
Research Article
Thromb Haemost Res. 2025; 9(1): 1099.
Corticosteroids to Manage Immune Thrombocytopenia: Are They Used Properly? The GEPTI Experience
Mingot-Castellano ME1*, Alcalde-Mellado P2, Martínez-Carballeira D3, Pedrote-Amador B4, Canaro-Hinryk M5, Zafra-Torres D6, Calo-Pérez A7, Pascual-Izquierdo C8, Caballero-Navarro G9, Canet-Maldonado M10, Gómez-del-Castillo- Solano MC11, Cuesta-García A12, García-Culebras M13, Chiclana-Rodríguez B14, García-Pallarols F15, Horrillo-Orejudo A16, Sánchez-Llorca P17, Casares- Aguiar D18, Pérez-Montes R19 and Sánchez- González B20
1Hospital Universitario Virgen del Rocío, Sevilla, Spain
2Hospital Universitario Virgen del Rocío, Sevilla, Spain
3Hospital Universitario Central de Asturias, Oviedo, Asturias, Spain
4Hospital Universitario Virgen del Rocío, Sevilla, Spain
5Hospital Universitario Son Espases, Palma de Mallorca, Mallorca, Spain
6Hospital 12 de Octubre, Madrid, Spain
7Hospital Universitario del Henares, Coslada, Madrid, Spain
8Hospital Universitario Gregorio Marañón, Madrid, Spain
9Hospital Universitario Miguel Servet, Zaragoza, Spain
10Hospital Mutua Terrassa, Terrassa, Barcelona, Spain
11Complexo Hospitalario Universitario A Coruña, A Coruña, Spain
12Hospital de Sierrallana, Torrelavega, Cantabria, Spain
13Hospital Universitario Virgen del Rocío, Sevilla, Spain
14Hospital Universitario Virgen del Rocío, Sevilla, Spain
15Hospital del Mar, Barcelona, Spain
16Hospital Universitario Son Espases, Palma de Mallorca, Mallorca, Spain
17Hospital 12 de Octubre, Madrid, Spain
18Hospital Universitario Gregorio Marañón, Madrid, Spain
19Hospital de Sierrallana, Torrelavega, Cantabria, Spain
20Hospital del Mar, Barcelona, Spain
*Corresponding author: Mingot-Castellano ME, Department of Hematology, University Hospital Virgen del Rocío, Instituto de Biomedicina de Sevilla (IBIS)/ CSIC, Universidad de Sevilla, Seville, Spain Tel: +34955012000; Email: mariae.mingot.sspa@juntadeandalucia.es
Received: June 17, 2025 Accepted: July 15, 2025 Published: July 18, 2025
Abstract
Corticosteroids (CTCs) are the first-line treatment to manage immune thrombocytopenia (ITP). Prolonged immunosuppression is associated with adverse events, especially infection and loss of bone mineral density. Guidelines recommend avoiding the use of prednisone/methylprednisolone and dexamethasone >6-8 weeks and >3 cycles, respectively. Prophylaxis is recommended in patients who undergo long CTC exposure. We analyzed if recommendations regarding CTC use are followed by practitioners experienced in ITP management after implementation of national guidelines. A nationwide, retrospective, observational study was performed by the Spanish ITP Group. Two-hundred and forty-seven ITP treatments with =12 month post-treatment follow-up were reported. CTCs were given as first-line therapy in 200/247 (81.0%) cases. Prednisone/methylprednisolone and dexamethasone were administered in 161 and 84 cases, respectively. The median (interquartile range) duration of prednisone/methylprednisolone first-line treatment was 64 (43-83) and 69 (39-144) days in newly-diagnosed (n=102) and chronic (n=32) ITP patients, respectively. Dexamethasone treatment lasted =4 cycles in 15/84 (17.9%) cases. Prophylaxis against herpes simplex virus/herpes zoster virus, Pneumocystis carinii and osteopenia was administered before/during CTC treatment to 1/187 (0.5%), 16/140 (11.4%) and 10/37 (27.0%) eligible patients, respectively. In this representative cohort, management of CTCs is far from compliance with recommendations. Actions should be taken to address this shortcoming.
Keywords: Immune thrombocytopenia; Corticosteroids; Guidelines; Treatment duration; Prophylaxis
Abbreviations
ITP: Immune Thrombocytopenia; CTCs: Corticosteroids; AEs: Adverse Events; GEPTI: Spanish ITP Group; SEHH: Spanish Society of Hematology and Hemotherapy; HSV: Herpes Simplex Virus; HZV: Herpes Zoster Virus; HBV: Hepatitis B Virus; T2DM: Type 2 Diabetes Mellitus; IQR: Interquartile Range; TMP-SMX: Trimethoprim- Sulfamethoxazole; HBsAg: Hepatitis B Surface Antigen; HBc: Core Antigen of Hepatitis B Virus; DVT: Deep Venous Thrombosis.
