Research Article
Austin J Surg. 2019; 6(4): 1168.
Prognostic Significance and Predictive Factors of Lymph Node Metastasis in Resectable Intrahepatic Cholangiocarcinoma
Navarro JG, Rho SY, Lee JH, Choi GH*, Han DH, Kim SK and Choi JS
Department of Surgery, Yonsei University College of Medicine, Korea
*Corresponding author: Choi GH, Department of Surgery, Yonsei University College of Medicine, Yonseiro, Seodaemun-gu, Korea
Received: December 20, 2018; Accepted: February 08, 2019; Published: February 15, 2019
Abstract
Background: Lymph node metastasis portend a worse prognosis following resection of Intrahepatic Cholangiocarcinoma (ICC); however, the role of lymphadenectomy is still controversial and not routinely performed. In this study, we investigated the oncologic significance and predictive factors of lymph node metastasis in patients with ICC, which can potentially influence decision making for the patient’s oncologic benefit with lymphadenectomy.
Materials and Methods: We retrospectively reviewed patients who underwent curative-intent surgery for intrahepatic cholangiocarcinoma between 2001 and 2014. The data was collected from the electronic medical record database of the hospital.
Results: A total of 168 patients were included in the study. Multivariable analysis revealed that: age >65 years, lymph node metastasis, tumor size >5 cm and periductal infiltrating tumor morphology, were independently associated with poor OS (P<0.05); moreover, tumor size >5 cm, periductal infiltrating tumor morphology, multiple tumor, vascular invasion, and lymph node metastasis, were independently associated with increased risk of tumor recurrence (P<0.05). The CT finding of enlarged lymph nodes and CA 19-9 >120 IU/ml were preoperative predictors for lymph node metastasis; however, the sensitivity and specificity were only 62.5% and 88.0%, respectively, to identify lymph node metastasis.
Conclusion: Lymph node metastasis is associated with poor overall survival and disease-free survival following curative-intent resection in patients with ICC. Routine lymph node dissection for preoperatively diagnosed ICC should be recommended to properly assess the lymph node status of patients with ICC.
Keywords: Intrahepatic cholangiocarcinoma; Lymph node; Survival
Abbreviations
ICC: Intrahepatic Cholangiocarcinoma; CA19-9: Carbohydrate Antigen 19-9; CEA: Carcinoembryonic Antigen; CT: Computed Tomography; MRCP: Magnetic Resonance Imaging with Cholangiography; PET: Positron Emission Tomography; AJCC: American Joint Committee on Cancer; OS: Overall Survival; DFS: Disease-Free Survival
Introduction
Intrahepatic Cholangiocarcinoma (ICC) is the second most common primary liver malignancy [1]. In Korea, it represents approximately 10% of liver cancers (8.8% for males and 10.6% for females) and this proportion increases by year with an estimated annual percentage change of 7.9% and 10.6% for males and females, respectively, from 1999 to 2005 [2]. Up to date, there is limited data regarding the effectiveness of adjuvant chemotherapy for ICC; therefore, surgical resection remains the only definitive treatment available [3]. Although survival has improved in the last decade, [4] the 5-year survival still ranges from 20-40%, [5-7] and the recurrence rates remain as high as 50-60% [8,9] even after complete surgical resection.
As a result, it is important to recognize prognostic factors associated with long-term survival for patients with ICC for risk stratification after surgery. Most prominently, the lymph node status which can be influenced by the surgeon through an adequate lymphadenectomy. Several reports have documented the incidence of lymph node metastasis in patients with ICC as high a 45-62% [10,11]. Moreover, although its oncologic significance is well-documented in different studies, [12] the role of lymphadenectomy is still controversial and not even routinely performed [13]. Therefore, we sought to investigate the prognostic factors of patients with ICC who underwent curative intent surgery. In addition, we sought to identify the preoperative determinants of lymph node metastasis in patients with ICCs as it might potentially influence the decision making and the patient’s oncologic benefit on lymphadenectomy.
Materials and Methods
Study design
A retrospective review of all patients with a histologically confirmed intrahepatic cholagiocarcinoma who underwent curative intent resection in Severance hospital, Yonsei University College of Medicine, Seoul, Korea, between 2000 and 2014. Patients with incomplete clinical and histologic data were excluded from this study. Also excluded were patients with other underlying malignancies. The study protocol was approved by the Yonsei Institutional Review Board.
