Research article
Austin J Surg. 2025; 12(1): 1346.
Management of Spitz Lesions in Children: What are the Best Practices for Suspected Spitz Tumor?
Coppola V¹*, Di Mento C¹, Cerulo M¹, Esposito G², Fornaro L³, Scalvenzi M³, Escolino M¹ and Ciro E¹
¹Pediatric Surgery Unit, Department of Medical Translational Science, University of Naples Federico II, Italy
²Internal Medicine Unit, Department of Medical Translational Science, University of Naples Federico II, Italy
³Dermatology Clinic, Department of Public Health, Pharmacology and Dermatology, University of Naples Federico II, Italy
*Corresponding author: Vincenzo Coppola, MD, University of Naples Federico II, 5, Pansini street, Naples, Italy Tel: 0039 0817463298; Fax: 0039 0817463293; Email: vincenzocoppola1992@gmail.com
Received: January 16, 2025; Accepted: February 03, 2025; Published: February 06, 2025
Abstract
Purpose: Spitzoid lesions are categorized into Spitz nevi, Spitz tumors and spitzoid melanomas. While the understanding of Spitz nevi has improved, the malignant potential of Spitz tumors remains uncertain, leading to diagnostic and management challenges. This study evaluates the surgical management of pediatric patients with suspected Spitz lesions.
Methods: Fifty-eight pediatric cases of suspected Spitz lesions were analyzed. Data on demographics, lesion characteristics, surgical indications, histopathology, and follow-up were collected. Histopathological groups included Spitz nevus (G1), Spitz nevus/tumor with mild atypia (G2), Spitz tumor with moderate atypia (G3), and spitzoid melanoma (G4). Subgroups were based on whether additional procedures (A) or follow-up only (B) were needed.
Results: The mean patient age was 14.6 years, with a female predominance (58%). Lesions were commonly located on the arts (51%), thorax (35%), and head (9%). Histopathological findings were: 37 (64%) Spitz nevus (G1), 9 (15%) Spitz nevus/tumor (G2), 11 (19%) Spitz tumor (G3), and 1 case of spitzoid melanoma (G4). Additional procedures were necessary in 5/9 (55%) in G2, 10/11 (91%) in G3, and 1/1 in G4, mostly involving margin widening. In all cases of G2 (5/5) and most of G3 (8/10), the requested margin expansion failed.
Conclusion: The diagnosis of suspected Spitz tumors in children often lacks clarity, leading to treatment decisions influenced by clinicians’ experience rather than standardized guidelines. Margin widening should be limited to cases with high-grade atypia Spitz tumors. Additionally, other factors, such as aesthetic considerations and potential complications, especially in visible areas, should always be taken into account.
Keywords: Spitz tumor; Nevi; Children; Wound; Tumor
Introduction
In recent decades, spitzoid melanocytic proliferations lesions have been classified into three types: Spitz nevi, atypical Spitz tumors, and spitzoid melanomas [1].
A Spitz Nevus is a melanocytic neoplasm of epithelioid and/or spindle cells that usually appears in childhood. These lesions are by nature benign, but their features such as the present of cellular atypia can sometimes make them difficult to distinguish from melanomas [2].
Atypical Spitz tumors are spitzoid melanocytic proliferations with atypical histopathologic features that are not sufficient for a melanoma diagnosis. The malignant potential of these lesions remains uncertain [2].
Spitzoid melanoma is a type of melanoma who shares many histopathologic features with Spitz nevus. The incidence rate of the two diseases is definitely different: while the former is more present in infants or young adults, the latter appears in adults/elderly people [5]. Despite that, still today It represents one of the most difficult lesions to diagnose in dermatophatology and a misdiagnosis of a melanoma as a Spitz nevus is one of the most frequent causes of malpractise lawsuits in surgical pathology and dermatopathology [3].
In literature are reported many review who analyzed clinical, dermoscopic, and histopathologic features of this lesions to try to optimize the diagnostic criteria [2,4,6,7,23].
In pediatrics, a fundamental role is played by the dermatoscopic examination [8]. The International Society of Dermatoscopy has recognized 3 most frequent patterns of representation of Spitz lesions: starburst pattern, a pattern of regularly distributed dotted vessels and globular pattern with reticular depigmentation [22].
Surgical excision of the lesion is requested to carry out histological analysis for malignant potential rate. Having clarified the absence (Spitz nevus) or fully presence (sptizoid melanoma) of malignancy, the management of paediatric patients with histo-pathological findings of cellular atypia of uncertain meaning (spitz tumor) is still very complex.
