrid, Spain
*Corresponding author: Miguélez-GonzÁlez M, Endocrinology Service, Fundación Jiménez Díaz Hospital, Madrid, Spain Email: mariamiguelezmd@gmail.com
Received: April 20, 2025 Accepted: April 30, 2025 Published: May 05, 2025
Abstract
Introduction: Rapidly growing thyroid tumours require a prompt and accurate diagnosis to ensure timely initiation of appropriate treatment, which significantly impacts patient’s outcome. The aim of this study is to present a series of cases of patients with rapidly growing thyroid masses to establish a diagnostic protocol to improve our understanding and oncologic outcomes.
Methods: Retrospective longitudinal study of patients with rapidly growing thyroid masses during the years 2022 to 2024. A total of 12 patients were included. Data were collected from each patient’s medical record, including age at diagnosis, sex, personal background, symptoms at diagnosis, imaging studies, pathological, treatment and follow-up data.
Results: Five patients were diagnosed with thyroid cancer (TC)- four poorly differentiated thyroid carcinoma (PDTC) and one locally advanced papillary thyroid carcinoma (PTC)-, six were diagnosed with primary thyroid lymphoma (PTL), two mucosa-associated lymphoid tissue (MALT) and four diffuse large B-cell lymphoma (DLBCL)- and one case was diagnosed with Riedel’s thyroiditis. Mean age was 64,4 years (range 34-84). Nine patients presented with compressive symptoms. Diagnostic tests revealed vocal cord paralysis in two cases, oesophageal and tracheal involvement in two others. Distant metastases were detected in three patients. Seven patients underwent surgical intervention, two were considered unresectable, and two cases of PTL were managed with chemotherapy. Two cases of PDTC underwent neoadjuvant treatment.
Conclusion: Rapidly growing thyroid tumours are uncommon and may have a poor prognosis. Molecular studies are essential. Optimal management requires a multidisciplinary team, and the development of protocols is crucial to improving oncological outcomes.
Keywords: Thyroid tumours; Lymphoma; Anaplastic; Poorly differentiated carcinoma; Management
Abbreviations
TC: Thyroid cancer; PTC: Papillary Thyroid Carcinoma; PDTC: Poorly Differentiated Thyroid Carcinoma; PTL: Primary Thyroid Lymphoma; MALT: Mucosa-associated Lymphoid Tissue; DLBCL: Diffuse Large B-Cell Lymphoma; CT: Computed Tomography; PETCT FDG: Positron Emission Tomography; CNB: Core Needle Biopsy; FANB: Fine-needle Aspiration; ATC: Anaplastic Thyroid Carcinoma; NGS: Next Generation Sequencing; EBUS: Endobronchial Ultrasonography; EUS: Esophagoscopy or Endoscopic Ultrasound.
Introduction
Neck masses are a common clinical presentation in adults, but often their underlying etiology is challenging to determine. Among these, malignant neoplasms are notably more prevalent than other etiologies [1]. Primary differential diagnoses in thyroid gland include locally advanced TC, PTL, and thyroiditis. Other important considerations include squamous cell carcinoma of the neck, salivary gland neoplasms, and parapharyngeal synovial sarcoma [2].
Rapidly growing thyroid tumors (< 2 months) require a prompt and accurate diagnosis to ensure timely initiation of appropriate treatment, which significantly impacts patient's outcome [3,4]. Following a standardized diagnostic protocol facilitates the selection of the most appropriate diagnostic tests [5] and effective management strategies. The aim of this study is to describe our experience with patients with rapidly growing thyroid tumors to establish a diagnostic protocol to improve our understanding and oncologic outcomes.
Material and Methods
Retrospective longitudinal study of patients with debut rapidly growing thyroid masses seen at the Fundación Jiménez Díaz in Madrid between 2022 and 2024. The patients came from both primary and specialised care. Based on the anatomopathological and the multidisciplinary committee registry, 17 patients with rapidly growing thyroid tumour debut were identified. Of these 17 patients, 5 had no treatment or follow-up in our hospital and were excluded. A total of 12 patients were included.