Clinicopathologic data
The data was collected from the electronic medical record database of the hospital. Patients’ demographic characteristics, clinical presentations, preoperative comorbidities, perioperative outcomes, imaging studies, and laboratory results were reviewed and analyzed. Preoperative evaluation including: carbohydrate antigen 19-9 (CA19- 9), Computed Tomography (CT), Magnetic Resonance Imaging with Cholangiography (MRCP) and Positron Emission Tomography (PET), were reviewed. Tumor characteristics such as tumor size, type, number, and the presence or absence of enlarged lymph nodes were obtained from the report of preoperative imaging studies. An enlarged lymph nodes on preoperative imaging was determined as exceeding the short axis diameter of 8 mm in the gastrohepatic ligament, 7 mm in the porta hepatis, 10 mm in the portocaval space, and 9 mm in the paraaortic region [14]. Enlarged lymph nodes on preoperative imaging were defined as clinical N1 whereas clinical N0 if no enlarged lymph nodes. The standard lymph node dissection in this cohort was defined as removal of the hilar and hepatoduodenal ligament (station 12) or more such as the retropancreatic, the common hepatic artery, the celiac axis, the left gastric artery, or the caval lymph nodes.
Pathology review was performed by an experienced hepatobiliary pathologist. Tumor growth type, grade, vascular invasion, multicentricity, and lymph node status were obtained. The tumor growth pattern was classified as being of mass-forming type, periductal infiltrating type, intraductal, and mixed type (massforming/ periductal infiltrating type) according to the Liver Cancer Study Group of Japan [15]. The pathological stage was defined according to the 8th edition of the American Joint Committee on Cancer 8th edition (AJCC) [16].
Study outcomes
The primary endpoints of this study were the long-term survival outcomes of patients with intrahepatic cholangiocarcinoma after curative-intent resection. Overall survival was calculated from the date of surgery to the date of death or last follow-up. Disease-free survival was calculated from the date of surgery to the date of recurrence or last follow-up. Patients were also followed up regularly for serum CA 19-9, Carcinoembryonic Antigen (CEA) level determination, and computed tomography scan to detect tumor recurrence.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics, version 22 (SPSS Inc., Chicago, IL, USA). Categorical variables were expressed as frequencies (%), whereas continuous variables were presented as means with their range or ±standard deviation. The means of continuous variables were compared using an independent sample t-test. Categorical variables were compared using the Pearson P2 test. The Receiver Operating Characteristic (ROC) curve analysis was used to determine the optimal cutoff value of CA 19-9 in determining lymph node metastasis. Overall survival and diseasefree survival were estimated using the Kaplan-Meier method and compared using a log-rank test. A multivariable Cox proportional hazards model was applied to identify the statistically significant independent prognostic factors for overall survival and disease-free survival. Association between the preoperative variables and lymph nodes metastases was tested, as continues and categorical, using logistic regression analysis. A P-value of <.05 was used to define statistical significance.
Results
Clinicopathologic characteristics
A total of 179 patients who underwent curative liver resection for ICC were initially identified. Among them, only 168 patients met the inclusion criteria. The median age was 64 years (range, 360-84 years). The characteristics of patients are summarized in (Table 1). With a median follow-up period of 21 months (range, 1-156 months), 88 patients (52%) had a recurrence and 53 patients (32%) died in this cohort. Moreover, among 88 patients with tumor recurrence, 77 (86.5%) recurred within 2 years of follow-up.
