Research Article
Austin J Surg. 2025; 12(2): 1349.
Kimura’s Disease of the Clinical Characteristics, Treatment, and Prognosis of 34 Cases: A Retrospective Cross-Sectional Study
Xue J1,2, Xin P3, Hu C1,2, Qin X1,2, Huang C1,2, Song K1,2 and Li W1,2*
¹Department of Stomatology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
²School of Stomatology, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
³Department of Stomatology, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Third Hospital of Shanxi Medical University, Taiyuan, China
*Corresponding author: Wenqiang Li, Department of Stomatology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, 430030, China Tel: +86 27 83663805; Email: liwenq2012@126.com
Received: April 06, 2025 Accepted: April 18, 2025 Published: April 23, 2025
Abstract
Background: This study aimed to explore the clinical characteristics, diagnosis, treatment, and prognosis of Kimura’s disease (KD), and to analyze the related factors of recurrence after treatment.
Methods: 34 patients with KD were diagnosed from Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan from June 2011 to March 2023. Retrospective analysis of clinical data, treatment, and prognostic information, recurrence was defined as the appearance of a mass at the treatment site, or the continued enlargement of the original mass, or the death of the patient.
Results: KD presents as a painless mass or regional lymph node involvement, with pruritus and hyperpigmentation of the skin. 26 patients showed peripheral blood eosinophilia, and 23 patients presented elevated eosinophil counts. Magnetic Resonance Imaging showed well-defined lymph nodular or ill-defined solid mass. 14 patients were treated by surgery alone, 16 patients received surgery combined with adjuvant therapy, 2 patients received chemotherapy and radiotherapy, and 1 received systemic corticosteroids alone. Follow-up data on 23 patients revealed that 8 patients recurred within 2 months to 3 years after the treatment, one of them died 4 months after radiotherapy. Univariate analysis showed duration and lymphocytes were related to recurrence. (P<0.05).
Conclusion: KD is characterized by painless masses or lymphadenopathy, with pruritus and hyperpigmentation, and laboratory tests show eosinophilia. After surgery, adjuvant therapy can be selected according to duration and lymphocytes to reduce the recurrence rate.
Keywords: Kimura’s disease; Eosinophilia; Diagnosis; Treatment; Recurrence
Background
KD was first reported by a Chinese doctor in 1937 as an “eosinophilic hyperplastic lymphogranuloma”, and described in detail by the Japanese scholar Kimura [1], and named the disease. KD is a rare chronic inflammatory disease that is much more prevalent in young males of Asian. The clinical characteristics are one or more painless masses with waxing and waning over time [2], some with pruritus and hyperpigmentation. It often involves the head and neck region, and also occurs in the salivary glands, subcutaneous tissues, regional lymph nodes, and other sites. Laboratory tests show peripheral blood eosinophilia and elevated immunoglobulin E (IgE) levels [3,4]. KD is a slowly progressive disease and the site, number of masses, and clinical presentation vary from patient to patient, which makes it difficult to diagnose. KD is rare, most of the clinical studies are case reports, and there is a lack of large, comprehensive clinical studies. Some treatments have been reported, including surgery, systemic steroids, immunosuppressive medications, and radiotherapy [5]. However, it is easy to recurrence and there is no current consensus on the optimal treatment.
KD is associated with pruritus for a long time, which affects people's quality of life. Furthermore, the disease is still prone to recurrence even after treatment. We reviewed the presentation and treatment of 34 patients and the relevant literature to deepen the understanding of this disease.
Methods and Analysis
Study Design and Procedures
This study was a retrospective cohort study and was approved by the ethics committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (TJ-JRB20231293). The need for obtaining informed patient consent will be waived due to the retrospective nature of this study.
Patients admitted to the hospital between June 2011 and March 2023. Inclusion criteria included (1) being treated in our hospital and diagnosed with Kimura's disease by pathology, (2) having complete medical history data. Exclusion criteria included (1) any mental illness, or other diseases that can lead to the legal recognition of restricted capacity, (2) life-threatening systemic diseases such as malignant tumors within 1 year before the consultation, (3) special infectious diseases such as Hepatitis B or HIV infection.