For this reason, new diagnostic guidelines have been proposed, which are showing promising results, although further evaluation is needed. Among these proposals, an additional, not well-defined group of Spitz tumours with limited metastatic potential was introduced. This represents those cases in which microscopic evaluation of the lesion may be inconclusive, resulting in a verdict of Spitz tumour of uncertain malignant potential (STUMP). STUMP would therefore represent a separate entity and should not be equated with Spitz tumors with limited metastatic potential [9].
Even today, however, the tendency of paediatric dermatologists is to manage all lesions with cellular atypia in the same way: surgical excision and the need for surgical enlargement of the lesion after histopathological confirmation of suspected spitz tumor. This study aims to retrospectively evaluate the management of children diagnosed with Spitz lesions following the new proposed clinical and histopathological evaluations, to understand whether the management of these patients occurs correctly.
Methods
We retrospectively analyzed the management of 58 children from 2014 to 2020 sent to our pediatric surgery center under dermatological indication following a suspicion of a Spitz lesion, to carry out a surgical exeresis.
By analysing the medical records we collected the demographic data of these children, the type of pattway of the lesion at dermatoscopic examination, the indication for surgical excision, the histopathological result, with an analysis focused above all on post-operative management. In fact, the needs to carry out further procedures such as surgical re-intervetion for enlargement of free margins were analysed. In this case, the time between the istopathological diagnosis and the surgical re-intervention was also taken into account.
Inclusion criteria included patients who had a post-excision follow-up of at least 4 years. The follow-up included one clinical and dermatoscopic check-up per year. Exclusion criteria were patients with post-excision diagnosis of non-Spitzoid lesion, mis-diagnosis, and patients who did not perform a follow-up at least 4 years later.
The elements considered by the histo-pathological findings were: macroscopy (organization into nodules), presence of Kamino bodies, irregularities of the dermis, mitotic activity, positivity to immunohistochemistry (BRAFV600E, MART1, HMB45, p16, Ki67) and free margin of healthy skin. We divided the patients according to the hystophatologic diagnosis of Spitzoid lesion they received: group G1 with a diagnosis of Spitz nevus, group G2 with a diagnosis of Spitz Nevus/Tumor (low degree of cellular atypia), group G3 with a diagnosis of Spitz tumor (moderate degree of cellular atypia), group G4 with Spitz tumor with high degree of cellular atypya or Spitzoid melanoma.
We then divided the post-excision management of each group into patients who underwent other procedures (including widening of the wound excision margins) (classified as subgroup A) and patients who only underwent follow-up (classified as subgroup B).
In case of widening of the excision margins we evaluated the subsequent result of the histopathologic analysis. In both subgroups (A and B), any post-operative complications were evaluated at the first and second surgical excision. In the case of widening of the excision margins, the histopathological response of the margins was evaluated.
Statistical Analysis
To compare the probability of being referral to a treatment (A or B) in each group and the probability of being referred to other procedures in case of A we used a binomial test. To compare the results obtained between the groups (G1vsG2, etc) we used the Fisher’s exact test, that is very accurate for smaller sample sizes. Lastly, to compare the probability of having a negative histophatology report in case of surgical widening we used the binomial test but with an adjusted baseline probability.
Results
The mean age of patients with suspected Spitz lesion was 14.6 years. 58% of cases were female and the most frequent localizations were the arts (51%), the thorax (35%) and finally the Head (9%) and others (5%). The most frequently reported pattern following the dermatoscopic examination was the starbarst type (67% cases).
In all 58 cases analyzed, a biopsy of the lesion was recommended for suspected spitz proliferation (100%). Furthermore, in all cases total excision procedure was carried out (100%) with a lesion-free margin of at least 1 mm on each side.
All demographic and preoperative evaluation data of the patients are reported in Table 1.
Total
Demographics Data
Age (years)
14.6 (9.1-16.9)
Site
- Arts
- Thorax
- Head
- Others
30 (51%)
20 (35%)
5 (9%)
3 (5%)Lesion Features
Morphology
- Starburst pattern
- Pattern of regularly distributed dotted vessels
- Globular pattern with reticular depigmentation
- Others
35/58
14/58
8/58
1/58Average diameter (mm)
15 (4-42)
Average time between initial suspicion and surgical excision (months)
1.4 (0.1 – 6.4)
Surgical Indication
Indication to biopsy
58/58 (100%)
Partial Biopsy
0/58 (0%)
Total Biopsy
58/58 (100%)
Table 1: Demographics data and lesion characteristics.