Introduction
Although new therapies have become available to manage immune thrombocytopenia (ITP) [1], corticosteroids (CTCs) remain the first-line of treatment [2-4]. However, the prolonged use of CTCs is associated with toxicity. More than 95% of treated ITP patients have reported adverse events (AEs) in survey studies [5]. There is consensus that the duration of this therapy has to be tightly controlled [2-4]. Prolonged immunosuppression places patients at a significant risk of severe conditions such as infection or ischemic events [6-8], and complications such as hyperglycemia or osteoporosis [9]. The risk is higher in elderly patients [8,10,11].
Safety of CTC therapy relies on two cornerstones: drug burden, associated with dose and therapy duration, and prophylaxis to prevent opportunistic infections and loss of bone mineral density. Guidelines regarding duration and dosage of treatments with prednisone, methylprednisolone or dexamethasone have long been available, and have not changed notably with successive updating [2-4,12-14]. Prophylaxis is recommended in long-term CTC treatment [15-17], although well-defined eligible patients and specific actions have only recently been included in treatment guides [2]. The Spanish ITP Group (GEPTI) of the Spanish Society of Hematology and Hemotherapy (SEHH) brings together clinicians with experience in ITP management. The GEPTI Registry is open to Spanish hematology practitioners to include patients [2,14,18-20]. Our objective was to assess if CTC-based therapies are properly and safely administered to ITP patients in a nationwide scenario.
Methods
Patients and Design
A nationwide, retrospective, multicenter, observational study was conducted. Those ITP patients who were treated with CTCs between January-2020 to April-2024 and had a minimum 12-month followup were included. All CTC-based treatments that were administered during this period were reported, regardless of whether more than one of them could have been administered to the same patient. Provided that enough information about dosing, prophylaxis and outcomes was available, treatments administered before the recruitment period were included. Inclusion criteria were age >18 years, and use of CTC treatment to manage an ITP, diagnosed according to updated ITP international consensus criteria [4]. Patients were monitored during a 12-month post-treatment follow-up period. Treatment response, relapse, toxicities and AEs were reported. The exclusion criterion was the simultaneous presence of thrombocytopenia-predisposing conditions other than ITP. The study (code 1104-N-23) was approved by the Medical Research Ethics Committees of all hospitals. All patients signed the Informed Consent Form, and their data were anonymized. The study was conducted in accordance with the Declaration of Helsinki.
Assessments
Type of CTC Treatment: CTC type, dose and duration were reported. Treatment duration in patients >75 years was also calculated [21]. In chronic ITP patients, CTC was considered as first-line treatment again when they suffered a relapse =2 years after the end of first-line treatment.
Effectiveness: Effectiveness was assessed according to the response criteria defined by the updated ITP international consensus report (Table S1) [2-4,22].
Prophylaxis: Compliance with prophylaxis according to the guidelines recently detailed by GEPTI investigators was analyzed [2]. The proportion of candidates who were administered prophylaxis against herpes simplex virus/herpes zoster virus (HSV/HZV), Pneumocystis carinii, hepatitis B virus (HBV) and/or osteopenia/ osteoporosis was calculated. The criteria to identify patients where prophylaxis would be recommended are outlined in Table S2.
Toxicities and AEs: Infections requiring hospitalization, ischemic events and bone fractures were considered as main AEs. Other toxicities were development/worsening of type 2 diabetes mellitus (T2DM), arterial hypertension, dyslipidemia, Cushing syndrome, myopathy and folliculitis.
Statistical Analysis
Discrete variables were summarized as numbers and percentages and continuous variables were described by median (interquartile range [IQR]). The chi-square test compared effectiveness and relapse among treatments. The Kruskal-Wallis test compared time to relapse among treatments. The Mann-Whitney U test compared duration of treatment according to subsequent development of infection.
Results
Baseline Features
Two-hundred and twelve ITP patients who had received treatment with CTCs between January-2020 and April-2024 were recruited (Figure 1). Seventy percent of patients had been newly diagnosed, and 59% were female. Thirty-six (17.0%) patients presented with another autoimmune disease, 26 of whom (72.2%) were female (Table 1).
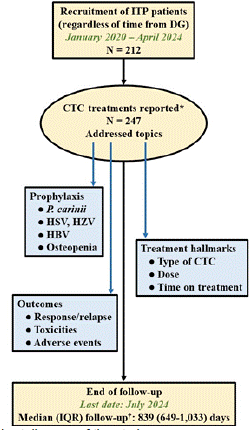
Figure 1: Flowchart diagram of the study.
*Those CTC treatments that were administered to these patients before the
recruitment period were also included, provided that enough information
about dosing, prophylaxis and outcomes was available (n=12).
†Between start of CTC treatment and last control date.
CTC, corticosteroid; DG, diagnosis; HBV, hepatitis B virus; HSV, herpes
simplex virus; HZV; herpes zoster virus; IQR, interquartile range; ITP,
immune thrombocytopenia; P. carinii, Pneumocystis carinii.