Analysis of clinical pathological features, treatment, and prognosis of KD. During follow-up, recurrence was defined as the appearance of a mass at the site of original treatment, the continued enlargement of the original mass, or the death of the patient.
Statistical Analysis
Continuous variables were analyzed using mean ±SD or median with IQR, while categorical variables were represented as counts and proportions. Univariate analysis, using the chi-square test or Fisher’s exact test, was conducted for each selected recurrence factor (gender, age, disease location, symptom, physical findings, duration, laboratory values, and Systemic disease history). Values of p<0.05 were considered statistically significant. All statistical analyses were performed using SPSS V.23.0 (IBM SPSS).
Results
Clinical Characteristics
Clinical features are summarized and 34 cases with Kimura disease are listed in Table 1. 29 cases being male. The duration of symptoms was 10 days to 30 years. Most cases (30 of the 34, 88.2%) presented on the head and neck, and 4 cases involved other parts of the body. For glands, involvement of the parotid gland in 16 cases, the submandibular gland in 4 cases, the eyelids in 3 cases, the palate in 1 case, and the buccal in 1 case. The lesions were presented unilaterally in 20 cases and bilaterally in 14 cases. There were 19 cases of multiple lesions, 11 cases of skin pruritus and 9 cases had hyperpigmentation (Figure 1). 6 cases had a history of smoking and 5 cases had drinking, 11 cases had allergic diseases (such as drug allergy, bronchial asthma, atopic dermatitis and chronic urticaria), 3 cases with hypertension and 1 case was complicated with nephrotic syndrome.
Patient No.
Age (yr)/ Gender
Duration of symptoms
Site
Clinical Presentation
size (cm)
boundary
pruritus
hyperpigmentation
1
28/M
2years
parotid gland, neck
painless mass
4*3
unclear
none
none
2
41/M
5years
parotid gland
painless mass
1.5*1.5
unclear
none
have
3
21/M
2years
parotid gland
painless mass
3.4*1.6
unclear
none
none
4
45/M
2years
parotid gland
painless mass
left2.5*2.5, right2*2.5
clear
none
none
5
11/M
1year
neck
painless mass
1.2*1
clear
have
none
6
66/M
1year
submandibular gland
lymphadenopathy
3*4
unclear
have
none
7
43/M
2months
submandibular gland
painless mass
2.0*1.7
clear
none
have
8
38/M
3months
parotid gland
painless mass
left3*4,right1*4
clear
none
none
9
50/M
3years
parotid gland
painless mass
2.5*3,1*1
unclear
have
have
10
50/M
20years
parotid gland
painless mass
right1*2,3*4.5, left3*4,2*4
unclear
none
none
11
39/M
10years
parotid gland
painless mass
2*2.5
unclear
have
have
12
29/M
4months
parotid gland
swelling
1.6*1
unclear
none
none
13
28/F
3months
parotid gland
painless mass
2* 1
unclear
none
none
14
5/M
3months
cheek
painless mass
1.5*1
unclear
none
none
15
39/M
10years
parotid gland
painless mass
2.5*2.5
clear
have
none
16
7/M
7months
eyelid
swelling
no
unclear
none
none
17
23/M
11years
submandibular gland, parotid gland
swelling
no
unclear
none
none
18
12/M
1year
neck, limb
painless mass
2.5*2.5
unclear
none
none
19
36/M
2months
groin, neck, brow
lymphadenopathy
2.5*2.5
unclear
have
have
20
48/F
10days
submandibular gland
swelling
no
no
none
none
21
8/M
3years
neck, groin, limb
lymphadenopathy
3*3
unclear
none
have
22
37/M
30years
parotid gland, neck
painless mass
left3*4,right1*1
clear
none
none
23
8/M
4years
eyelid, neck, limb
swelling,lymphadenopathy
no
unclear
have
have
24
8/M
2years
neck
painless mass
4*4
unclear
none
none
25
66/M
2years
parotid gland
painless mass
2.5*2.5
unclear
have
none
26
47/M
1month
neck
lymphadenopathy
3*3
unclear
none
none
27
50/M
4years
occiput, parotid gland, neck, limb
painless mass
5*5, 3*3, 1*1.5
unclear
have
have
28
3.2/M
20days
neck
painless mass
3*4
unclear
none
none
29
46/M
15days
parotid gland
painless mass
3*5
unclear
none
none
30
67/M
15days
palate
painless mass
2*3
clear
none
have
31
67/M
2months
parotid gland
painless mass
left2.5*3,1.5*2.,right2.5*2.5,1*1
unclear
none
none
32
60/F
10years
parotid gland
painless mass
left4*4,1*1,right2*2
unclear
have
ne
33
58/M
1year
parotid gland
painless mass
2.0*0.8
unclear
none
none
34
15/M
3years
eyelid
painless mass, swelling
1.0*0.8
unclear
none
None
M, male; F, female.