Retrospective longitudinal study of patients with debut rapidly growing thyroid masses seen at the Fundación Jiménez Díaz in Madrid between 2022 and 2024. The patients came from both primary and specialised care. Based on the anatomopathological and the multidisciplinary committee registry, 17 patients with rapidly growing thyroid tumour debut were identified. Of these 17 patients, 5 had no treatment or follow-up in our hospital and were excluded. A total of 12 patients were included.
The study has been approved by our hospital’s ethics committee.
Results
Twelve patients were included, five were diagnosed with thyroid carcinoma (four PDCT and one locally advanced PTC), six were diagnosed with PTL (two MALT and four DLBCL) and one case was diagnosed with Riedel’s thyroiditis. Only two cases were Hispanic or Asian, being the mayority caucasian. Nine were females (75%), three were men (25%), and the mean age was 64,4 years (range 34-84). Nine patients (75%) presented with compressive symptoms (dysphagia, dysphonia, dyspnea or cervical pain). There were not any cases of phrenic nerve paralysis, Horner's syndrome, jugular vein compression, thrombosis, or superior vena cava syndrome. Epidemiological and clinical characteristics of the patients are presented in Table 1.
Case
Age
Gender
Medical history
Symptoms
Physical examination
Time since
onset symptoms (weeks)Time to
diagnosis ( weeks)1
71
F
Hashimoto
Cervical pain, odynophagia
Goiter. Fixed left mass
3
8*
2
48
F
Hashimoto
None
Goiter
-
*
3
84
F
MNG HTA
Dyspnea, dysphagia, dysphonia,
constitutional syndrome, weight lossGoiter
4
2
4
73
M
Lymphoma HTA
constitutional syndrome, weight loss
Goiter
-
3
5
53
F
Hashimoto
Dysphonia
Right nodule
12
*
6
58
F
Cervical sarcoma
Cervical pain
Mass in right lobule
-
4
7
43
F
Sjogren syndrome
Cervical pain, odynophagia
Fixed mass
3
<1
8
34
F
None
Cervical pain, odynophagia, dysphagia, dysphonia
Painful goiter
5
8
9
70
F
Hashimoto HTA
Dyspnea, dysphagia, dysphonia, odynophagia, constitutional syndrome, weight loss
Goiter
1,5
<1
10
83
F
None
Dyspnea, dysphagia, cervical pain
-
4
<1
11
78
M
HTA
Dysphagia, dysphonia, odynophagia
-
6
3
12
81
M
Asthma Parkinson
None
Goiter. Fixed left mass
-
3
*Postoperative diagnosis, Time to diagnosis from first consultation. MNG: Multinodular goiter HTA: hypertension.
Table 1: Epidemiological and clinical characteristics of the patients.
Six cases were classified as Ti-RADS 5 on cervical ultrasound, two as Ti-RADS 4, and two as Ti-RADS 3. Laryngoscopy was performed on eight patients, revealing vocal cord paralysis in two cases. All patients, except two, underwent cervical computed tomography (CT), with a mean mass diameter of 6 cm (range: 3–11 cm). Positron emission tomography (PET-CT FDG) was performed on eleven patients, showing a mean SUV max of 20.3 (range: 2.2–46.4). Cases of TC exhibited a higher SUV max compared to PTL (mean SUV max: 25.62 vs. 17.42). Endoscopic ultrasound and assessment of tracheal involvement were performed in four patients, identifying oesophageal and tracheal involvement in two of them. Distant metastatic involvement was observed in four patients at the time of diagnosis: in two cases of TC in the bone and lung and liver, and in two cases of PTL in the peritoneum. Seven patients underwent surgical intervention, two were considered unresectable, and two cases of PTL were managed with chemotherapy. Among the surgically treated patients, hemithyroidectomy was performed in two of them and total thyroidectomy in five cases. Central lymphadenectomy was conducted in two patients, and one of them additionally underwent lateral cervical lymphadenectomy
Four patients diagnosed with PTL completed treatment with R-CHOP, with one case requiring additional radiotherapy. The two cases of MALT lymphoma did not require further treatment after surgery. Two cases of PDTC underwent neoadjuvant treatment with Lenvatinib. One of these cases continued therapy with Lenvatinib because of the metastatic status. After tumor progression this patient received second-line treatment with cabozantinib as part of a clinical trial. Another case of PDTC was treated with sorafenib and radiotherapy.