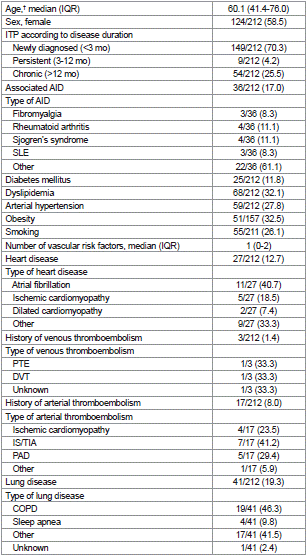
Table 1: Baseline characteristics of the study cohort (n=212)*.
CTC Treatments
Table 2 summarizes times of drug exposure and doses used with CTC-based therapies. Two-hundred and forty-seven treatments were documented. The median (IQR) follow-up period between treatment starts and the last control visit was 839 (649-1,033) days. Prednisone was chosen in more than half of the cases. Dexamethasone and methylprednisolone were used in 34.0% and 8.5% of treatments, respectively. Deflazacort was administered to 2 patients. Most patients treated with prednisone or methylprednisolone received a daily dose of 1 mg/kg. Treatment lasted >21 and >28 days in 146 (91.2%) and 139 (86.9%) out of 160 cases, respectively. When prednisone or methylprednisolone were given as first-line treatment in newly diagnosed patients, the treatment lasted for 64 (43-83) days (n=102). When one of these schemes was used in chronic ITP patients but =2 years had passed since ITP diagnosis and/or the end of the last treatment, duration was 69 (39-144) days (n=32). The median (IQR) time on prednisone/methylprednisolone in patients >75 years was 67 (48-91) days (n=55). Ten out of 12 (83.3%) patients who achieved no response to prednisone/methylprednisolone were administered this treatment for >3 weeks (median [IQR]: 62 [29-91] days).
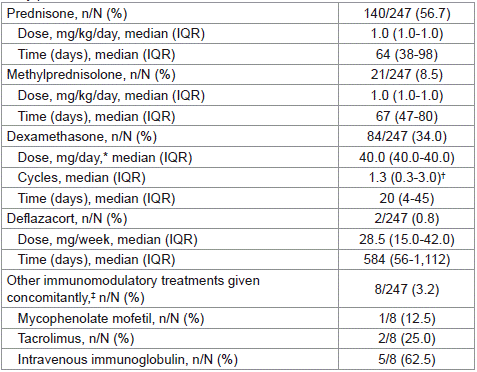
Table 2: Characteristics of all reported CTC treatments documented during the
study period.
Seventy-eight out of 84 (92.9%) patients who were treated with dexamethasone, 9 of whom were >75 years old, were administered the dose of 40 mg a day for 4 consecutive days. Doses higher than this were given in 3 cases only. Thirty-three out of 84 (39.3%) dexamethasonetreated patients received only one cycle of treatment. In 15 out of 84 (17.9%) cases, the treatment lasted =4 cycles.
Concomitant ITP treatments, mostly consisting of intravenous immunoglobulin, were administered in 3.2% of cases, because of moderate to severe bleeding or high bleeding risk according to the investigators’ criteria.
Treatment Effectiveness
Seventy-eight percent of patients responded to treatment in the overall cohort. There were no significant differences among treatments regarding effectiveness (p=0.641 when applying the chi square test to compare proportions of no response/CTC dependence versus response/complete response with prednisone, methylprednisolone or dexamethasone). Although the proportion of no responses was slightly higher with dexamethasone, the number of patients who had CTC dependence was, in turn, slightly higher with prednisone and methylprednisolone. The overall relapse rate after a median (IQR) follow-up period of 837 (640-1,003) days was close to 50%, with no remarkable differences among treatments (p=0.793, chi square test). Although the median time to relapse was shorter with methylprednisolone, there were no significant differences among treatments regarding this variable (p=0.405, Kruskal-Wallis test) (Table 3).
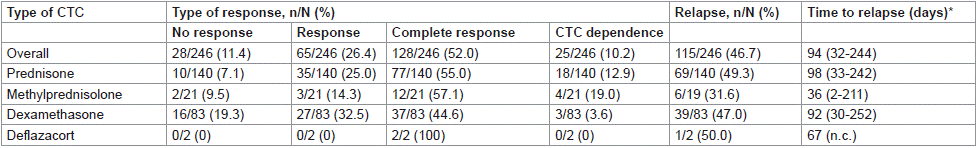
Table 3: Outcomes after CTC treatment.
Prophylaxis
The proportion of patients who were administered prophylaxis is shown in Figure 2. All treatments were included for calculations (n=247). Patients were categorized considering prophylaxis eligibility or non-eligibility according to defined criteria (Table S1).