Variables
N=168
Age
<65
87 (51.8%)
>65
81 (48.2%)
Sex
Male
97 (57.7%)
Female
71 (42.3%)
CA 19-9 IU/ml
<72
75 (44.6)
>72
70 (41.7)
Tumor size (cm)
<5
80 (47.6%)
>5
88 (52.4%)
Number of lesions
Solitary
137 (81.5%)
Multiple
31 (18.5%)
Biliary invasion
92 (54.8%)
Perineural invasion
Vascular invasion
105 (62.5%)
Tumor Grade
Well/Moderately differentiated
129 (76.8%)
Poorly/Undifferentiated
29 (12.7%)
Metastatic lymph nodes (AJCC 8th ed., pN)
Nx
29 (17.3%)
N0
96 (57.1%)
N1
43 (25.6%)
Tumor Morphology
Mass forming
115 (68.5%)
Periductal infiltrating
14 (8.3)
Intraductal growth
21 (12.5%)
Mixed type
17 (10.1%)
Positive resection margin (R1)
24 (14.3%)
TNM stage (AJCC 8th edition)
0
11 (6.5%)
IA
15 (8.9%)
IB
8 (4.8%)
II
62 (36.9%)
IIIB
43 (25.6%)
Unknown
29 (17.3%)
Surgical resection
Less than hemihepatectomy
35 (20.8%0
Right hemihepatectomy
56 (33.3%)
Left hepatectomy
57 (33.9%)
Central bisectionectomy
4 (2.4%)
Extended hemihepatectomy
15 (8.9%)
Lymph node dissection (LND)
No LND
29 (17.3%)
Standard LND
139 (82.7%)
CA: Cancer Antigen; AJCC: American Joint Committee on Cancer; LND: Lymph Node Dissection
Table 1: Clinipathologic characteristics of patients.
Factors associated with Overall Survival (OS)
The cumulative 1-year, 3-year, and 5-year OS rates were 86.9%, 67.9%, and 54.9%, respectively (Figure 1A). The median OS was 65 months. Factors associated with poor OS included age >65 years, CA19-9 >72 U/ml, vascular invasion, perineural invasion, bile duct invasion, tumor size >5 cm, periductal infiltrating tumor morphology, and metastatic lymph nodes (pN1) (P < 0.05). On multivariable analysis, Age >65 years (HR, 2.307; 95% CI, 1.264-4.210; P=0.006), lymph node metastasis (HR, 2.222; 95% CI, 1.115-4.427; P=0.023), tumor size >5 cm (HR, 2.330; 95% CI, 1.186-4.575; P=.014), and periductal infiltrating tumor morphology (HR, 3.961; 95% CI, 1.496- 10.486; P=0.006) were independently associated with poor OS. Table 2 detailed the clinicopathologic factors associated with OS.
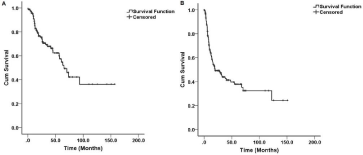
Figure 1: (A) Overall survival Kaplan-Mier curve of patients with ICC after curative-intent resection, (B) Disease-free survival.
Clinicopathologic factors
N
Univariable
Multivariable
Means (Months)
P value
P value
HR
95% CI
Age (years)
<65
87
105.6
>65
81
56.1
0.0001
0.006
2.31
1.264-4.210
Sex
Male
97
78.2
Female
71
86
0.621
CA 19-9 (U/mL)
<72
75
84.2
>72
70
65.6
0.039
0.345
1.34
0.730-2.460
Tumor size (cm)
<5
80
94.5
>5
88
68.3
0.007
0.014
2.33
1.186-4.575
Number of lesions
137
84.5
Solitary
31
51.3
0.186
Multiple
Bile duct invasion
No
92
97.9
Yes
76
52
0.027
0.458
1.27
0.677-2.376
Vascular invasion
No
63
107.6
Yes
105
62.6
0.0001
0.093
2.01
0.891-4.523
Perineural invasion
No
103
94
Yes
65
58.5
0.001
0.838
1.08
0.536-2.158
Metastatic lymph
No
96
99.9
0.001
0.023
2.22
1.115-4.427
Yes
43
30.5
Tumor morphology
0.002
0.006
Intraductal growth
21
110.9
Ref
Ref
Mass forming
115
90.6
0.141
0.828
Periductal infiltrating
14
28.6
0.002
0.006
3.96
1.496-10.486
Mixed type
17
48.9
0.025
0.758
Tumor Grades
Well/Moderately differentiated
129
82.1
Poorly/Undifferentiated
29
50.5
0.151
Resection margin
R0
144
80.5
R1
24
70.9
0.443
CA: Cancer Antigen; CI: Confidence Interval; HR: Hazard Ratio; AJCC: American Joint Committee on Cancer
Table 2: Clinicopathologic factors associated with overall survival.