The pathological analysis and molecular study, treatment and follow up data are presented in Tables 2, 3 and 4. The median followup period was 22months (2 - 60 months). One patient was lost to follow-up. Three patients died during the study period: two due to the disease (PDTC) and one from COVID-19 (PTL)
Case
Histologycal type
IHQ/ molecular test
Ki67%
Tumor size
Clinical stage
Treatment
Follow up time
2
PTL: MALT
MNDA, CD20 +
-
30
Hemithyroidectomy
24 months
3
PTL: DLBCL- NON GCB
CD45, CD20, BCL2, BCL6, cmyc(focal), CD10(focal), MUM1(focal)PD1(focal)P 53(focal)+
EBER, CD3, TDT, HMB45, MNDA, PAX8, Thyroglobulin, TTF1, CD56 (-)
Mutation L265P in MYD88
Not MYC, BCL6, BCL2 Translocation80%
80
IA
Thyroidectomy(R2)
R-miniCHOP
RT4 years
4
PTL: MALT
PAX5, CD79a, CD20, BCL 2+
CD10, BCL6, MNDA, Cyclin D1, P53, (-)
IGH Clonally rearranged<10%
11
Thyroidectomy
15 months
5
PTL: DLBCL- GCB
CD20, CD79, PAX5, CD10, BCL6, CD23,
P53(focal) (+)
BL2, CD5, CYCLIN D1, CD30(-)
Not clonally rearranged70%
20
IV A
Hemityroidectomy R-CHOP
5 years
9
PTL: DLBCL- GCB
CD20, CD10, BCL6, LMO2, MYC+
MUM1/ciclinaD1, TDT, EBER –
Not MYC, BCL6, BCL2
TranslocationNot Done
Biopsy (1,6cm)
IV B
R.CHOP
11 months
10
PTL: DLBCL- NON GCB
CD20, BCL6, MUM1, MYC (focal)+, CD10, CD30, EBER, TDT (-)
BCL6 Translocation
Not MYC, BCL2
Translocation80%
Biopsy (1,1cm)
IV
R-miniCHOP
7 months
*PTL: Primary Thyroid Lymphoma, *MALT: mucosa associated lymphoid tissue lymphoma, * DLBCL: difuse large B cell lymphoma. * MNDA: myeloid nuclear differentiation antigen.
Table 2: Primary thyroid Lymphoma- Histology, treatment, and follow up data of patients.
Case
Histologycal type
IHQ/ molecular test
Previous operation
Tumor size
Treatment
Distant metastases
Follow up time
1
PDTC (insular)
CK7, BCL2,
TTF1, Tiroglobulin +
BRAF -Surgery biopsy
42
Total Thyroidectomy
No
33 months
6
PDTC
TTF1, Tiroglobulin, PAX8, CKAE1/AE3 +
1,4
Sorafenib
+Total thyroidectomy
+ RTyes
46 months
7
PDTC (Folicular carcinoma)
BRAF -
PAX8: PPARG- CREB3L2: PPARG -
10,6
Lenvatinib +
total thyroidectomy+
Cabothyroid(C T)No
23 months
11
PDTC (DHGT)
TTF1, PAX8,
CKAE1/AE3, Vimentin, CyclinD1 (+) Thyroglobulin, MelanA, HMB45, EMA, NapsinA,
CD45, Calcitonin (-)
P53 aberrant (mutational type), Ki67 40%
5
Lenvatinib
yes
2 months
12
PTC
No
Lenvatinib
No
6 months
*PDTC: poorly differentiated thyroid cancer, *PTC: Papillary thyroid carcinoma.