The histopathological report (Table 2) of these patients showed 37 diagnoses (64%) of Spitz Nevus (G1), 9 cases (15%) of Spitz Nevus/ tumor (low atypia reported) (G2), 11 cases (19%) of Spitz tumor (moderate atypia reported) (G3) and 1 case (0.01%) of Spitzoid Melanoma (G4).
Total
Organization in nodules
40/58
Kamino bodies
38/58
Immunoistochimicy (BRAFV600E, MART1, HMB45, p16, Ki67)
1/58
Irregular maturation of Derma
11/58
Relevant Mitotic Activity
2/58
Widening of Surgical margins at least 5 mm
0/58 (0%)
Widening of surgical margins at least 1 mm
58/58 (100%)
Histopathology diagnosis
- Spitz nevus (G1)
- Spitz nevus/tumor (G2)
- Spitz tumor (G3)
- Spitzoid melanoma (G4)
37/58 (64%)
9/58 (15%)
11/58 (19%)
1/58 (0.01%)
Table 2: Result of Histopathologic report after total excision of the lesion.
In G1 all patients did not undergo further procedures later (0/37 G1A and 37/37 G1B) and were followed only with one follow-up per year.
In G2 in 5 patients an additional procedure was required (G2A 55.6%), represented in all cases by widening the surgical margins, while in 4 patients nothing was required, and they were only followed up clinically (G2B 44.4%). In G3 in 10/11 cases further procedures were indicated (G3A 91%), represented in all cases only by widening of the surgical margins, and just 1/10 patient did not receive nothing more (G3B 9%). In the G4 group in the only patient analysed, both widening of surgical margins and sentinel lymph node search were requested (G4A 100%).
All data relating to diagnosis and post-operative management are reported in Table 3.
Post-operative management type A
Post-operative management type B
p
Other procedures performed
in case of Ap
Widening of excision margins
Search for the sentinel lymph-node
Others
G1
0/37
(0%)37/37
(100%)1.46 x10-11
0/37
0/37
0/37
n/a
G2
5/9
(55.6%)4/9
(44,4%)1
5/5
(100%)0/5
0/5
0.0041
G3
10/11
(91%)1/11
(9%)0.012
10/10
(100%)0/10
0/10
1.69x10-5
G4
1/1
(100%)0/1
(0%)1
1/1
(100%)1/1
(100%)0/1
0.333
Statistical Analysis with fisher’s test for comparison between each group.
G1
G2
G3
G4
G1
n/a
9.19-5
1.68-9
0.026
G2
9.19-5
n/a
0.127
1
G3
1.68-9
0.127
n/a
1
G4
0.026
1
1
n/a
Table 3: Management of patients after histopathologic result.
Histopathological analysis following the second widening of the excision margins (Table 4) reported: in G2A 5/5 cases (100%) of "absence of further cellular abnormalities", in G3A 8/10 cases (80%) of "absence of further cellular abnormalities", 1/10 cases (10%) of "uncertain result" and 1/10 (10%) cases of "deep dermal abnormalities in the absence of appreciable mitotic activity". In G4A it reported 1/1 case (100%) of "no further cellular abnormalities". Sentinel lymph node search in G4A was negative.
G1A
G2A
G3A
G4A
Absence of further cellular abnormalities
n/a
5/5
8/10
1/1
Uncertain result
n/a
0/5
1/10
0/1
Deep dermal abnormalities in absence of appreciable mitotic activity
n/a
0/5
1/10
0/1
p
n/a
0.0041
0.0034
0.333
Table 4: Results of histopathology after second re-surgery for widening of wound margins.
Regarding postoperative complications, no complications occurred after the first removal procedure. In patients who were referred for further surgical procedures (G2A + G3A + G4A), 3/20 complications were reported, such as 1 case of surgical wound infection, managed at home with antibiotic therapy, and 2 cases of surgical wound dehiscence, which required ordinary hospitalization.
From the 4-year follow-up carried out in all patients, 0 cases of recurrence or appearance of new lesion on the surgical scar were detected.
Discussion
The management of children with spitzoid melanocytic lesions has always been very complex [7]. Clinical evaluation is the first and most important element for diagnostic suspicion.
The age of onset of the lesion and the size of the lesion are two important factors that strongly direct the prognosis [10]. In fact, spitzoid lesions are more frequent in childhood and adolescence.