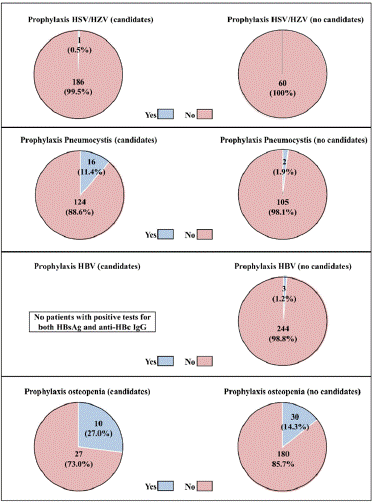
Figure 2: Prophylaxis according to guidelines to avoid CTC-related infection
and osteopenia.
The patients who were candidates for prophylaxis of Pneumocystis carinii with
TMP-SMX, HSV and HZV with acyclovir, HBV with entecavir and osteopenia
with calcium and vitamin D supplementation, were identified following the
guidelines reported in the most recent update of the recommendations for
the clinical approach to ITP developed by the GEPTI investigators.2 Then,
the proportion of patients who were administered prophylaxis was calculated
within the subgroups of candidates and no candidates for prophylaxis.
Calculations were performed considering all treatments reported during the
study period (n=247).
CTC, corticosteroid; GEPTI, Spanish ITP Working Group; HBV, hepatitis B
virus; HSV, herpes simplex virus; HZV; herpes zoster virus; ITP, immune
thrombocytopenia; TMP-SMX, trimethoprim-sulfamethoxazole.
HSV/HZV: Preventive treatment with acyclovir was reported in 1 out of the 187 cases that fulfilled criteria for HSV/HZV prophylaxis. Some patients eligible for prophylaxis may not have been detected, since information about history of infection with HSV or HZV was lacking. In the 60 CTC treatments where patients did not meet the criteria for antiviral prophylaxis, acyclovir was not used.
Pneumocystis Carinii: There were 140 cases where treatment to prevent infection with Pneumocystis carinii was recommended. Sixteen of them (11.4%) were administered trimethoprim-sulfamethoxazole (TMP-SMX). In most cases, TMP-SMX was given three times a week, dosed at 15-20 mg/kg of the trimethoprim component. This prophylaxis was also used in 2 (1.9%) of the 107 cases where such procedure was not a priority. In most cases, prophylaxis started when the first CTC dose was administered, or shortly afterwards. In 4 cases, TMP-SMX treatment started at least 4 weeks after CTC therapy start.
HBV: None of the patients, in none of the reported CTC treatments, had positive tests for both hepatitis B surface antigen (HBsAg) and antibodies against core antigen of hepatitis B virus (HBc). Nevertheless, prophylaxis with entecavir was applied to 3 patients, 1 and 2 of whom presented with a positive test for HBsAg and anti-hepatitis B surface antibody, respectively.
Osteopenia/osteoporosis: According to the guidelines, prophylaxis to minimize loss in bone mineral density was recommended in up to 37 CTC treatments, 24 and 13 of which corresponded to postmenopausal women and >50 years old males on CTC treatment for >3 months, respectively. Prophylaxis was applied in 10 (27.0%) cases, 8 female and 2 males. Simultaneous supplementation with vitamin D and calcium was co administered in 4 out of 10 cases. Prophylaxis against osteopenia was also applied in 30 out of 210 (14.3%) cases where prophylaxis would not be strictly required according to guidelines. Among 8 cases who had a history of fracture, 5 and 1 of them received prophylaxis with both vitamin D and calcium or vitamin D only, respectively. Three and 2 patients had a diagnosis of osteopenia and osteoporosis, respectively, according to densitometry procedures performed years before. All except one patient with osteopenia had been receiving prophylaxis ever since, 2 with vitamin D and calcium and 2 with vitamin D only. Osteopenic treatments, mainly protein pump inhibitors, were simultaneously administered with CTCs in 35 cases. In this subgroup, vitamin D, with or without calcium supplements, was administered in 3 out of 8 patients, who were furthermore eligible for prohylaxis, and in 5 out of 27 non-eligible patients.
AEs
Table 4 summarizes the main AEs reported during the followup period [839 (649-1,033) days, median (IQR)]. Twenty (8.1%) infections requiring hospitalization were reported among the 247 CTC treatments studied. In this subgroup, the duration of CTC treatment was not longer than that observed in the other cases: 57 (30-77) and 50 (23-81) days respectively, p=0.788, Mann-Whitney U test. When this comparison was performed according to type of treatment (prednisone/methylprednisolone or dexamethasone), differences were not observed either (not shown). One patient who had not received prophylaxis with acyclovir despite being eligible (age >60 years) had an eye infection with HZV. No infections caused by Pneumocystis carinii, HSV or HBV were reported. Thirty percent of infections were caused by SARS-CoV-2.
