
Research Article
Austin Therapeutics. 2015; 2(2): 1022.
Fabrication and Characterization of Hexagonal Mesoporous Silica (HMS) for Impregnating Lornoxicam as a Drug Carrier
Dange VU, Vibhute SK, Kendre PN and Pande VV*
Department of Pharmaceutics, Sanjivani College of Pharmaceutical Education and Research, India
*Corresponding author: Vishal Pande, Department of Pharmaceutics, Sanjivani College of Pharmaceutical Education and Research, Kopargaon, Maharashtra, India
Received: November 04, 2015; Accepted: December 04, 2015 Published: December 07, 2015
Abstract
The aim of the present work was fabrication and characterization of Mesoporous Silica Barbara Amorphous-15 (SBA -15) as a template to improve dissolution behavior and ultimately bioavailability of Lornoxicam (LX), it is a non steroidal anti-inflammatory drug belongs to Biopharmaceutical Classification System - Class II i.e. Low Solubility and High Permeability. The 2D hexagonal mesoporous silica SBA-15 was synthesized using Pluronic P-123 and TEOS in acidic condition. The synthesized Hexagonal Mesoporous Silica SBA-15 has large specific surface area (381.58m2/g) with an average particle size 13 nm. The impregnation of Lornoxicam in SBA-15 was determined by calculating the drug loading and it was found to be 52.16%. The plain SBA-15 and LX loaded SBA-15 samples were characterized by FT-IR, Scanning and Transmission Electron Microscopy, X-Ray Diffraction and Mercury Porosimetry. Dissolution profile of LX loaded SBA-15 shows improvement in drug release (i.e. 76%) as compared to crystalline drug LX (41%) within 180min in phosphate buffer (pH 6.8). The TEM provide direct evidence that the material comprises well-ordered hexagonal arrays of mesopores and a 2D hexagonal structure. Hence the Hexagonal mesoporous silica template can be used as a platform to fabricate novel drug delivery system by impregnating water insoluble drugs.
Keywords: Mesoporous silica; Lornoxicam; Dissolution; Bioavailability; Inclusion
Introduction
Lornoxicam (LX) is Non Steroidal Anti-Inflammatory Drug (NSAID) belongs to Oxicam class with Biopharmaceutical Classification System Class II [1,2] i.e. LX is highly permeable through biological membrane but exhibit low aqueous solubility. As compared to other Oxicams, LX has short elimination half life (3-5h), potent anti inflammatory, analgesic and antipyretic activity [3]. It is potent inhibitor of prostaglandin synthesis and reduced risk of side effects associated with Gastrointestinal Tract (GIT). LX has low aqueous solubility and poor dissolution in GI fluid which is rate limiting step in absorption through biological membrane [4,5]. As a consequence, many approaches [6-9] have been proposed to improve dissolution performance of LX which ultimately enhances bioavailability. The aim of present work was to fabricate hexagonally ordered mesoporous material SBA-15 as a drug carrier to improve dissolution performance of poorly water soluble drug.
Mesoporous silica such as MCM-41(Mobile Crystalline Matter) and SBA-15 (Santa Barbara Amorphous) are comprised of honeycomb like porous network which are capable to adsorb or entrap or encapsulate large amount of drug molecules [6,10]. Tunable particle size from 50 to 300nm [11] , high hydrothermal stability, high surface area (<900m2/g) and pore volume (0.9cm3/g) and thin pore wall as compared to MCM-41 make them potentially suitable and advantageous for efficient drug delivery system [10].
Recent studies of mesoporous material demonstrated that the drugs lose their crystallinity when they are deposited or entrapped into surface of mesoporous silicates, which improved their dissolution rate [12-14]. Heikkila and co-workers investigated the cytotoxic effects of Ordered Mesoporous Silica MCM-14 and SBA-15 microporous on undifferentiated human colon carcinoma cell line, considering the feasibility of using mesoporous silicate materials in oral drug formulations. The MCM-41 and SBA-15 microparticles caused cytotoxic effects on the Caco-2 cells, at most tested concentrations and incubation times. The effects on the cells included weakened cell membrane integrity, diminished cell metabolism and increased apoptotic signaling. They suggested to control the microparticle size of MCM-41 and SBA-15 used in an oral formulation and to avoid the use of the smallest microparticles [15].