Another element is the presence of congenital lesions, which often present with histopathological features of both a spitz nevus and a superficial congenital melanocytic nevus [11]. The clinical history including the age of the patient should be always considered for a correct diagnosis [12].
As with all pigmented lesions of the skin, the dermatoscopic description of the lesion's characteristics, and any changes over time, is of fundamental importance. In fact, there are dermatoscopic criteria that can characterise spitz nevus, such as starburst, globular, homogeneous, reticular and multicomponent pattern, or the presence of dotted vessels, whether combined with an inverted network [13].
The indication nowadays when Spitz melanosis is suspected is to perform a surgical excision. The data from our study confirm that clinical knowledge of these lesions among dermatologist has considerably increased and excisions are indicated correctly. The same cannot yet be said regarding the management of patients posthistopathological characterization, especially in doubtful cases of suspected Spitz tumor.
The high statistical significance of the probability of being totally referred for follow-up in the G1 group demonstrates how there is better knowledge of these lesions.
The stark contrast yields between G1 and G3 group (p=1.68-9) shows a highly significant result, emphasizing a pronounced difference in referral patterns between these two groups.
This demonstrates how the management of patients diagnosed with Spitz nevus and that with Spitz tumor is contrasting, demonstrating how there is a tendency to treat Spitz tumor as if it were a certainly malignant lesion.
The uncertain malignant potential of these lesions has always been a cause for concern [4,10] but we believe it is necessary to clarify this to avoid mismanagement.
Our study shows how the management in case of suspected malignancy is always the same: the widening of the margins, limiting the search for the sentinel lymph node only to certain cases of spitoid melanoma, as also suggested several times by the guidelines of the Italian dermatology society.
In this regard, analysis of the histopathological features of the lesion should play a diriment role [15].
Unfortunately, we are not yet at a complete level of clarity in this respect. Although the benign nature of Spitz's nevi is fully elucidated, the debate on the nature of Sptiz's tumours is still open, as demonstrated by a very recent study conducted by Dika [16].
This implies that many spitz nevi with pronounced atypical cells are described as suspected tumors when in fact they are not. This leads the clinician to question whether or not to proceed with surgical enlargement.
The most interesting data, in fact, is related to the comparison of the G1 and G2 (p=9.19-5), where in this second case there is a mixed/ unclear histopathological diagnosis. In this case there is no univocal management, but the subsequent therapeutic decision depends on other factors, first of all the clinician's experience.
However, the negative histopathological result of the majority of those re-operated in G2A demonstrates once again that it is not necessary to recommend this procedure in case of doubtful results.
In an interesting study by Gelbrand et all, the management of these lesions by one thousand American dermatologists was evaluated. It was seen that 93% of them recommended biopsy in the case of Sptiz nevi, which for 43% of the specialists should be totally excisional. Interestingly, 9% of the dermatologists only considered excisions to be valid if they had a lesion-free margin of more than 4 mm [14]. This finding confirms our study's assumption that there is still a tendency to perform a margin enlargement if there are any doubts.
Research is moving in this direction and numerous studies have attempted to define the histo-pathological features of these lesions for proper post-operative management [2,6,15].
Molecular analysis has always played a key role in this [17].
New tools, such as immunohistochemical stains, comparative genomic hybridization, and fluorescence in situ hybridization, have been used to provide further insight into these controversial lesions and to aid in their evaluation [18]. Despite that, not all the new technique suggested are available in all hysto-pathologic units.
A study by Raghavan et al. assessed the degree of PRAME expression in this type of lesion. The diffuse PRAME expression typical of melanoma is very rarely expressed in spitzoid lesions, whether in spitz nevus or spitz tumour with atypia [19].
Moreover, the proposal of new classification forms such as STUMP [9,18] would bring more information to the clinician for the correct management of these patients. Our idea is that there is still a tendency of pathologist-analysts to describe a suspected malignancy picture when there is the slightest doubt.
This is no longer tolerable considering that certain important elements are not included when dealing with the paediatric patient. First, the site of these lesions. The data of our study are in line with those reported in the literature [20], which report a greater tendency of Spitz lesions in females with more affected sites such as lower limbs and trunk. However, areas such as the face, which have a great aesthetic impact when it comes to excision and eventual widening of the excision margins, should not be excluded. Figure 1 shows the aesthetic outcome of the removal of a Spitz's lesion at the sternal site (along the breast line) in a 15-year-old girl, where a 5 mm widening of the margins was required due to the presence of cell atypia as per Spitz's tumor. We believe that in such cases the aesthetic and consequently psychological impact should also be considered before choosing any enlargement based on mere suspicion.