Factors associated with Disease-Free Survival (DFS)
The median DFS was 19.7 months, and the cumulative 1-year, 3-year, 5-year DFS rates were 63.5%, 43.8%, 37.8%, respectively (Table 3 & Figure 1B). Factors associated with increased risk of recurrence included CA19-9 >72 IU/ml, multiple tumor, poorly/undifferentiated tumor, vascular invasion, perineural invasion, tumor size >5 cm, periductal infiltrating tumor morphology, and lymph node metastasis (P<0.05). On multivariable analysis, tumor size >5 cm (HR, 2.137; 95% CI, 1.176-3.884; P=0.013), periductal infiltrating tumor morphology (HR, 4.679; 95% CI, 1.247-10.318; P=0.0001), multiple tumor (HR, 2.255; 95% CI, 1.254-4.070); P=0.007), vascular invasion (HR, 2.307; 95% CI, 1.201-4.431); P=0.012) and lymph node metastasis (HR, 2.145; 95% CI, 1.247-3.688; P=0.006) were independently associated with increased risk of tumor recurrence.
Clinicopathologic factors
N
Univariable
Multivariable
Means (Months)
P value
P value
HR
95% CI
Age
<65
87
56
>65
81
64.6
0.965
Sex
Male
97
51.6
0.405
Female
71
66.2
CA 19-9 (U/mL)
<72
75
67.8
0.031
0.37
1.28
0.749-2.172
>72
70
48.7
Tumor size (cm)
<5
80
71.2
0.0001
0.013
2.14
1.176-3.884
>5
88
38.8
Number of lesions
Solitary
137
66.2
0.001
0.007
2.26
1.254-4.053
Multiple
31
21.8
Bile duct invasion
No
92
67.9
0.116
Yes
76
32.1
Vascular invasion
No
63
88.8
0.0001
0.012
2.31
1.201-4.431
Yes
105
37.8
Perineural invasion
No
103
69.7
0.0001
0.189
1.46
0.831-2.548
Yes
65
37.3
Metastatic lymph node
No
96
61.2
0.0001
0.006
2.15
1.247-3.688
Yes
43
18.2
Tumor morphology
Intraductal growth
21
97.2
Ref
Ref
Mass forming
115
57.8
0.002
0.243
0.47
0.132-1.668
Periductal infiltrating
14
12
0.0001
0.0001
4.68
2.122-10.318
Mixed type
17
40.7
0.014
0.22
0.61
0.275-1.346
Tumor Grades
Well/Moderately differentiated
129
69.4
0.0001
0.198
1.48
0.813-2.710
Poorly/Undifferentiated
29
17
Resection margin
R0
144
59.9
0.861
R1
24
50.9
Table 3: Clinicopathologic factors associated with disease-free survival.
Preoperative factors associated with lymph node metastasis
Among 139 patients who underwent standard lymph node dissection, 42 (30.2%) had positive lymph node metastasis on final histopathology report. To identify the preoperative predictive factors for lymph node metastasis in patients with ICC, we compared the preoperative variables among patient with lymph node metastasis and no lymph node metastasis by using logistic regression analysis. We included age >65 years, gender, CA 19-9, and preoperative CT scan tumor size >5 cm, tumor morphology (mass forming, periductal infiltrating, and intraductal growth) and clinically enlarged lymph node on CT scan. A Receiver Operating Characteristic (ROC) curve analysis was used to determine the optimal cutoff value of CA 19-9 to predict pathologic lymph node. The optimal cutoff value was 120 IU/ ml (sensitivity, 55.3%; and specificity, 70.2%) with an Area under the Curve (AOC) 0.619 (95% CI, 0.505-0.733, P=0.035).
The CT findings of enlarged lymph nodes (clinical N1) and CA 19-9 >120 IU/ml were predictive of pN1 in univariable analysis, and remained independent predictors in multivariable analysis (Table 4). Furthermore, we identified 16 patients with cN1 and CA 19-9 >120 IU/ml (high risk group), 28 patients with cN0 and CA 19-9 >120 IU/ ml (intermediate risk group), 20 patients with cN1 and CA 19-9 <120 IU/ml (intermediate risk group), and 50 patients cN0 and CA 19-9 <170 IU/ml (low risk group). Table 5 summarizes the incidence of lymph node metastasis according to the CA 19-9 and clinical lymph node status. Notably, the high-risk group is associated with 62.5% risk of lymph node metastasis following curative resection compared to 30-35% in the intermediate risk groups and 12.0% in the low-risk group (P=0.001); however, the sensitivity was only 62.5% for the high risk group to identify lymph node metastasis and specificity 88.0% for the low risk group to rule out lymph node metastasis.