Table 1: Clinical information of 34 patients with Kimura disease.
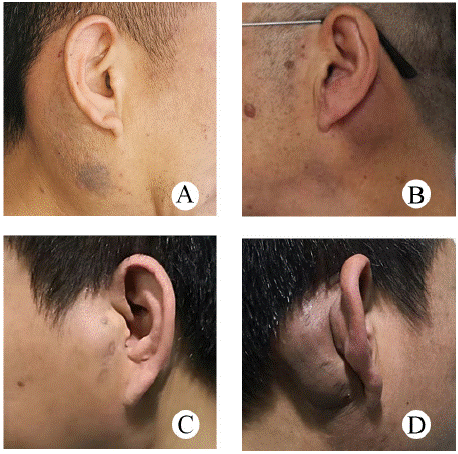
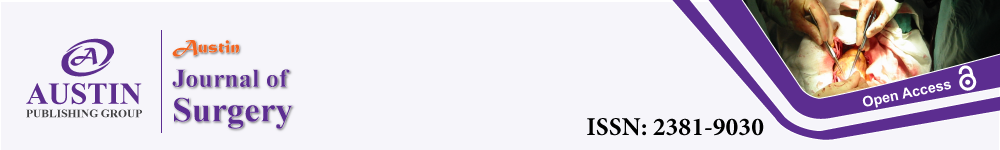
Figure 1: Preoperative picture of typical Kimura disease (A) Patient 1,
unilateral mass with skin hyperpigmentation. (B) Patient 2, unilateral
mass with skin hyperpigmentation. (C.D) Patient 3, Bilateral mass with
hyperpigmentation on the right.
Laboratory Tests
Among the 30 cases included, 26 cases showed elevated peripheral blood eosinophils, 23 cases had elevated eosinophil counts, 14 cases had decreased neutrophils, 4 cases had decreased neutrophil counts, 3 had decreased lymphocytes, and 2 showed decreased lymphocyte counts (Table 2). Preoperative bone marrow puncture in 5 cases showed that granulocyte hyperplasia was active and the eosinophils increased, accounting for 20%~36%, and a few cells had vacuoles. Additionally, 2 cases showed decreased lymphocytes.
indicator
case(%)
Eosinophils (%)
normal
4(13.33)
elevated
26(86.67)
Eosinophil count (109/L)
normal
7(23.33)
elevated
23(76.67)
Neutrophils (%)
decreased
14(46.67)
normal
15(50)
elevated
1(3.33)
Neutrophil count (109/L)
decreased
4(13.33)
normal
23(76.67)
elevated
3(10)
Lymphocytes (109/L)
decreased
3(10)
normal
25(83.33)
elevated
2(6.67)
Lymphocyte count (109/L)
decreased
2(6.67)
normal
24(80)
elevated
4(13.33)
Table 2: Laboratory vales.
Magnetic Resonance Imaging Characteristics
Magnetic resonance imaging (MRI) was performed in 10 cases. 4 cases showed T1-weighted sequences isointense signal and hyperintense signal on T2-weighted sequences, 4 cases showed T1-weighted sequences hyperintense and T2-weighted sequences hyperintense, 1 case showed T1-weighted sequences hypointense and heterogeneously hyperintense lesion on T2-weighted sequences. 7 cases showed nodular lesions, 3 cases patchy or lobulated lesions were seen. The boundary was well-defined in 5 cases and ill-defined in 5 cases. Among them, 2 cases of multiple lesions were partially fused (Figure 2), and 2 cases only showed beaded changes of lymph nodes (Figure 3). 1 case showed diffuse atrophy of subcutaneous fat on T1-weighted sequences. Contrast-enhanced magnetic resonance imaging (CE-MRI) was performed in 10 cases, 3 cases were patchy heterogeneous enhancement and 1 case showed nodular homogeneous enhancement lesion. 4 cases were indicated Inflammation.