The synthesis of mesoporous silica is based on a sol-gel process, the particles size and volume pores can be tunable during synthesis process [16]. The pore size can be tailored by using swelling agents such as Trimethyl benzene, hexane and ethanol by penetrating the cores of surfactant micelles to enlarge the size [11,17-19]. The SBA -15 materials is easily synthesized by non ionic triblock copolymer Pluronic P-123 as a structure directing agent and Tetraethyl Orthosilicate as an inorganic silica precursor in strongly acidic condition [20]. As compared to MCM-41, the SBA-15 has higher hydrophilic character due to high amount of silanol groups that can interact with many compounds forming hydrogen bonds. Since SBA- 15 can adsorbs drug molecules on its large surface by forming weak interactions which can be easily broken in biological fluids and allow rapid drug release [20,21].
Materials and Methods
Materials
All the chemicals used in this work were of AR grade. The Pluronic P-123 and Tetraethyl Orthosilicate (TEOS) were purchased from Research Lab Fine Chem Industries Mumbai. The drug Lornoxicam was obtained as a gift sample from Glenn mark India Ltd, Nasik. Ethanol and Hydrochloric Acid were obtained from Yarrow hem Pvt. Ltd. Mumbai.
Method of Synthesis of Hexagonal Mesoporous silica SBA - 15
Initially 4g of Pluronic P123 was dissolved in 120ml of 0.2M HCl and 30ml of deionized water and stirred at 25oC on magnetic stirrer for 2h. Then 8.5g of tetraethyl Orthosilicate was added in drop wise manner into dissolved solution of Pluronic P123 and stirred at 35oC for 20h. This solution kept at 80oC without stirring for 48h. The final product was washed and filtered with water and ethanol. The washed product dried at 50- 60oC and finally claimed at 700oC for 6h in muffle furnace to remove the polymer from the pores of synthesized SBA-15 [11].
Characterization
Infrared spectroscopy
The compatibility study of SBA-15 and drug was carried out by IR spectroscopy. FT-IR spectra were recorded at ambient temperature using FTIR 8400-S, Shimadzu (Japan) over wave number range of 4000-400 cm-1. The samples were prepared by grinding with dry KBr powder [22].
Scanning Electron Microscopy (SEM)
The morphology of synthesized SBA-15 was examined by Scanning Electron Microscopy (SEM; JEOL; 5400; Tokyo; Japan). The samples were loaded on studs and applied fine gold coating with gold for 5min at 10mA ion current under a pressure of 0.1 Torr using ion sputter. The coated pellets were scanned and the micrographs were examined [23].
Transmission Electron Microscopy (TEM)
The hexagonal porous structure of synthesized SBA-15 was characterized by using Technai G2 20 (Philips, Holland). The sample was prepared on 300 mesh copper grid and scanned using TEM; operated at 200 kV [24].
Mercury porosimetry
The pore size, particle size distribution and surface area were determined by the mercury porosimetry technique by using the Pascal Mercury Porosimeter (Pascal 440; Thermo Electron Corporation; Italy) The intruded mercury volume was referred to the sample mass unit and was plotted versus applied pressure in Mpa. The sample was degassed in an oven at 120oC for 1h [25].
Powder X-ray diffraction
Powder X-ray Diffraction analysis was performed with a D5000 diffractometer (40 kV; 40 mA; Siemens/Bruker) using Cu-Ka radiation (l = 0.154 nm) with scan speed 5 deg/min. About 500 mg sample was ground in a mortar and put on silicon sample holders with zero background prior to analysis [26].