Vairables
Univariable
(P value)Multivariable
(P value)Hazard ratio
95% Confidence interval
Age =65 years
0.418
Sex (male/Female)
0.105
CA 19-9 >120 U/mL
0.004
0.015
4.309
1.451-12.793
Tumor size > 5 cma
0.837
Multiple tumora
0.562
Clinical N1a
0.001
0.009
2.936
1.236-6.976
Tumor morphology (periductal infiltrating tumor)a
0.474
Table 4: Logistic regression analysis of factors predictive for pathologic lymph node metastasis.
Risk factors
Pathologic N0 (%)
Pathologic N1 (%)
High risk
Clinical N0 + CA 19-9 > 120 IU
6 (37.5%)
10 (62.5%)
Intermediate risk
Clinical N1 + CA 19-9 <120 IU/ml
14 (70%)
6 (30%)
Clinical N0 + CA 19-9 >120 IU/ml
18 (64.3%)
10 (35.7%)
Low risk
Clinical N0 + CA 19-9 < 120 IU/ml
44 (88.0%)
6 (12.0%)
Sensitivity of High risk: 62.5%
Specificity of low risk: 88.0%
Positive predictive value: 62.5
Negative predictive value: 88.0%
Accuracy: 75.2%
Table 5: Incidence of lymph node metastasis according to the presence or absence of risk factors.
Discussion
The standard of care for intrahepatic cholangiocarcinoma remains complete surgical resection. However, due to the rarity of the disease, most of the reports on the management of ICC are from small retrospective reviews of data from individual institutions; therefore, intrahepatic cholangiocarcinoma after surgery is still associated with poor prognosis. Moreover, the reported 5-year overall survival of ICC after complete surgical resection in studies ranges from 20- 40% [5-7,17]. Our present study however, had demonstrated a high 5-year survival rate of 54.9%, slightly higher than reported in studies. However, despite our aggressive surgical approach for patients with ICC, we were able to achieve 85.6% R0 resection rate but the 5-year DFS remains low (37.8%). Notably, although R1 resection was not associated with poor survival outcome in this cohort, several reports had demonstrated a poor DFS and OS with R1 resection [18-20].
Our present cohort has identified various clinicopathologic factors associated with poor long-term survival after curative-intent surgery. It is important to identify these prognostic factors for patients’ risk stratification, close follow-up monitoring, and future planning of adjuvant therapy. We have found that independent predictors of OS were age >65 years, the presence of lymph node metastases, tumor size > 5 cm, and periductal infiltrating growth type tumor. Moreover, independent factors associated with decrease DFS were tumor size > 5 cm, periductal infiltrating growth type tumor, multiple tumor, vascular invasion, and lymph node metastasis. These factors, however, had been demonstrated as important determinant of OS and DFS in different studies [5,6,17,21-23].
Frequently cited as important prognostic factors for overall survival for patients with intrahepatic cholagiocarcinoma are the presence of vascular invasion and multiple tumor lesions [24,25]. AJCC 7th and 8th edition emphasizes the clinical importance of these two factors in the T stage classification for intrahepatic cholangiocarcinoma. On the contrary, multiple tumors were not associated with poor overall survival in this cohort; furthermore, although it was proven to be associated with poor overall survival in the univariate analysis, vascular invasion was not found to be an independent factor associated with overall survival. Nevertheless, both these factors were found to be an independent variable for decreased disease-free survival after curative-intent resection. These findings are the same as Weber et al. [26]. reported that together with tumor size, vascular invasion, and multiple lesions were associated with increased risk of tumor recurrence after curative-intent resection.
Moreover, tumor size of >5 cm and the periductal infiltrating type of tumor is found to be associated with poor overall survival, as well disease-free survival in this present cohort. Tumor size was found to be associated with poor pathologic outcome such as vascular invasion and poor tumor differentiation [27] and hence associated with poor prognosis [28,29]. Some studies however, had shown that tumor size was not a significant predictor of overall survival [30,31]. Periductal infiltrating tumor is also a well-recognized prognostic factor associated with poor long-term survival [15,32]. In AJCC 7th edition, it was used to identify the T classification into T4, however, it was no longer part of the T classification in the AJCC 8th edition.