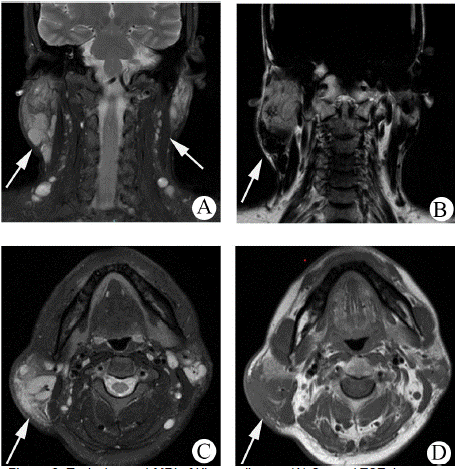
Figure 2: Typical case 1 MRI of Kimura disease (A) Coronal TSE, images
show a lobulated heterogeneously hyperintense lesion, and beaded
changes of lymph nodes. (B) Coronal TSE, images show diffuse atrophy of
subcutaneous fat in the right parotid mass. (C, D) Axial TSE, the boundary
of the lesion on the right is ill-defined, partial fusion, and vascular flow void
signal can be seen.
Figure 3: Typical case 2 MRI of Kimura disease (A, B) Coronal TSE, (C, D)
Axial TSE. Only the glands and cervical lymph nodes are increased. The
lymph nodes were elliptical, with a smooth margin and a relatively welldefined
outline.
Treatment and Outcome
Among the 34 cases, 14 cases were treated by surgery alone, 6 cases with surgical combined with radiotherapy, 2 cases were treated with combined surgery, radiotherapy, and chemotherapy, 8 cases with surgery and steroid, 2 cases underwent combined surgery, steroid, and chemotherapy, 1 case with steroid only, and 1 case did not undergo any treatment after diagnosing KD by the cytological puncture. For 23 cases with follow-up data, 8 cases experienced recurrence, with a recurrence rate of 34.78%. Among recurrence cases, 4 cases were treated with surgery alone, 1 case was treated with combined surgery, steroid and, chemotherapy, 2 cases underwent surgical combined with steroid, 1 was treated by surgery and radiotherapy, died 4 months after radiotherapy. 7 cases recurred within 2 months to 3 years after treatment. 5 were showed pruritus, and 2 showed hyperpigmentation (Figure 4). 34 cases of Kimura's disease were treated. Methods and prognosis (Table 3).
Treatment
Total
Follow-up
Recurrence
Surgical
14
10
4
Surgery + Radiotherapy
6
4
1
Surgery + Radiotherapy + Chemotherapy
2
1
0
Surgery + Steroid
8
6
2
Surgery + Steroid + Chemotherapy
2
1
1
Steroid
1
0
0
Table 3: Treatment and Outcome of 34 patients with Kimura disease.
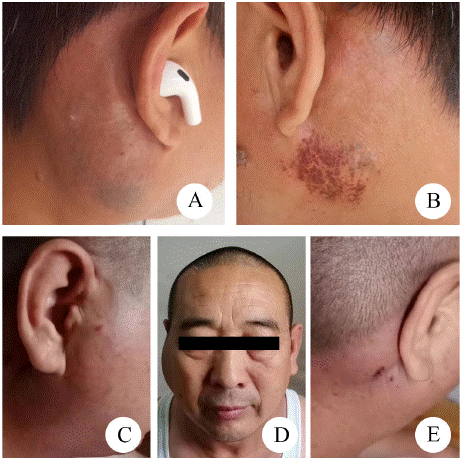
Figure 4: Recurrence after treatment for Kimura disease. (A, B) Patient
1, was treated by surgery and steroid, hyperpigmentation and pruritus of
bilateral parotid glands showed 6 months after treatment. (C, D, E) Patient
2, was treated by surgery alone, relapsed two months after treatment, and
manifested as bilateral painless masses.