Loading of Lornoxicam SBA-15 and quantification of the drug
The incipient wetness impregnation method used for loading of LX in SBA-15. The drug (500mg) was dissolved in 10ml methanol. The SBA-15 sample (500mg) was added into 10 ml of methanol solution and kept on magnetic stirring. The dissolved solution of LX in methanol was successively added to methanolic solution of SBA- 15. The mixture was kept under magnetic stirring for 48h at 25oC. Finally the methanol was removed by evaporation leaving LX loaded SBA-15 material. This final product was kept in desiccator to remove moisture completely [19]. The Lornoxicam loaded SBA-15 samples was determined by the UV spectrophotometer. The 10 mg of the LX: SBA-15 was dissolved in 100ml of methanol and sonicated for 30 minutes. The sample was filtered through cellulose membrane and the amount of the drug was determined by UV spectrophotometer (UV1650, Shimadzu, Japan). The drug calibration curve was previously prepared in methanol solution (λ=380). The drug loading efficiency was calculated by using following [27].
% D.E. = (Actual drug loaded / Theoretical drug loaded) X 100
In vitro drug release study
The dissolution rate of drug loaded silica and free drug was determined by using USP Type II dissolution apparatus (ELECTROLAB USP TDT - 08L) with 900ml of phosphate buffer (pH 6.8) dissolution media at 100rpm, under sink condition at 37oC. A weighed quantity of pure drug LX and LX loaded silica material containing equivalent mass of pure drug was added into dissolution media. The aliquots were withdrawn at defined time interval and also replaced the equal volume of fresh dissolution medium to maintain sink condition. The withdrawn samples were filtered and absorbance was taken on UV Spectrophotometer at 380nm λ max [28].
Results and Discussion
FT-IR study
IR Spectra of pure drug LX (A), LX loaded SBA-15 (B) and SBA- 15 (Figure 1).
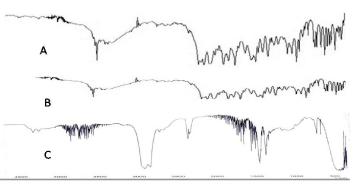
Figure 1: IR Spectra of pure drug LX (A), LX loaded SBA-15 (B) and SBA-15.
The major peaks observed in IR spectrum of mesoporous silica SBA-15 are shown in (Table 1, 2 and 3) [29].
Sr. No.
Functional Groups
Peaks observed in SBA-15 (cm-1)
1
S=O
1124
2
C=O
1646
3
N-H
2923
Table 1: Major peaks observed in LX.
Sr. No.
Functional Groups
Peaks observed in SBA-15 (cm-1)
01
Si-O-Si stretching
1106
03
C-C stretch
1500
05
C–H stretch
2850
06
O-H stretching
3200
07
Si -OH
3750
Table 2: Major peaks observed in SBA-15.
Sr. No.
Functional Groups
Peaks observed in SBA-15 (cm-1)
01
S=O
1106
03
C=O
1650
05
N-H
2923
06
O-H stretching
3200
07
Si -OH
3650
Table 3: Major peaks observed in LX loaded mesoporous silica SBA-15.
Scanning Electron Microscopy (SEM)
The SEM image (Figure 2) shows the morphology of synthesized SBA-15 samples with many aggregated particles. In one of previous study investigators found similar morphology [30].
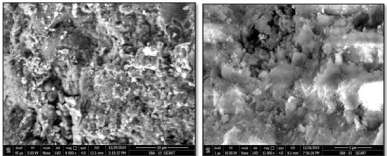
Figure 2: SEM images of SBA-15.
Transmission Electron Microscopy (TEM)
The TEM images of the mesoporous silica SBA-15 (Figure 3) reveal that the pores of the SBA-15 particles exhibits honey comb like 2D hexagonal and well defined inter connected mesoporous structure. Zhu et al. [31] have similar results with more magnification.
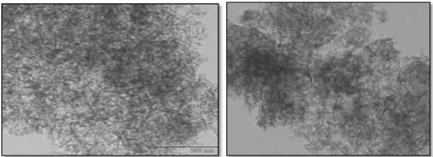
Figure 3: TEM images of the Mesoporous Silica.