Table 3: Thyroid Carcinoma- Histology, treatment, and follow up data of patients.
Case
Extent of thyroidectomy
Extent of CND
Extent of LND
Infltration pattern
Resection margin status
N status
1
Total Thyroidectomy
Yes
No
Prethyroid muscles
R1
N1a
pT3b pN1a
6
Total thyroidectomy
No
No
Muscles
R0
Nx
ypT1b
7
Total thyroidectomy
yes
yes
Muscle, tumour thrombus yugular left vein
R1
No
ypT4a ypN0
Table 4: Surgical treatment and staging.
Discussion
Rapidly enlarging thyroid masses are defined as a clinically evident enlargement of the anterior compartment of the neck, occurring in less than one month, and usually associated with compressive symptoms. Locally advanced TC, PTL and thyroiditis are the main entities. Differential diagnosis requires rapid and protocolized assessment. In our study, five patients were diagnosed with TC six with PTL and one case was diagnosed with Riedel’s thyroiditis.
An exhaustive initial evaluation is essential to guide subsequent therapeutic interventions. The first step is performing cervical ultrasound, which serves for characterizing the lesion. Furthermore, it allows to obtain tissue samples for pathological and molecular assessments. Whenever feasible, a core needle biopsy (CNB) should be prioritized over fine-needle aspiration (FNAB) due to its significant diagnostic advantages. Ha et al. reported that CNB is a superior diagnostic tool for anaplastic thyroid carcinoma (ATC) and PTL, demonstrating a sensitivity of 87.5% and a positive predictive value of 100%. In this study, PTL was misdiagnosed in one case of CNB compared to 16 cases of FNAB [6]. CNB reduces the need for diagnostic surgeries and provides sufficient tissue for molecular analyses. In cases where a PTL is suspected, the sample must be referred for flow cytometry [6,7]. In the pathological assessment for PDCT, locally advanced PTC and ATC, the first diagnostic approach is the immunohistochemistry study [8]. Subsequently, Next Generation Sequencing (NGS) is recommended to assess BRAF mutations, NTRK fusions, RET alterations (point mutations or fusions), ALK translocation or m-TOR mutations to assess targeted therapies [9]. Within trial specific studies of other therapeutic targets such as TMB, MSI, dMMR, RAS, TERT, STK11, PTEN, TP53 or IDH1 will be considered within specific protocols [10].
Although cervical ultrasonography is considered the primary imaging modality for the evaluation of thyroid masses, additional complementary imaging studies were required [5,11,12]. A total of 54.5% of our patients were classified as TiRADS-5. However, the initial cervical ultrasound did not raise suspicion of PTL or aggressive TC in any of the reported cases.
Several other tools are available for the diagnosis and staging of thyroid masses, particularly in complex cases. These include CT and PET-CT FDG, which provide detailed imaging to assess the extent of disease, and potential metastases. Furthermore, procedures such as laryngoscopy, endobronchial ultrasonography (EBUS) and esophagoscopy or endoscopic ultrasound (EUS), are highly valuable for evaluating local invasion and involvement of adjacent structures [13,14].
Published studies emphasize the critical role of a multidisciplinary approach and the implementation of standardized diagnostic algorithms in the management of thyroid masses [11,15]. The results of our case series show the impact of the absence of standardized protocols, emphasizing their development as a key objective of this study. A rapidly growing thyroid mass should not proceed to surgery without prior diagnosis and staging. This is particularly significant in cases of advanced TC, where the initial surgical intervention plays a pivotal role in patient outcomes [5]. In the present study, four patients were diagnosed following the initial surgical procedure. Of these, two cases were diagnosed intraoperatively, one with PTL and the other with PDTC. The remaining two cases were identified based on the final pathological results.