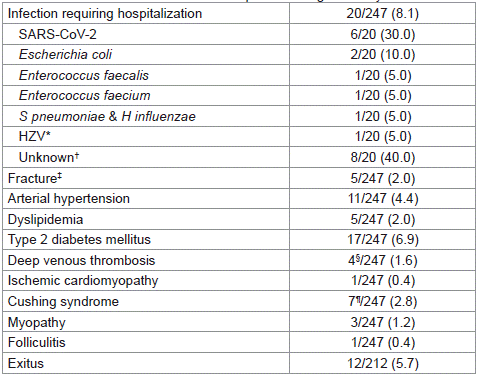
Table 4: Toxicities and adverse events reported during the study.
Once immunosuppressor therapy had finished, fracture was documented in 5 (2.0%) cases between 1 and 26 months after the last CTC dose. Four out of the 5 patients had been treated with prednisone or methylprednisolone for 46 (31-105) days, median (IQR). This period of time was no longer than that of those prednisone/ methylprednisolone-treated patients who did not report fracture, which lasted to 64 (41-94) days. Two patients who had a history of previous fracture and were receiving prophylaxis with both vitamin D and calcium reported a fracture.
In 4 (1.6%) cases, deep venous thrombosis (DVT) was reported. All were treated with dexamethasone for 20 (6-48) days, median (IQR), at a daily dose of 40 (40-77) mg, and had no history of ischemic events. One 20-year-old smoker patient with obesity and no history of heart disease or ischemic events developed ischemic cardiomyopathy and died 2.5 months after the end of a treatment with prednisone which lasted for 60 days.
In up to 17 (6.9%) cases, patients developed T2DM. The CTC had been prednisone or methylprednisolone in 15 of these. Other AEs documented in >2% of cases were arterial hypertension (4.4%) and Cushing syndrome (2.8%).
Exitus was reported in 12 (5.7%) patients. The causes are summarized in Table 5. Nine of them (75.0%) had been treated with prednisone/methylprednisolone for =8 weeks. Infection was present in 7 (58.3%) cases, either as direct or underlying cause. The median (IQR) age of patients who died of infection was 87 (68-94) years. One of them, aged 87, was being treated with methylprednisolone when the infection occurred.
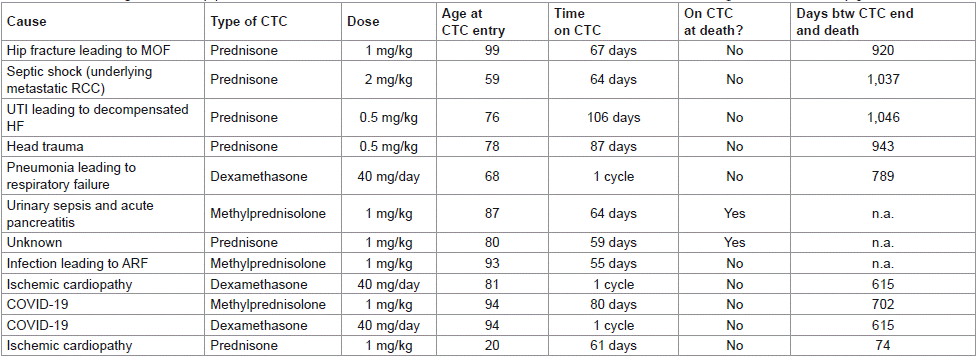
Table 5: Exitus during the follow-up period.
Discussion
We examined whether a nation-wide group of clinicians with experience in ITP management comply with the recommendations provided by guides regarding CTC-based therapy. The effectiveness of CTCs has long been described [23]. In the present study, the rate and type of response, relapse and time to relapse, were in line with those found in previous reports. More than 70% of patients responded, but half of them relapsed in the subsequent months. We consider that our cohort is representative of real-world patients and thus serves as a valid means of assessing the aforementioned objective.
The recommendation that CTC-based therapies, when standard doses are used, should never be prolonged beyond 6-8 weeks, regardless of response, had been suggested long before the treatments analyzed herein were administered [12,13,19,24]. However, in our cohort the median time of use of prednisone/methylprednisolone was >60 days, even in patients older than 75 years. In fact, the treatment lasted >3 weeks in 91% of cases. Furthermore, despite guidelines recommending CTC tapering when response is not achieved after 2 weeks, treatment was prolonged long beyond 21 days in up to 80% of those cases where no response to prednisone or methylprednisolone was reported. Dexamethasone overuse was not so frequent. Nevertheless, this treatment lasted for >3 cycles in about one-fifth of cases, even though such a practice does not provide additional benefit beyond this period of time [2-4].