Mercury porosimetry
The porosity of material can affect it physical nature and ultimately its use. According to IUPAC classification of adsorption isotherms; Figure 4 shows Type IV isotherm having well defined H1 hysteresis loop which are typical for mesoporous materials. The presence of H1 hysteresis type confirms that they have open ended cylindrical mesoporous. The fact that both isotherms have hysteresis loop with sharp branches indicates narrow pore size distribution [28]. The characteristic feature of the Type IV isotherm is its hysteresis loop which is associated with capillary condensation taking place in mesopores and it gives Type I Hysteresis loop i.e. agglomerates (assembly of particles rigidly joined together) of cylindrical pore channels [32]. By measuring the volume of mercury that intrudes into the sample material with each pressure change, the volume of pores in the corresponding size class is known. The Figure 4 indicates pore size distribution of SBA-15 i.e. pore diameter (micron) vs. Cumulative Volume (cc/g). By mercury porosimetry analysis of SBA- 15; Total Cumulative Volume, Total Specific Surface Area, Average Pore Diameter and Total porosity (%) were found to be 0.74cm3/g, 381.58m2/g, 13nm and 94.88% resp.
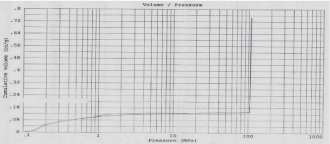
Figure 4: Mercury intrusion-extrusion curve.
The X-Ray diffraction analysis (XRD)
The XRD spectrum of pure drug Lornoxicam (Figure 5) shows high intense peak of 2θ value at 21.58°, 23°, 24.76° and 25.46° with intensity 2561, 2179, 5862 and 1733 resp. which indicates that the pure drug LX is in crystalline state. Whereas the LX loaded SBA-15 XRD spectrum (Figure 6) shows 2θ value at 21.58°, 23°, 24.76° and 25.46° with intensity 913, 720, 1740 and 230 resp. From this two spectra it is concluded that LX loaded SBA-15 shows less intense peak than pure drug LX. Decrease in intensity of characteristic peaks can be correlated to decrease in crystalline nature of drug [31,33].
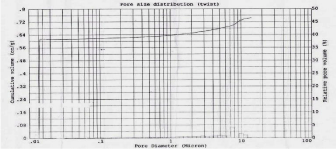
Figure 5: Pore size distribution of SBA-15.
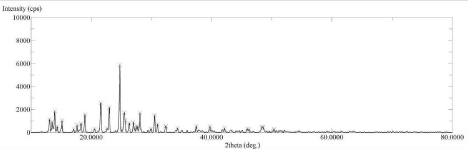
Figure 6: XRD Spectra of Lornoxicam.
Drug quantification/ Drug loading efficiency
The Drug Loading Efficiency was determined by UV spectroscopic method. The drug loading efficiency is the ratio of actual drug loaded to the theoretical drug loaded into mesoporous material [31]. The drug Loading Efficiency was found to be 52.16%.
In vitro dissolution data
The mesoporous silica acts as a drug carrier or template to enhance dissolution rate of poorly soluble drugs in biological fluids. Generally after immersing the drug loaded mesoporous silica in biological media the following steps take place: (1) Absorption of aqueous medium into mesoporous material by capillary action (2) Dissolution of drug molecules into the release medium inside the pores (3) Diffusion of drug molecules out of the meso pores owing to concentration gradient (4) Diffusion and convectional transport of drug molecules in the release medium [16]. The dissolution data of LX and LX: SBA-15 is shown in Figure 7. As compared to pure drug LX, the LX: SBA-15 shows greater release in 180min. The release of pure LX is 41%; whereas LX: SBA-15 shows 76% drug release within same period of time. The improved dissolution of LX is due to inclusion of the drug (LX) within the pore channels of SBA-15 (Figure 8) which resulted in an efficient particle size reduction and the decrease in crystalline nature of LX.
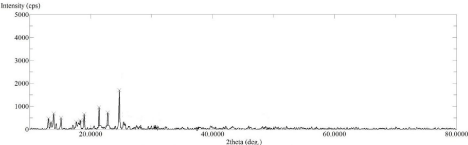
Figure 7: XRD Spectra of LX loaded SBA-15.