PTL is a rare tumor (1-5% of all thyroid malignancies). The most common subtype is DLBCL (50-70%) and MALT lymphoma (10-50%) [12,16]. It is known that the main risk factor for PTL is Hashimoto’s thyroiditis [17,18]. Fifty percent of our patients with PTL had a history of this condition. Five patients were women and one man, with a mean age of 68,5 (range: 48-84), similar as describes in the literature. Uptake in FDG-PET is usually higher in CT than in PTL. In aggressive varieties, PET/CT is useful for both staging and response to treatment [12].
The role of surgery is limited [12]. It is often complex due to tumor infiltration that increases the risk of injury to the recurrent laryngeal nerve and other cervical structures [19]. Moreover, various studies have shown that surgery does not improve survival compared to chemotherapy or radiotherapy, stages IE or IIE included [20]. However, in certain cases, a definitive diagnosis is achieved through histopathological analysis of the surgical specimen, either because TC was suspected or due to indeterminate results from FNAB or CNB. In a case series reported by Sakhri et al, all patients underwent surgery without a confirmed prior diagnosis. In our series, two patients underwent surgery, one due to indeterminate FNAB results and other due to suspect TC; another case was operated on to confirm a PTL diagnosis, while one patient presented with compressive symptoms caused by a goiter. Nevertheless, more recent cases have been managed with corticosteroids to relieve compressive symptoms and chemotherapy [21].
Recent studies have highlighted the optimal treatment for PTL, particularly in DLBCL subtype. The standard frontline treatment of DLBCL remains chemo-immunotherapy with R-CHOP with or without radiotherapy according to disease stage and clinical risk factors [6,18,22].
TC is characterized by having a good prognosis. Unfortunately, certain cases debut as a locally advanced disease or aggressive tumor subtypes, such PDTC, ATC or locally advanced medullary cancer [23]. These forms are associated with significant morbidity and mortality, due to cervical structures invasion (airway, great vessels, esophagus) and therapeutic limitations [2,24,25]. In the present study, five cases were diagnosed as TC, including four PDTC and one case of locally advanced PTC. Surgery remains the cornerstone of treatment for TC. However, in locally advanced cases, surgical intervention must be carefully weighed against its potential impact on quality of life, disease control, recurrence rate, and survival benefits [5]. In our series, four cases underwent surgery, one of which was performed following neoadjuvant therapy. However, two cases were deemed unresectable due to extensive infiltration involving the trachea and esophagus. Other therapeutic modalities include radioactive iodine, external beam radiotherapy, and systemic therapies. Neoadjuvant treatment regimens included Lenvatinib (multikinase inhibitor), dabrafenib + trametinib (selective BRAF and MEK inhibitors), selpercatinib (selective RET inhibitor), vandetanib (multikinase inhibitor), crizotinib, and alectinib (both ALK inhibitors). The use of neoadjuvant therapy for inoperable TC is a promising area and currently under investigation [26]. In the study by Iwasaki et al., six initially inoperable cases were treated with lenvatinib, enabling surgical intervention upon completion of the therapy. Similarly, Yeo et al. reported a series of four cases with favorable outcomes, demonstrating that lenvatinib was effective in reducing the extent of the primary tumor, potentially facilitating limited surgical resection by local disease control [27]. Our experience is limited to a single case.
In summary, it is important to protocolize the management of rapidly enlarging neck masses as time is urgent (Figure 1). It is essential to go beyond ultrasound with the performance of a PETCT scan, and to go beyond FNA with the performance of a CNB, in order to obtain a molecular study that will also allow us to direct the treatments. Optimal management of rapidly growing thyroid tumors requires a multidisciplinary team to evaluate diagnostic findings, therapeutic options, and individual patient needs, ensuring the selection of the most appropriate treatment strategy. The development of standardized diagnostic and therapeutic protocols is crucial to improving oncological outcomes.