Pathological Characteristics
In the study, the pathology report suggested partial glandular destruction, lymphoid follicular hyperplasia with massive eosinophilic infiltration, and prominent eosinophilic micro-abscesses and follicular lymphoid hyperplasia [6] (Figure 5). The immunohistochemical analysis of 16 cases, a lot of CD3-positive were present in 13 cases and a large number of CD20-positive in 13 cases, partial CD21 positivity can be seen in 9 cases, Bcl-6 was positive in 8 cases and Bcl-2 was positive in 2 cases. A few IgG-positive were observed in 8 cases. In situ hybridization, 8 cases showed EBER CISH negative and 2 cases showed Braf (V600E) negative.
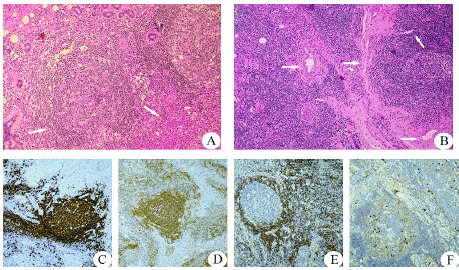
Figure 5: Histologic features (Hematoxylin–eosin staining magnification:
×100) (A) Prominent eosinophilic infiltrate with micro-abscesses formation,
and lymphoid follicular hyperplasia. (B) Increased vessel. (C) A large number
of CD20-positive. (D) Partial CD21 positivity. (E) Bcl-2 was positive; F: A few
IgG4-positive cells.
Recurrence Predictors
Follow-up data on 23 patients revealed that and 8 patients recurred within 2 months to 3 years after the treatment, the recurrence rate was 34.78%. Table 4 shows predictors of recurrence. Univariate analysis showed duration (P=0.006) and lymphocytes (P=0.021) were associated with recurrence. There were no significant recurrence-related differences in gender, age, location, multiplicity, laterality, maximum size, symptom, eosinophils, eosinophil count, neutrophils, lymphocyte count, smoking and drinking history, past medical history (P<0.05).
factor
Total
recurrence
P-value
Gender
0.482
Male
19
6
Female
4
2
Age
0.879
<40
11
4
>=40
12
4
Duration
0.006
<1
10
1
1~5
9
3
>5
4
4
Smoking history
0.955
none
20
7
have
3
1
Alcohol history
0.651
none
19
7
have
4
1
Site
0.214
Head and neck
20
6
Head, neck and other parts
3
2
Multiplicity
0.363
Single
17
5
Multiple
6
3
Laterality
0.181
Unilateral
18
5
Bilateral
5
3
Maximum size, cm
0.113
<3cm
12
4
=3cm
9
4
swelling
2
0
Pruritus
0.591
None
16
5
Have
7
3
Hyperpigmentation
0.363
None
17
5
Have
6
3
Boundary
0.433
Clear
5
1
Unclear
18
7
Eosinophils (%)
0.175
Normal
3
0
Elevated
20
8
Eosinophil count (109/L)
0.175
Normal
3
0
Elevated
20
8
Neutrophils (%)
0.591
Decreased
7
3
Normal
16
5
Elevated
Lymphocytes (109/L)
0.021
Decreased
3
2
Normal
20
6
Elevated
0
0
Lymphocyte count (109/L)
0.175
Decreased
3
0
Normal
20
8
Elevated
0
0
Cardiovascular history
0.455
None
22
8
Have
1
0
Allergic reaction history
0.591
None
16
5
Have
7
3
Table 4: Recurrence predictors.
Discussion
Kimura's disease is a chronic inflammatory disease and presents as a painless mass or subcutaneous nodule with pruritus occasionally. The lesions are single or multiple, and may appear in different locations over several years [7-11]. KD often involves the salivary glands, and cervical lymph nodes, and also involves other areas such as the armpits and groin. When salivary glands and cervical lymph nodes are involved, they need to be differentiated from malignant lymphoma, and parotid tumors such as Warthin's tumor.