CTC overuse is a common finding in ITP management. Overuse has been described in two large real-world US healthcare databases, where the median duration of treatment was 76 days, often with higher-than-recommended dosages [25]. A previous nationwide study conducted in Korea reported CTC treatment duration of >3 months in 75% of patients [26]. On the other hand, the use of CTCs as first-line of treatment in second and subsequent ITP episodes deserves attention. Treatment duration should be shorter than that recommended for newly diagnosed patients. This therapy should facilitate a rapid recovery of platelet count while serving as a bridge to second-line treatment options. However, in our series, the duration of either prednisone/methylprednisolone or dexamethasone therapy in this condition was fully comparable to that reported in newly diagnosed patients.
CTC overuse has also been observed beyond first-line therapy. As mentioned above, TPO-RAs, and other agents such as fostamatinib or rituximab, should be considered as second-line treatment [2-4]. However, in our series, CTCs were used beyond the first-line in 47 cases. CTCs were also overused in second and third-line therapy in the real-world US-based study, where these drugs were administered in >80% of cases [25].
Prophylactic strategies have been suggested in long-term CTC treatment [15-17], but they were not explicitly included in Spanish ITP management guidelines until 2023 [2]. Therefore, most treatments were administered in the absence of specific guidelines to prevent infection or osteopenia. Nevertheless, the risk of infection by opportunistic pathogens and the loss of bone mineral density have been reported to be consistently elevated among patients on CTC treatment, for whom preventive measures have long since been encouraged [27,28]. We identified those patients eligible for prophylaxis against opportunistic pathogens, such as Pneumocystis carinii, HSV, HZV, and hepatitis virus in patients with antecedents. The proportion of patients who received correct prophylaxis was always remarkably low. Nevertheless, only one patient reported infection with HZV, and no infection was reported with the other pathogens. Regarding prophylaxis against Pneumocystis carinii, it is worth noting that TMP-SMX has been associated with thrombocytopenia [29]. However, CTC-caused platelet count recovery was reported in 16 out of 18 cases where this prophylaxis was applied.
The loss of bone mineral density induced by prolonged CTC treatment was described long ago [30], and how to manage this condition has been discussed [31]. Exposure to CTCs may result in osteopenia, osteoporosis and fracture, even with low doses [32,33]. Furthermore, one frequent ITP patient profile corresponds to >50-year-old women [34]. The use of bisphosphonates has been described for managing osteoporosis in adult ITP patients [9]. However, few studies address bone prophylaxis in ITP patients undergoing CTC treatment. Indeed, supplementation with vitamin D and calcium is the gold standard to prevent bone loss [35]. We identified risk profiles according to the recently reported guidelines [2], and observed than <30% of eligible patients were administered vitamin D and/or calcium prior to or while on CTC treatment. In the case of postmenopausal women, only 7 out of 22 of those who were to be administered CTCs during >90 days received bone prophylaxis. Conversely, prophylaxis was applied to most patients with previous fracture or a diagnosis of osteopenia/osteoporosis. Our findings highlight the risk associated with a history of fracture(s). Almost half the fractures reported after CTC exposure occurred in patients who fitted this profile. In these cases, the fracture occurred more than one year after treatment suspension and even though patients were receiving continuous prophylaxis with vitamin D and calcium.
Other AEs were reported. The most common infectious disease leading to hospitalization was COVID-19. Whether or not CTC treatment increases the risk of SARS-CoV-2 infection remains controversial [36,37]. Importantly, more than half of the deaths were directly or indirectly related with infection. Most patients who died of infection had been treated with CTCs during a period of time longer than recommended, and one of them was on treatment at the moment of death. Infection occurred predominantly in the elderly, which highlights the need to be compliant when using CTC therapy to treat these patients.
Five ischemic events were documented. The four reported DVT episodes occurred in dexamethasone-treated patients. Oral CTCs have been associated with the risk of first and recurrent venous thromboembolic events [38]. Nevertheless, a recent randomized trial found no concerning thrombotic risk when adult, newly diagnosed ITP patients were treated with standard prednisone doses or pulsed high-dose dexamethasone [39].
Most cases of Cushing syndrome occurred in patients treated with prednisone or methylprednisolone. This AE is associated with therapy duration, which is usually longer with the mentioned agents than with dexamethasone [40]. The occurrence of hypertension and hyperglycemia, which was documented in <10% of treatments, has been widely reported [5,41]. The hyperglycemic effects manifest rapidly, often within hours of administration [42], and may be a cause of concern because healthcare professionals often underestimate their consequences [43].
Our study has limitations inherent to its retrospective design. Furthermore, the occurrence of AEs other than those typically linked to CTC administration cannot be ruled out.
Conclusion
In summary, we analyzed how professionals experienced in ITP management administer CTC-based therapies, and found causes for concern. Treatments are often prolonged beyond recommendations, thus putting patients at risk of severe immunosuppression-related side effects. Prophylactic measures against opportunistic pathogens or loss of bone mineral density are only taken in a small proportion of eligible patients. The recently released guidelines endorsed by the GEPTI investigators may contribute to improve safety during CTCbased ITP management. Studies are ongoing to answer this question.