Furthermore, in this present cohort, lymph node metastasis portends a poor long-term survival. This finding was consistently reported in various studies [29,33]. As such, in order to properly determine the prognosis of patient with intrahepatic cholangiocarcinoma, a thorough evaluation of the lymph node status by adequate lymph node dissection must be emphasized. Several reports had documented as high as 45-62% incidence of lymph node metastasis in patients with ICC [10,11] and its oncologic significance was also well-documented in different studies [34]. However, until recently, not all patients with ICC underwent lymph node dissection [10]. Thus, in our cohort, we tried to identify those preoperative determinants of lymph node metastasis in patients with ICCs as it might potentially influence the decision making about who might benefit from lymphadenectomy. Our result had demonstrated that patient with clinically lymph node metastasis (cN1) on CT scan and elevated CA 19-9 >120 IU/ml significantly associated with pathologic lymph node metastasis in univariable and multivariable analysis. We classify these patients as high risk and has a 65.2% risks of lymph node metastases. Patients with either one of these 2 risk factors were classified as intermediate risk with 30-36% risk of lymph node metastasis whereas neither of these 2 risk factors was associated with 12% risk of lymph node metastasis. The same observation reported by Nanashima et al. [35] that the combination of positive CT findings and high CA19-9 level had the highest positive rate for node metastasis for biliary and pancreatic carcinoma. Although CT scan has a low accuracy in detecting lymph node metastasis in patients with cholangiocarcinoma, [36-38] CA 19-9 was notably associated with advanced disease and lymph node metastasis in some studies [39,40]. However, given that this model has a sensitivity of only 62.5% and more importantly a specificity of only 88.0%, we still advocate a routine lymph node dissection considering that 12% of patient categorized as low risk may harbor an occult lymph node metastasis. As such, up to date, there is still no valuable tools with high sensitivity and specificity to identify preoperatively the lymph node metastasis for intrahepatic cholangiocarcinoma.
This study had several limitations. First, the selection bias that influenced the choice of treatment among our patients. For instance, patients with clinically small size tumor and clinically no lymph node on CT scan (cN0) were possibly selected to undergo liver resection only which might undoubtedly affect the survival outcome. Second, it is a single institution with a relatively small sample size. However, given the rarity of the ICC, this single institution study is one of the largest series that was reported. Nonetheless, our result should be further validated in a multicenter or randomized prospective study.
In summary, lymph node metastasis is associated with poor overall survival and disease-free survival in patients with ICC after curative-intent resection. A combination of preoperative CT scan and CA 19-9 for predicting lymph node metastasis has a sensitivity and specificity of 62.5% and 88.0% respectively. As such, routine lymph node dissection for preoperatively diagnosed intrahepatic cholangiocarcinoma should be recommended to properly assess the lymph node status of patients with intrahepatic cholangiocarcinoma.
References
- Khan SA, Toledano MB, Taylor-Robinson SD. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB (Oxford). 2008; 10: 77-82.
- Shin HR, Oh JK, Lim MK, Shin A, Kong HJ, Jung KW, et al. Descriptive Epidemiology of Cholangiocarcinoma and Clonorchiasis in Korea. J Korean Med Sci. 2010; 25: 1011-1016.
- Weber SM, Ribero D, O'Reilly EM, Kokudo N, Miyazaki M, Pawlik TM. Intrahepatic Cholangiocarcinoma: expert consensus statement. HPB. 2015; 17: 669-680.
- Nathan H, Pawlik TM, Wolfgang CL, Choti MA, Cameron JL, Schulick RD. Trends in Survival after Surgery for Cholangiocarcinoma: A 30-Year Population-Based SEER Database Analysis. J Gastrointest Surg. 2007; 11: 1488-1497.
- Cho SY, Park SJ, Kim SH, Han SS, Kim YK, Lee KW, et al. Survival analysis of intrahepatic cholangiocarcinoma after resection. Ann Surg Oncol. 2010; 17: 1823-1830.
- Hyder O, Marques H, Pulitano C, Marsh JW, Alexandrescu S, Bauer TW, et al. A nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma: an Eastern and Western experience. JAMA Surg. 2014; 149: 432-438.