In the study, the average duration of KD was 3.8 years, and the longest case was up to 30 years. 58.82% occurred unilaterally and 55.88% showed multiple. 30 cases developed in the head and neck region, of these, 53% involved the parotid gland, and 13.3% showed the eyelids. It may be considered that there are more lymph nodes in the head and face, so the parotid glands, eyelids, and other upper cervical lymph nodes were involved earlier. Gao et al [12] reported that increased eosinophils in the subcutaneous tissue induced the release of relevant cytokines and neurotransmitters, when delivered to the sensory nerve fibers in the subcutis, resulting in pruritus. In our study, all cases with pruritus were accompanied by elevated eosinophils, similar to our results. We found that the lesion skin was accompanied by hyperpigmentation, which has not been reported previously.
MRI helps identify the location and size of the mass and the diagnosis. Previously reported that KD was considered when MRI demonstrated an ill-defined lesion and increased lymph nodes, which is similar to this study [13,14]. In our study, it can be classified into two types according to the MRI, one is a well-defined nodular lesion and the other is an ill-defined patchy or lobulated lesion. Boundaries may be related to the duration of inflammatory infiltration around the lesion. In addition, increased lymph nodes and diffuse atrophy of subcutaneous fat at the lesion are characteristic imaging findings of KD.
The pathogenesis of KD is not clear. According to Munemura et al [15], when the body's immunity is disordered, a large number of T helper (Th) cells differentiate into type 2 helper T (Th2) cells and release cytokines, collectively activating eosinophils and causing serum eosinophilia. In our study, bone marrow puncture and blood routine showed eosinophils, which may be related to pathogenesis. Pathology is the gold standard for diagnosis, characteristics of KD include dense lymphoid follicular hyperplasia, massive eosinophilic infiltration, accumulation of micro-abscesses, venular hyperplasia, and differing extents of fibrosis [16-18]. Bi et al [19] reported of KD involving the eyelids, a lot of CD3-positive and partial CD20 positivity were present in the lymphoid follicles, and CD20-positive were observed in all areas. Gurram et al [20] showed CD3 and CD20 positivity in the submandibular region. BCL-6 is specific for B lymphocytes, and is often identified with reactive follicular hyperplasia, lymphomas, and malignant tumors [21]. In the study, it was found that a few IgG positives were because of the synthesis of B cells induced by cytokines released by Th cells [15,22,23].
Definite diagnosis is for better treatment, many treatment modalities have been proposed, including surgery, steroid, chemotherapy, radiotherapy, and combined treatment modalities, but there has been no consensus up to now. Some [24,25] reported recommending surgical resection with negative margins is preferred. Although it is considered to be a benign disease, KD is sensitive to radiotherapy and is the first choice for recurrent cases or patients with incomplete resections [26]. Multiple cases should be treated with steroids, but it is easy to relapse during the clinical course [27- 30], therefore, it is a second-line regimen in the treatment of KD. In the study, the recurrence rate for those who were treated by surgery alone was 17.39%, the recurrence rate for those who were treated by surgery and steroid was 8.69%, the recurrence rate for those who underwent combined surgery, steroid and, chemotherapy was 4.35%, the recurrence rate for those who was 4.35%, and 1 case were treated by surgery and radiotherapy who died 4 months after radiotherapy.
Recurrence rates are high even after treatment, which is related to ill-defined lesions and increased lymph nodes. In previous reports, we found that eosinophilia, maximum size of the mass, and pruritus are associated with recurrence [31]. Yang et al [14] followed up on 29 patients and found that symptom duration, bilateral, size, eosinophilia, and ill-defined mass were the main predictors of recurrence. Our study shows that duration longer than or equal to 5 years and lymphocyte decrease are associated with recurrence, which helps us to better reduce the recurrence rate.
Conclusions
In the study of 34 patients, our study confirmed that KD is considered with painless masses or lymph node involvement, accompanied by pruritus and hyperpigmentation, and eosinophilia. MRI showed well-defined nodular lesions or ill-defined patchy or lobulated lesions, increased lymph nodes and diffuse atrophy of subcutaneous fat at the lesion are characteristic imaging findings of KD. Pathology is the best way of diagnosis, immunohistochemistry showed diffuse expression of CD3, CD20, and CD21, as well as partial expression of Bcl-6 positivity and weak IgG4 positivity. After surgery, we can choose appropriate adjuvant treatment according to duration and lymphocytes to control recurrence.