References
- Mingot-Castellano ME, Bastida JM, Caballero-Navarro G, Entrena Ureña L, González-López TJ, González-Porras JR, et al, on behalf of the GEPTI. Novel therapies to address unmet needs in ITP. Pharmaceuticals (Basel). 2022; 15: 779.
- Mingot-Castellano ME, Canaro Hirnyk M, Sánchez-González B, álvarez- Román MT, Bárez-García A, Bernardo-Gutiérrez á, et al. Spanish Immune Thrombocytopenia Group (GEPTI). Recommendations for the Clinical Approach to Immune Thrombocytopenia: Spanish ITP Working Group (GEPTI). J Clin Med. 2023; 12: 6422.
- Neunert C, Terrell DR, Arnold DM, Buchanan G, Cines DB, Cooper N, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019; 3: 3829-3866.
- Provan D, Arnold DM, Bussel JB, Chong BH, Cooper N, Gernsheimer T, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019; 3: 3780-3817.
- Cuker A, Liebman HA. Corticosteroid overuse in adults with immune thrombocytopenia: Cause for concern. Res Pract Thromb Haemost. 2021; 5: e12592.
- Moulis G, Palmaro A, Sailler L, Lapeyre-Mestre M. Corticosteroid Risk Function of Severe Infection in Primary Immune Thrombocytopenia Adults. A Nationwide Nested Case-Control Study. PLoS One. 2015; 10: e0142217.
- Moulis G, et al. Infections in non-splenectomized persistent or chronic primary immune thrombocytopenia adults: risk factors and vaccination effect. J Thromb Haemost. 2017; 15: 785-791.
- Ruggeri M, Tosetto A, Palandri F, Polverelli N, Mazzucconi MG, Santoro C, et al. Gruppo Italiano Malattie EMatologiche dell'Adulto (GIMEMA) Anemia and Thrombocytopenias Working Party. GIMEMA Study ITP0311. Thrombotic risk in patients with primary immune thrombocytopenia is only mildly increased and explained by personal and treatment-related risk factors. J Thromb Haemost. 2014; 12: 1266-1273.
- Mannering N, Hansen DL, Moulis G, Ghanima W, Pottegård A, Frederiksen H. Risk of fractures and use of bisphosphonates in adult patients with immune thrombocytopenia-A nationwide population-based study. Br J Haematol. 2024; 204: 1464-1475.
- Crickx E, Mahévas M, Michel M, Godeau B. Older Adults and Immune Thrombocytopenia: Considerations for the Clinician. Clin Interv Aging. 2023; 18: 115-130.
- Cunningham JM, Kessler CM. Immune thrombocytopenia in the elderly: immunosenescent and clinical diversity. Br J Haematol. 2022; 196: 1134- 1136.
- Neunert C, Lim W, Crowther M, Cohen A, Solberg L Jr, Crowther MA; American Society of Hematology. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011; 117: 4190-4207.
- Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, Bussel JB, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010; 115: 168-186.
- Mingot-Castellano ME, Román MTá, Fernández Fuertes LF, González-López TJ, Guinea de Castro JM, Jarque I, et al. Management of Adult Patients with Primary Immune Thrombocytopenia (ITP) in Clinical Practice: A Consensus Approach of the Spanish ITP Expert Group. Adv Hematol. 2019; 2019: 4621416.
- Malpica L, Moll S. Practical approach to monitoring and prevention of infectious complications associated with systemic corticosteroids, antimetabolites, cyclosporine, and cyclophosphamide in nonmalignant hematologic diseases. Hematology Am Soc Hematol Educ Program. 2020; 2020: 319-327.
- Hill QA, Stamps R, Massey E, Grainger JD, Provan D, Hill A; British Society for Haematology. The diagnosis and management of primary autoimmune haemolytic anaemia. Br J Haematol. 2017; 176: 395-411.
- Jäger U, Barcellini W, Broome CM, Gertz MA, Hill A, Hill QA, et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: Recommendations from the First International Consensus Meeting. Blood Rev. 2020; 41: 100648.
- Pascual-Izquierdo C, Sánchez-González B, Canaro-Hirnyk MI, García-Donas G, Menor-Gómez M, Gil-Fernández JJ, et al. Spanish ITP Group (GEPTI) of the Spanish Society of Hematology and Hemotherapy (SEHH). Avatrombopag in immune thrombocytopenia: A real-world study of the Spanish ITP Group (GEPTI). Am J Hematol. 2024; 99: 2328-2339.
- Lozano ML, Sanz MA, Vicente V, Grupo Español de PTI (GEPTI). Guidelines of the Spanish ITP Group for the diagnosis, treatment and follow-up of patients with immune thrombopenia. Med Clin (Barc). 2021; 157: 191-198.
- Mingot-Castellano ME, Alcalde-Mellado P, Pascual-Izquierdo C, Perez Rus G, Calo Pérez A, Martinez MP, et al. on behalf GEPTI (Grupo Español de Trombocitopenia Inmune). Incidence, characteristics and clinical profile of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection in patients with pre-existing primary immune thrombocytopenia (ITP) in Spain. Br J Haematol. 2021; 194: 537-541.