- Konstadoulakis MM, Roayaie S, Gomatos IP, Labow D, Fiel MI, Miller CM, et al. Fifteen-year, single-center experience with the surgical management of intrahepatic cholangiocarcinoma: operative results and long-term outcome. Surgery. 2008; 143: 366-374.
- Doussot A, Gonen M, Wiggers JK, Groot-Koerkamp B, DeMatteo RP, Fuks D, et al. Recurrence Patterns and Disease-Free Survival after Resection of Intrahepatic Cholangiocarcinoma: Preoperative and Postoperative Prognostic Models. J Am Coll Surg. 2016; 223: 493-505.
- Hyder O, Hatzaras I, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery. 2013; 153: 811-818.
- Amini N, Ejaz A, Spolverato G, Maithel SK, Kim Y, Pawlik TM. Management of lymph nodes during resection of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: a systematic review. J Gastrointest Surg. 2014; 18: 2136-2148.
- Tsuji T, Hiraoka T, Kanemitsu K, Takamori H, Tanabe D, Tashiro S. Lymphatic spreading pattern of intrahepatic cholangiocarcinoma. Surgery. 2001; 129: 401-407.
- Guglielmi A, Ruzzenente A, Campagnaro T, Valdegamberi A, Bagante F, Bertuzzo F, et al. Patterns and Prognostic Significance of Lymph Node Dissection for Surgical Treatment of Perihilar and Intrahepatic Cholangiocarcinoma. J Gastrointest Surg. 2013; 17: 1917-1928.
- Adachi T, Eguchi S. Lymph node dissection for intrahepatic cholangiocarcinoma: a critical review of the literature to date. J Hepatobiliary Pancreat Sci. 2014; 21: 162-168.
- Dorfman RE, Alpern MB, Gross BH, Sandler MA. Upper abdominal lymph nodes: criteria for normal size determined with CT. Radiology. 1991; 180: 319-322.
- Sakamoto Y, Kokudo N, Matsuyama Y, Sakamoto M, Izumi N, Kadoya M, et al. Proposal of a new staging system for intrahepatic cholangiocarcinoma: Analysis of surgical patients from a nationwide survey of the Liver Cancer Study Group of Japan. Cancer. 2016; 122: 61-70.
- Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. AJCC Cancer Staging Manual. 8 ed: Springer International Publishing; 2017.
- Choi SB, Kim KS, Choi JY, Park SW, Choi JS, Lee WJ, et al. The Prognosis and Survival Outcome of Intrahepatic Cholangiocarcinoma Following Surgical Resection: Association of Lymph Node Metastasis and Lymph Node Dissection with Survival. Ann Surg Oncol. 2009; 16: 3048.
- DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, et al. Cholangiocarcinoma: Thirty-one-Year Experience With 564 Patients at a Single Institution. Ann Surg. 2007; 245: 755-762.
- Spolverato G, Yakoob MY, Kim Y, Alexandrescu S, Marques HP, Lamelas J, et al. The Impact of Surgical Margin Status on Long-Term Outcome After Resection for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol. 2015; 22: 4020-4028.
- Yeh CN, Hsieh FJ, Chiang KC, Chen JS, Yeh TS, Jan YY, et al. Clinical effect of a positive surgical margin after hepatectomy on survival of patients with intrahepatic cholangiocarcinoma. Drug Des Devel Ther. 2015; 9: 163-174.
- Chang ME, Lei HJ, Chen MH, Yeh YC, Li CP, Hung YP, et al. Evaluation of prognostic factors and implication of lymph node dissection in intrahepatic cholangiocarcinoma: 10-year experience at a tertiary referral center. J Chin Med Assoc. 2017; 80: 140-146.
- Guglielmi A, Ruzzenente A, Campagnaro T, Pachera S, Valdegamberi A, Nicoli P, et al. Intrahepatic cholangiocarcinoma: prognostic factors after surgical resection. World J Surg. 2009; 33: 1247-1254.
- Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and Prognosis for Patients with Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg. 2014; 149: 565-574.
- Ohtsuka M, Ito H, Kimura F, Shimizu H, Togawa A, Yoshidome H, et al. Results of surgical treatment for intrahepatic cholangiocarcinoma and clinicopathological factors influencing survival. Br J Surg. 2002; 89: 1525-1531.