Abbreviations
KD: Kimura's disease; TIVA: Total intravenous anesthesia; Ig E: Immunoglobulin E; MRI: Magnetic resonance imaging.
Authors’ Contributions
JX wrote the paper. PFX carried out data collection. CYH, CMH and KS were involved in statistical analysis. WQL and XQ modified the paper and designed this study concepts. All authors read and approved the final manuscript.
Funding
The authors received financial supports from Hubei Provincial Natural Science Foundation of China (2022CFB706, 2023AFB765), Fund of Hubei Province Key Laboratory of Oral and Maxillofacial Development and Regeneration (2022kqhm003) and Huazhong University of Science and Technology Teaching Research Project (2021158).
Availability of Data and Materials
The raw data are confidential and cannot be readily shared. Researchers need to obtain permission from the institutional Review Board and apply for data access to The Ethics Committee of The study was approved by the ethics committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology.
Declarations
Ethics Approval and Consent to Participate
The study was approved by the ethics committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (TJ-JRB20231293). All participants volunteered for the study, were informed about the scope of the study and provided written consent.
Author Details
1Department of Stomatology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China. 2School of Stomatology, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China. 3Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences Tongji Shanxi Hospital, Third Hospital of Shanxi Medical University, Taiyuan, 030032, China.
References
- Kimura T. On the unusual granulations combined with hyperplastic changes of lymphatic tissue. Trans Soc Pathol Jpn. 1948; 37.
- Kapoor NS, O’Neill JP, Katabi N, Wong RJ, Shah JP. Kimura disease: diagnostic challenges and clinical management. Am J Otolaryngol. 2012; 33: 259-262.
- Sahu P, Jain S, Kaushal M. Kimura’s disease: A short study of cytomorphologic features with its differential diagnosis and review of literature. Cytojournal. 2022; 19: 50.
- Ao S, Huang G, Tang X, Zhu Z, Han J, Wang F, Zhai W. Anti-immunoglobulin E provides an additional therapy to conventional steroids for Kimura’s disease. J Dermatol. 2024; 51: 602-606.
- Luo SY, Zhou KY, Wang QX, Deng LJ, Fang S. Kimura’s disease treated with dupilumab: A case report and literature review. Int Immunopharmacol. 2024; 131: 111895.
- Bonfils P, Moya-Plana A, Badoual C, Nadéri S, Malinvaud D, Laccourreye O. Intraparotid Kimura disease. Eur Ann Otorhinolaryngol Head Neck Dis. 2013; 130: 87-89.
- Laguna J, Rodríguez-García M, Molina A, Merino A. Kimura disease as an uncommon cause of persistent hypereosinophilia: a diagnostic challenge. Biochem Med (Zagreb). 2023; 33: 020801.
- Lyu Y, Cui Y, Ma L, Guan L, Wen Z, Huang J, Shi M, Hou S. Dupilumab combined with corticosteroid therapy for Kimura disease with multiple systemic masses: a case report and literature review. Front Immunol. 2024; 15: 1492547.
- Zou A, Hu M, Niu B. Comparison between Kimura’s disease and angiolymphoid hyperplasia with eosinophilia: case reports and literature review. J Int Med Res. 2021; 49: 3000605211040976.
- Yang B, Liao H, Wang M, Long Q, Zhong H, Luo L, Liu Z, Cheng X. Kimura’s disease successively affecting multiple body parts: a case-based literature review. BMC Ophthalmol. 2022; 22: 154.
- Wang X, Ma Y, Wang Z. Kimura’s Disease. J Craniofac Surg. 2019; 30: e415-e418.
- Gao Y, Chen Y, Yu GY. Clinicopathologic study of parotid involvement in 21 cases of eosinophilic hyperplastic lymphogranuloma (Kimura’s disease). Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 102: 651-658.
- Sangwan A, Goyal A, Bhalla AS, Kumar A, Sharma R, Arava S, Dawar R. Kimura Disease: A Case Series and Systematic Review of Clinico-radiological Features. Curr Probl Diagn Radiol. 2022; 51: 130-142.