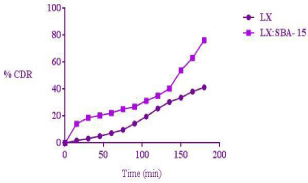
Figure 8: Drug release profile of LX and LX: SBA-15.
Conclusion
In the present work the hexagonal mesoporous silica template was synthesized successfully to enhance the dissolution behaviour of poorly water soluble drug Lornoxicam. Mesoporous silica materials may have further application potential, such as a carrier for efficient drug delivery platform for poorly soluble drug. The 2D hexagonal mesoporous silica particles have up to 52% drug loading capacity. The dissolution release profile of LX loaded SBA-15 sample compared with pure crystalline LX. It can be concluded that improvement in dissolution behaviour was related with reduction of crystalline nature by mesoporous material SBA-15.
References
- Balfour JA, Fitton A, Barradell LB. Lornoxicam. A review of its pharmacology and therapeutic potential in the management of painful and inflammatory conditions. Drugs. 1996; 51: 639-657.
- Bansal AK, Pande V. Development and Evaluation of Dual Cross-Linked Pulsatile Beads for Chronotherapy of Rheumatoid Arthritis. J Pharm (Cairo). 2013; 2013: 906178.
- Dandagi PM, Desai GA, Gadad AP, Desai VB. Formulation and evaluation of nanostructured lipid carrier of lornoxicam. Int. J. Pharm. Pharm. Sci. 2014; 6: 73-77.
- Patil AL, Patil SS. Modification of physicochemical characteristics of Lornoxicam using cyclodextrine as modulator. Int J Pharm Tech Res. 2012; 4: 201-207.
- Srivastava D, Srivastava S, Nimisha, Prajapat V. Liquisolid Technique for enhancement of dissolution properties of Lornoxicam. Indo Glo J Pharm Sci. 2014; 4: 81-90.
- Chemate S, Chowdary K. A factorial study on enhancement of solubility and dissolution rate of lornoxicam employing hp-ß-cyclodextrin and surfactants. Int J Pharm Sci and Res. 2012; 3: 2252-2256.
- Sri Raviteja N, Nagalatha D, Sowmya B, Prasada Rao V, Renuka Tejasvi P. Improving the solubility and dissolution rate of poorly water soluble Lornoxicam by solid dispersion method and to compare effectiveness of hydrophilic polymers. Global Journal of Pharmacology. 2014; 8: 601-608.
- Yadav N, Chhabra G, Pathak K. Enhancement of solubility and dissolution rate of a poorly water soluble drug using single and double hydrophilization approach. Int J Pharm Pharm Sci. 2012; 4: 395-405.
- Kulkarni U, Raghavendra Rao N. Design and development of multi-application Lornoxicam fast dissolving tablets by novel drug - drug solid dispersion technique with Ranitidine. J Pharm Sci Biosci Res. 2012; 2: 103-108.
- Dange V, Pande V, Vibhute S. Inventi Rapid: Pharmaceutical Process Development. 2015; 2015: 1-8.
- Panage S, Pande V, Patil S, Borbane S. Design and synthesis of mesoporous silica for inclusion of poorly water soluble drug sertaconazole nitrate as a drug delivery platform. Der Pharmacia Lettre. 2014; 6: 159-168.
- Van Speybroeck M, Mols R, Mellaerts R, Thi TD, Martens JA, Van Humbeeck J, et al. Combined use of ordered mesoporous silica and precipitation inhibitors for improved oral absorption of the poorly soluble weak base itraconazole. Eur J Pharm Biopharm. 2010; 75: 354-365.
- Heikkilä T, Salonen J, Tuura J, Kumar N, Salmi T, Murzin DY, et al. Evaluation of mesoporous TCPSi, MCM-41, SBA-15, and TUD-1 materials as API carriers for oral drug delivery. Drug Deliv. 2007; 14: 337-347.
- Van Speybroeck M, Barillaro V, Thi TD, Mellaerts R, Martens J, Van Humbeeck J, et al. Ordered mesoporous silica material SBA-15: a broad-spectrum formulation platform for poorly soluble drugs. J Pharm Sci. 2009; 98: 2648-2658.