- Michel M, Rauzy OB, Thoraval FR, Languille L, Khellaf M, Bierling P, et al. Characteristics and outcome of immune thrombocytopenia in elderly: results from a single center case-controlled study. Am J Hematol. 2011; 86: 980-984.
- Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009; 113: 2386-2393.
- Samson M, Fraser W, Lebowitz D. Treatments for Primary Immune Thrombocytopenia: A Review. Cureus. 2019; 11: e5849.
- Pizzuto J, Ambriz R. Therapeutic experience on 934 adults with idiopathic thrombocytopenic purpura: Multicentric Trial of the Cooperative Latin American group on Hemostasis and Thrombosis. Blood. 1984; 64: 1179- 1183.
- Cuker A, Tkacz J, Manjelievskaia J, Haenig J, Maier J, Bussel JB. Overuse of corticosteroids in patients with immune thrombocytopenia (ITP) between 2011 and 2017 in the United States. EJHaem. 2023; 4: 350-357.
- Lee JY, Lee JH, Lee H, Kang B, Kim JW, Kim SH, et al. Epidemiology and management of primary immune thrombocytopenia: a nationwide populationbased study in Korea. Thromb Res. 2017; 155: 86-91.
- Youssef J, Novosad SA, Winthrop KL. Infection Risk and Safety of Corticosteroid Use. Rheum Dis Clin North Am. 2016; 42: 157-176.
- Anastasilaki E, Paccou J, Gkastaris K, Anastasilakis AD. Glucocorticoidinduced osteoporosis: an overview with focus on its prevention and management. Hormones (Athens). 2023; 22: 611-622.
- Hashimoto M, Hiraiwa M, Uchitani K, Ueda M, Tanaka M, Nishiyama N, et al. Sulfamethoxazole-trimethoprim for pneumocystis pneumonia prophylaxis, causes of discontinuation and thrombocytopenia observed during administration: A single-center retrospective study. J Infect Chemother. 2024; 30: 141-146.
- Hahn TJ. Corticosteroid-induced osteopenia. Arch Intern Med. 1978; 138 Spec No: 882-885.
- Kobza AO, Herman D, Papaioannou A, Lau AN, Adachi JD. Understanding and Managing Corticosteroid-Induced Osteoporosis. Open Access Rheumatol. 2021; 13: 177-190.
- Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007; 18: 1319- 1328.
- Naganathan V, Jones G, Nash P, Nicholson G, Eisman J, Sambrook PN. Vertebral fracture risk with long-term corticosteroid therapy: prevalence and relation to age, bone density, and corticosteroid use. Arch Intern Med. 2000; 160: 2917-2922.
- Mannering N, Hansen DL, Pottegard A, Frederiksen H. Survival in adult patients with chronic primary and secondary immune thrombocytopenia: a population-based study. Transfusion. 2023; 63: 415-426.
- Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997; 337: 670.
- Magder LS, Fava A, Goldman D, Petri MA. Does the use of corticosteroids and immunosuppressants increase the risk of COVID-19 infection among people with systemic lupus erythematosus? Lupus Sci Med. 2023; 10: e000961.
- Sechrist SJ, Tang E, Arnold BF, Acharya NR. Association between immunosuppressive medications and COVID-19 hospitalisation and death: a retrospective cohort study. BMJ Open. 2024; 14: e087467.
- Orsi FA, Lijfering WM, Geersing GJ, Rosendaal FR, Dekkers OM, le Cessie S, et al. Glucocorticoid use and risk of first and recurrent venous thromboembolism: self-controlled case-series and cohort study. Br J Haematol. 2021; 193: 1194-1202.
- Mazzucconi MG, Rodeghiero F, Avvisati G, De Stefano V, Gugliotta L, Ruggeri M, et al. Prednisone vs high-dose dexamethasone in newly diagnosed adult primary immune thrombocytopenia: a randomized trial. Blood Adv. 2024; 8: 1529-1540.
- Nieman LK, Castinetti F, Newell-Price J, Valassi E, Drouin J, Takahashi Y, et al. Cushing syndrome. Nat Rev Dis Primers. 2025; 11: 4.
- Barker HL, Morrison D, Llano A, Sainsbury CAR, Jones GC. Practical Guide to Glucocorticoid Induced Hyperglycaemia and Diabetes. Diabetes Ther. 2023; 14: 937-945.
- Schneiter P, Tappy L. Kinetics of dexamethasone-induced alterations of glucose metabolism in healthy humans. Am J Physiol. 1998; 275: E806-E813.
- Cho JH, Suh S. Glucocorticoid-Induced Hyperglycemia: A Neglected Problem. Endocrinol Metab (Seoul). 2024; 39: 222-238.