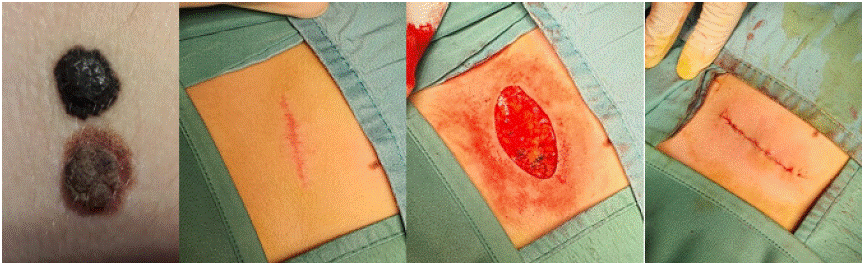
Figure 1: Suspected spitz lesion in the thoracic region at first dermatoscopic evaluation with associated melanocytic nevus (a). Aesthetic result of the first
surgical removal at 3 months (b). Request for surgical widening of the wound margins due to Spitz nevus/tumor diagnosis with marked atypia (c). Scar result
after one week the second surgery (d).
Any surgical complications that may occur during wound healing, such as infection or dehiscence of a surgical wound, should also be considered. The latter condition can have a negative impact on the hospitalisation and rehabilitation of these patients and can also cause major problems [21]. Therefore, the indication for a second surgery in the pediatric population should be carefully evaluated.
Despite this, our data confirm that the tendency to date is to recommend surgical wound augmentations to have a lesion-free margin of more than 5 mm.
Our data confirm that, regardless of the free margin, the incidence of new lesion at 5 years is completely negligible. Probably, the tendency of histopathologists to describe lesions as suspiciously atypical as soon as they have an undefined morphology still influences the management of these patients. This would also explain how such a high number of atypical lesions is possible in the pediatric population, where the incidence should be very rare.
However, the following study has its limitations. First of all, the heterogeneity of the clinicians and surgeons involved, whose management could be influenced by their degree of experience. Furthermore, the possibility that additional factors could influence the decision to perform a re-intervention, such as initial lesion size, familiarity, patients' willingness, etc., was not considered.
Another element is the loss of many patients in the follow-up. It’s important to emphasize that our study was not designed to take into account the incidence of disease or the likelihood of receiving one type of diagnosis over another. In fact, many of the patients studied in our center were lost to follow-up after lesion exeresis, especially them with diagnoses of Spitz nevi. It’s our opinion that they should still be followed up over time to acquire further clinical data in this regard.
The development of further studies with long-term follow-up could indeed be a clear and diriment element to clarify the evolution of these lesions.
This study aims to demonstrate the need for further investigations in the histopathological field, suggesting the drafting of guidelines, and not to neglect other important factors when dealing with pediatric patients.
In conclusion, we believe that only in case of spitzoid tumors with melanoma-like patterns or high mitotic index should be surgically widened. For all the rest of the lesions, sure of total excision, the best indication in children would be to perform a clinical follow-up for the first few years with dermoscopy and possible biopsy of the scar in case of doubt. This would have a strong impact on patient management and would not expose the patient to any risks that would be unnecessary.
Financial Disclosure Statement
All authors declare that they have no conflicts of interest. The authors report that they did not participate in an organization or entity with a financial or non-financial interest in the subject matter or materials discussed in this manuscript.
References
- Brown A, Sawyer JD, Neumeister MW. Spitz Nevus: Review and Update. Clin Plast Surg. 2021; 48: 677-686.
- L Sainz-Gaspar, J Sánchez-Bernal, L Noguera-Morel, A Hernández-Martín, I Colmenero, A Torrelo. Spitz Nevus and Other Spitzoid Tumors in Children -Part 1: Clinical, Histopathologic, and Immunohistochemical Features. Actas Dermosifiliogr (Engl Ed). 2020; 111: 7-19.
- Hideko Kamino. Spitzoid melanoma. Clin Dermatol. 2009; 27: 545-555.
- Melissa Gill, Jason Cohen, Neil Renwick, Joan M Mones, David N Silvers, Jülide Tok Celebi. Genetic similarities between Spitz nevus and Spitzoid melanoma in children. Cancer. 2004; 101: 2636-2640.