- Heikkilä T, Santos HA, Kumar N, Murzin DY, Salonen J, Laaksonen T, et al. Cytotoxicity study of ordered mesoporous silica MCM-41 and SBA-15 microparticles on Caco-2 cells. Eur J Pharm Biopharm. 2010; 74: 483-494.
- Xu W, Riikonen J, Lehto VP. Mesoporous systems for poorly soluble drugs. Int J Pharm. 2013; 453: 181-197.
- Corma A, Kan Q, Navarro MT, Perez-Pariente J, Rey F. Synthesis of MCM- 41 with different pore diameters without addition of auxiliary organics. Chem Mat. 1997; 9: 2123-2126.
- Kruk M, Cao L. Pore size tailoring in large-pore SBA-15 silica synthesized in the presence of hexane. Langmuir. 2007; 23: 7247-7254.
- Boissiere C, Martines MA, Tokumoto M, Larbot A, Prouzet E. Mechanisms of pore size control in MSU-X mesoporous silica. Chem. Mat. 2003; 15: 509-515.
- Ambrogi V, Perioli L, Pagano C, Marmottini F, Ricci M, Sagnella A, et al. Use of SBA-15 for furosemide oral delivery enhancement. Eur J Pharm Sci. 2012; 46: 43-48.
- Ma Y, Qi L, Ma J, Wu Y, Liu O, Cheng H. Large-pore mesoporous silica Spheres: synthesis and application in HPLC. Colloids Surf A. 2003; 229: 1-8.
- Ramli Z, Saleh R. Preparation of ordered mesoporous alumina particles via simple precipitation method. J. Funda. Sci. 2008; 4: 435-443.
- Hartmann S, Sachse A, Galarneau A. Challenges and strategies in the synthesis of mesoporous alumina powders and hierarchical alumina monoliths. Mat. 2012; 5: 336-349.
- Liu J, Huang F, Liao X, Shi B. Synthesis of thermally stable mesoporous alumina by using bayberry tannin as template in aqueous system. Bull Korean Chem Soc. 2013; 34: 2651-2656.
- Giesche H. Mercury Porosimetry: A general practical overview. Part Part Syst Charact. 2006; 23: 1-9.
- Ishii Y, Nishiwaki Y, Al-zubaidi A, Kawasaki S. Pore Size Determination in Ordered Mesoporous Materials Using Powder X-ray Diffraction. J Phys Chem C. 2013; 117: 18120-18130.
- Borbane SA, Pande VV, Vibhute SK, Kendre PN, Dange VU. Design and Fabrication of Ordered Mesoporous Alumina Scaffold for Drug Delivery of Poorly Water Soluble Drug. Austin Therapeutics. 2015; 2: 1-5.
- Pavia D, Lampman GM, Kriz GS, Vyvyan JR. Spectroscopy. Cengage Learning. Indian Edition. 2007; 40-87.
- Mellaerts R, Mols R, Jammaer JA, Aerts CA, Annaert P, Van Humbeeck J, et al. Increasing the oral bioavailability of the poorly water soluble drug itraconazole with ordered mesoporous silica. Eur J Pharm Biopharm. 2008; 69: 223-230.
- Zhu W, Wan L, Zhang C, Gao Y, Zheng X, Jiang T, et al. Exploitation of 3D face-centered cubic mesoporous silica as a carrier for a poorly water soluble drug: influence of pore size on release rate. Mater Sci Eng C Mater Biol Appl. 2014; 34: 78-85.
- Sing KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Rouquérol J, et al. Reporting physiosorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl Chem. 1985; 57: 603-619.
- Wang S. Ordered mesoporous materials for drug delivery. Micro Meso Mat. 2009; 117: 1-9.
- Ahern RJ, Hanrahan JP, Tobin JM, Ryan KB, Crean AM. Comparison of fenofibrate-mesoporous silica drug loading processes for enhanced drug delivery. Euro J Pharm Sci. 2013; 50: 400-409.