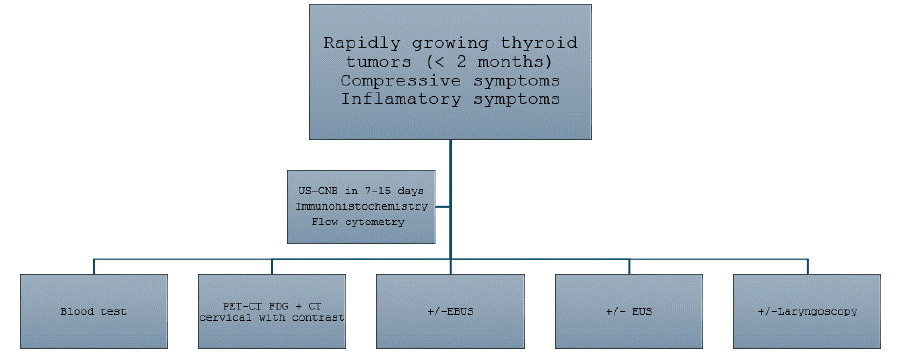
Figure 1: Algorithm for the management of Rapidly Growing Thyroid Masses.
Author Contributions
MM, OI, FA, QH, AM had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of data analysis.
MM, OI, FA are chief investigators and act as guarantors for this work.
Concept and design: MM, OI.
Drafting of the manuscript: MM, OI.
Critical revision of the manuscript for important intellectual content: MM, OI, FA, QH, AM.
Statistical analysis: OI.
Availability of Data and Materials
The data presented in this study are available on request from the corresponding author.
Ethics Approval and Consent to Participate
The study has been approved by our hospital’s ethics committee. The committee agreed to the exemption of informed consent, as this was a retrospective study and a pathology with a poor prognosis.
References
- Pynnonen MA, Gillespie MB, Roman B, Rosenfeld RM, Tunkel DE, Bontempo L, et al. Clinical Practice Guideline: Evaluation of the Neck Mass in Adults. Otolaryngol Head Neck Surg. 2017; 157: S1–30.
- Sitges-Serra A. The rapidly growing thyroid mass. Br J Surg. 2006; 93: 647– 649.
- Jannin A, Escande A, Al Ghuzlan A, Blanchard P, Hartl D, Chevalier B, et al. Anaplastic Thyroid Carcinoma: An Update. Cancers (Basel). 2022; 14: 1061.
- King AD, Ahuja AT, King W, Metreweli C. The role of ultrasound in the diagnosis of a large, rapidly growing, thyroid mass. Postgrad Med J. 1997; 73: 412–414.
- Sessa L, De Crea C, Voloudakis N, Pennestri’ F, Revelli L, Gallucci P, et al. Single Institution Experience in the Management of Locally Advanced (pT4) Differentiated Thyroid Carcinomas. Ann Surg Oncol. 2024; 31: 5515–5524.
- Ha EJ, Baek JH, Lee JH, Kim JK, Song DE, Kim WB, et al. Core needle biopsy could reduce diagnostic surgery in patients with anaplastic thyroid cancer or thyroid lymphoma. Eur Radiol. 2016; 26: 1031–1036.
- Ahmed S, Ghazarian MP, Cabanillas ME, Zafereo ME, Williams MD, Vu T, et al. Imaging of Anaplastic Thyroid Carcinoma. AJNR Am J Neuroradiol. 2018; 39: 547–551.
- Bible KC, Kebebew E, Brierley J, Brito JP, Cabanillas ME, Clark TJ, et al. 2021 American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer. Thyroid. 2021; 31: 337–386.
- Shonka DC, Ho A, Chintakuntlawar AV, Geiger JL, Park JC, Seetharamu N, et al. American Head and Neck Society Endocrine Surgery Section and International Thyroid Oncology Group consensus statement on mutational testing in thyroid cancer: Defining advanced thyroid cancer and its targeted treatment. Head Neck. 2022; 44: 1277–1300.
- Song YS, Park YJ. Genomic Characterization of Differentiated Thyroid Carcinoma. Endocrinol Metab (Seoul). 2019; 34: 1–10.
- Raffaelli M, Voloudakis N, Barczynski M, Brauckhoff K, Durante C, Gomez- Ramirez J, et al. European Society of Endocrine Surgeons (ESES) consensus statement on advanced thyroid cancer: definitions and management. Br J Surg. 2024; 111: znae199.