- Zhao F, Zhou M, Mao A, Zhang Y, Chen Y. Kimura Disease: A Detailed Analysis of Clinical and Radiological Manifestations in a Retrospective Case Series. J Inflamm Res. 2024; 17: 3371-3381.
- Munemura R, Maehara T, Murakami Y, Koga R, Aoyagi R, Kaneko N, et al. Distinct disease-specific Tfh cell populations in 2 different fibrotic diseases: IgG(4)-related disease and Kimura disease. J Allergy Clin Immunol. 2022; 150: 440-455.
- Bishop C, Wilhelm A, Watley D, Olobatuyi F, Coblens O, Joshi R. Kimura Disease: A Rare and Difficult to Diagnose Entity. Head Neck Pathol. 2022; 16: 278-281.
- Solomon L, Modak K, Paul PAM, John J. A common presentation of an uncommon pathology: Kimura disease. Trop Doct. 2023; 53: 512-516.
- Zhuang S, Chen X, Chen W, Li C, Wang T, Lin Q, Wang D. A retrospective study of 20 patients with Kimura’s disease from China. Acta Otolaryngol. 2022; 142: 357-362.
- Bi YW, Cai RR, Wang SY, Zhu XZ. [The clinicopathologic features and differential diagnosis of ocular Kimura disease and epithelioid hemangioma]. Zhonghua Yan Ke Za Zhi. 2021; 57: 689-695.
- Gurram P, Chandran S, Parthasarathy P, Thiagarajan MK, Ramakrishnan K. KIMURA’S Disease - An E[X]clusive Condition. Ann Maxillofac Surg. 2019; 9: 183-187.
- Wei X, Niu X. T follicular helper cells in autoimmune diseases. J Autoimmun. 2023; 134: 102976.
- Lee CC, Yu KH, Chan TM. Kimura’s disease: A clinicopathological study of 23 cases. Front Med (Lausanne). 2022; 9: 1069102.
- Wu X, Wang A, Zhang S, Wang X, Guo D, Zhu W, et al. Multiomic landscape of immune pathogenesis in Kimura’s disease. iScience. 2023; 26: 106559.
- Morinaga S, Yamamoto N, Hayashi K, Takeuchi A, Miwa S, Igarashi K, et al. Kimura’s Disease Diagnosed in the Department of Orthopedic Surgery Treated With Wide Excision: Report of Two Cases. In Vivo. 2023; 37: 1373- 1378.
- Hui PK, Chan JK, Ng CS, Kung IT, Gwi E. Lymphadenopathy of Kimura’s disease. Am J Surg Pathol. 1989; 13: 177-186.
- Kottler D, Barète S, Quéreux G, Ingen-Housz-Oro S, Fraitag S, Ortonne N, et al. Retrospective Multicentric Study of 25 Kimura Disease Patients: Emphasis on Therapeutics and Shared Features with Cutaneous IgG4-Related Disease. Dermatology. 2015; 231: 367-377.
- Zhang L, Yao L, Zhou WW, Ma JN, Zhang CQ. Computerized tomography features and clinicopathological analysis of Kimura disease in head and neck. Exp Ther Med. 2018; 16: 2087-2093.
- Zhang X, Jiao Y. The clinicopathological characteristics of Kimura disease in Chinese patients. Clin Rheumatol. 2019; 38: 3661-3667.
- Fan L, Mo S, Wang Y, Zhu J. Clinical, Pathological, Laboratory Characteristics, and Treatment Regimens of Kimura Disease and Their Relationships With Tumor Size and Recurrence. Front Med (Lausanne). 2021; 8: 720144.
- Moon Y, Kim H, Park H. Ear lobule reduction using a sub-antitragal groove technique in patients with angiolymphoid hyperplasia with eosinophilia on the earlobe: a case report and literature review. Arch Craniofac Surg. 2024; 25: 192-196.
- Lee CC, Feng IJ, Chen YT, Weng SF, Chan LP, Lai CS, Lin SD, Kuo YR. Treatment algorithm for Kimura’s disease: A systematic review and metaanalysis of treatment modalities and prognostic predictors. Int J Surg. 2022; 100: 106591.