- Okabayashi T, Yamamoto J, Kosuge T, Shimada K, Yamasaki S, Takayama T, et al. A new staging system for mass-forming intrahepatic cholangiocarcinoma: analysis of preoperative and postoperative variables. Cancer. 2001; 92: 2374-2383.
- Weber SM, Jarnagin WR, Klimstra D, DeMatteo RP, Fong Y, Blumgart LH. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg. 2001; 193: 384-3891.
- Spolverato G, Ejaz A, Kim Y, Sotiropoulos GC, Pau A, Alexandrescu S, et al. Tumor size predicts vascular invasion and histologic grade among patients undergoing resection of intrahepatic cholangiocarcinoma. J Gastrointest Surg. 2014; 18: 1284-1291.
- Morimoto Y, Tanaka Y, Ito T, Nakahara M, Nakaba H, Nishida T, et al. Long-term survival and prognostic factors in the surgical treatment for intrahepatic cholangiocarcinoma. Journal of Hepato-Biliary-Pancreatic Surgery. 2003; 10: 432-440.
- Shen WF, Zhong W, Xu F, Kan T, Geng L, Xie F, et al. Clinicopathological and prognostic analysis of 429 patients with intrahepatic cholangiocarcinoma. World J Gastroenterol. 2009; 15: 5976-5982.
- Choi SB, Kim KS, Choi JY, Park SW, Choi JS, Lee WJ, et al. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol. 2009; 16: 3048-3056.
- de Jong MC, Nathan H, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011; 29: 3140-3145.
- Shimada K, Sano T, Sakamoto Y, Esaki M, Kosuge T, Ojima H. Surgical outcomes of the mass-forming plus periductal infiltrating types of intrahepatic cholangiocarcinoma: a comparative study with the typical mass-forming type of intrahepatic cholangiocarcinoma. World J Surg. 2007; 31: 2016-2022.
- Nakagawa T, Kamiyama T, Kurauchi N, Matsushita M, Nakanishi K, Kamachi H, et al. Number of lymph node metastases is a significant prognostic factor in intrahepatic cholangiocarcinoma. World J Surg. 2005; 29: 728-733.
- Guglielmi A, Ruzzenente A, Campagnaro T, Valdegamberi A, Bagante F, Bertuzzo F, et al. Patterns and prognostic significance of lymph node dissection for surgical treatment of perihilar and intrahepatic cholangiocarcinoma. J Gastrointest Surg. 2013; 17: 1917-1928.
- Nanashima A, Sakamoto I, Hayashi T, Tobinaga S, Araki M, Kunizaki M, et al. Preoperative Diagnosis of Lymph Node Metastasis in Biliary and Pancreatic Carcinomas: Evaluation of the Combination of Multi-detector CT and Serum CA19-9 Level. Dig Dis Sci. 2010; 55: 3617-3126.
- Watadani T, Akahane M, Yoshikawa T, Ohtomo K. Preoperative assessment of hilar cholangiocarcinoma using multidetector-row CT: correlation with histopathological findings. Radiat Med. 2008; 26: 402-407.
- Kim HJ, Lee DH, Lim JW, Ko YT. Multidetector computed tomography in the preoperative workup of hilar cholangiocarcinoma. Acta Radiologica. 2009; 50: 845-853.
- Adachi T, Eguchi S, Beppu T, Ueno S, Shiraishi M, Okuda K, et al. Prognostic Impact of Preoperative Lymph Node Enlargement in Intrahepatic Cholangiocarcinoma: A Multi-Institutional Study by the Kyushu Study Group of Liver Surgery. Ann Surg Oncol. 2015; 22: 2269-2278.
- Hu HJ, Mao H, Tan YQ, Shrestha A, Ma WJ, Yang Q, et al. Clinical value of preoperative serum CA 19-9 and CA 125 levels in predicting the resectability of hilar cholangiocarcinoma. Springer Plus. 2016; 5: 551.
- Coelho R, Silva M, Rodrigues-Pinto E, Cardoso H, Lopes S, Pereira P, et al. CA 19-9 as a Marker of Survival and a Predictor of Metastization in Cholangiocarcinoma. GE Port J Gastroenterol. 2017; 24: 114-121.