- Sakhri S, Zemni I, Ayadi MA, Kamoun S, Chargui R, Ben Dhiab T. Primary thyroid lymphoma: a case series. Journal of Medical Case Reports. 2024; 18: 108.
- Klamt AL, Neyeloff JL, Santos LM, Mazzini G da S, Campos VJ, Gurski RR. Echoendoscopy in Preoperative Evaluation of Esophageal Adenocarcinoma and Gastroesophageal Junction: Systematic Review and Meta-analysis. Ultrasound Med Biol. 2021; 47: 1657–1669.
- Zaric B, Stojsic V, Sarcev T, Stojanovic G, Carapic V, Perin B, et al. Advanced bronchoscopic techniques in diagnosis and staging of lung cancer. J Thorac Dis. 2013; 5: S359–370.
- Durante C, Hegedüs L, Czarniecka A, Paschke R, Russ G, Schmitt F, et al. 2023 European Thyroid Association Clinical Practice Guidelines for thyroid nodule management. Eur Thyroid J. 2023; 12: e230067.
- Travaglino A, Pace M, Varricchio S, Insabato L, Giordano C, Picardi M, et al. Hashimoto Thyroiditis in Primary Thyroid Non-Hodgkin Lymphoma. Am J Clin Pathol. 2020; 153: 156–164.
- Otsuka Y, Yasuda M, Tokumasu K, Hasegawa K, Otsuka F. Hashimoto’s thyroiditis and primary thyroid lymphoma. QJM: An International Journal of Medicine. 2020; 113: 691–692.
- Suzuki N, Watanabe N, Noh JY, Yoshimura R, Mikura K, Kinoshita A, et al. The Relationship Between Primary Thyroid Lymphoma and Various Types of Thyroid Autoimmunity: A Retrospective Cohort Study of 498 Cases, Including 9 Cases with Graves’ Disease. Thyroid. 2022; 32: 552–559.
- Meyer-Rochow GY, Sywak MS, Reeve TS, Delbridge LW, Sidhu SB. Surgical trends in the management of thyroid lymphoma. European Journal of Surgical Oncology (EJSO). 2008; 34: 576–580.
- Pyke CM, Grant CS, Habermann TM, Kurtin PJ, van Heerden JA, Bergstralh EJ, et al. Non-Hodgkin’s lymphoma of the thyroid: is more than biopsy necessary? World J Surg. 1992; 16: 604–609; discussion 609-610.
- Lee JS, Shin SJ, Yun HJ, Kim SM, Chang H, Lee YS, et al. Primary thyroid lymphoma: A single-center experience. Front Endocrinol (Lausanne). 2023; 14: 1064050.
- Susanibar-Adaniya S, Barta SK. 2021 Update on Diffuse large B cell lymphoma: A review of current data and potential applications on risk stratification and management. Am J Hematol. 2021; 96: 617–629.
- Yeo JJY, Stewart K, Maniam P, Arman S, Srinivasan D, Wall L, et al. Neoadjuvant tyrosine kinase inhibitor therapy in locally advanced differentiated thyroid cancer: a single centre case series. J Laryngol Otol. 2023; 137: 1237–1243.
- Ibrahimpasic T, Ghossein R, Shah JP, Ganly I. Poorly Differentiated Carcinoma of the Thyroid Gland: Current Status and Future Prospects. Thyroid. 2019; 29: 311–321.
- Kunte S, Sharett J, Wei W, Nasr C, Prendes B, Lamarre E, et al. Poorly Differentiated Thyroid Carcinoma: Single Institution Series of Outcomes. Anticancer Res. 2022; 42: 2531–2539.
- Russell M, Gild ML, Wirth LJ, Robinson B, Karcioglu AS, Iwata A, et al. Neoadjuvant therapy to improve resectability of advanced thyroid cancer: A real-world experience. Head Neck. 2024; 46: 2496–2507.
- Whittaker DK, Williams-Jones DG. Biopsy techniques for electron microscopy. J Pathol. 1971; 103: 61–63.