- Benjamin A Wood. Paediatric melanoma. Pathology. 2016; 48: 155-165.
- L Sainz-Gaspar, J Sánchez-Bernal, L Noguera-Morel, A Hernández-Martín, I Colmenero, A Torrelo. Spitz Nevus and Other Spitzoid Tumors in Children. Part 2: Cytogenetic and Molecular Features. Prognosis and Treatment. Actas Dermosifiliogr (Engl Ed). 2020; 111: 20-25.
- Mirna Situm, Zeljana Bolanca, Marija Buljan, Davor Tomas, Marijana Ivancic. Nevus Spitz--everlasting diagnostic difficulties--the review. Coll Antropol. 2008: 32 Suppl 2: 171-176.
- I Neri, E Dika, G M Ravaioli, A Patrizi. Spitz nevi: defining features and management in children. G Ital Dermatol Venereol. 2014; 149: 675-682.
- FD Menezes, W J Mooi. Spitz Tumors of the Skin. Surg Pathol Clin. 2017; 10: 281-298.
- CB Ko, S Walton, EH Wyatt, HP Bury. Spitz nevus. Int J Dermatol. 1993; 32: 354-357.
- MN Harris, RM Hurwitz, LJ Buckel, HR Gray. Congenital spitz nevus. Dermatol Surg. 2000; 26: 931-935.
- G Argenziano, M Scalvenzi, S Staibano, B Brunetti, D Piccolo, M Delfino, et al. Dermatoscopic pitfalls in differentiating pigmented Spitz naevi from cutaneous melanomas. Br J Dermatol. 1999; 141: 788-793.
- M. Scalvenzi, C. Costa, G. De Fata Salvatores, M. Cappello, A. Villani. Clinical and dermoscopic features of Spitz naevus by sex, age and anatomical site: a study of 913 Spitz naevi. British Journal of Dermatology. 2018; 79: 769–770.
- Sandra N Gelbard, Jackie M Tripp, Ashfaq A Marghoob, Alfred W Kopf, Karen L Koenig, John Y Kim, Robert S Bart. Management of Spitz nevi: a survey of dermatologists in the United States. J Am Acad Dermatol. 2002; 47: 224-230.
- Tiffany W Cheng, Madeline C Ahern 1, Alessio Giubellino. The Spectrum of Spitz Melanocytic Lesions: From Morphologic Diagnosis to Molecular Classification. Front Oncol. 2022: 12: 889223.
- Emi Dika, Martina Lambertini, Federico Venturi, Giulia Veronesi, Simona Mastroeni, Bor Hrvatin Stancic, Aleksandra Bergant-Suhodolcan. A Comparative Demographic Study of Atypical Spitz Nevi and Malignant Melanoma. Acta Dermatovenerol Croat. 2023; 31: 165-168.
- PD Da Forno, A Fletcher, JH Pringle, GS Saldanha. Understanding spitzoid tumours: new insights from molecular pathology. Br J Dermatol. 2008; 158: 4-14.
- Wynnis L Tom, Jessica W Hsu, Lawrence F Eichenfield, Sheila Fallon Friedlander. Pediatric “STUMP” lesions: evaluation and management of difficult atypical Spitzoid lesions in children. J Am Acad Dermatol. 2011; 64: 559-572.
- Shyam S. Raghavan, Jennifer Y. Wang, Shirley Kwok, Kerri E. Rieger, Roberto A. Novoa, Ryanne A. Brown. PRAME expression in melanocytic proliferations with intermediate histopathologic or spitzoid features. J of Cutaneous Pathology. 2020.
- L Le Saché-de Peufeilhoux , I Moulonguet, B Cavelier-Balloy, A Biaggi- Frassati, S Leclerc-Mercier, C Bodemer, et al. Clinical features of Spitz naevus in children: a retrospective study of 196 cases. Ann Dermatol Venereol. 2012; 139: 444-451.
- Kylie Sandy-Hodgetts 1, Keryln Carville 1 2, Gavin D Leslie. Determining risk factors for surgical wound dehiscence: a literature review. Int Wound J. 2015; 12: 265-275.
- A Lallas, Z Apalla, D Ioannides, E Lazaridou, A Kyrgidis, P Broganelli, et al. Update on dermoscopy of Spitz/Reed naevi and management guidelines by the International Dermoscopy Society. Br J Dermatol. 2017; 177: 645-655.
- Iwei Yeh, Klaus J Busam. Spitz melanocytic tumours - a review. Histopathology. 2022; 80: 122-134.