1Department of Surgery, Okitama Public General Hospital, Japan
2Department of Surgery, Yonezawa City Hospital, Japan
3Department of Pathology, Okitama Public General Hospital, Japan
*Corresponding author: Toshio Hashimoto, Department of Surgery, Yonezawa City Hospital, 6-36 Aioicyo, Yonezawa City, Yamagata, Japan
Received: October 06, 2014; Accepted: November 27, 2014; Published: November 28, 2014
Citation: Hashimoto T, Toyono M and Fuyama S. A Case of Mammary Pagetoid Carcinoma with a Coexisting Fibroadenoma. Austin J Surg. 2014;1(9): 1043. ISSN: 2381-9030.
The patient was a 60-year-old woman who had been undergoing long-term follow-up for Fibroadenoma (FA) of the left breast, which was discovered through Mammography (MMG) for breast cancer screening. She visited our hospital with a principal complaint of erosion of the left nipple. Medical examination was performed, and a rare case of pagetoid carcinoma with coexistence of FA was found. Paget Disease (PD) consists of atypical cells that present in the intraepithelium of the nipple with invasive ductal carcinoma or ductal carcinoma in situ. The Japanese General Rules for Clinical and Pathological Recording of Breast Cancer classifies PD with invasive ductal carcinoma as pagetoid disease and differentiates it from PD with ductal carcinoma in situ. FA is a benign breast tumor that is common in young women. FA does not develop into a malignancy. However, breast cancer cases that occur at the FA epithelium are rarely reported. Based on the Magnetic Resonance Imaging (MRI) findings and pathological diagnosis, the FA had a connection with the invasive ductal carcinoma with an extensive ductal component. Therefore, invasive ductal carcinoma may originate from the FA epithelium.
Keywords: Breast cancer; Fibroadenoma coexistence; Pagetoid carcinoma; Paget’s disease
Paget Disease (PD) of the breast is a rare disease, accounting for 1–3% of all breast malignancies. PD is characterized pathologically by the presence of round intraepidermal cells of the nipple [1–5]. Although most cases of Paget disease will have an underlying breast malignancy [1–4], previous studies of patients treated with mastectomy have identified two distinct clinical presentations with associated histological findings [1,5]. Approximately 90% of patients who present with a palpable or mammographic mass will have an underlying invasive carcinoma. Conversely, 66–86% of patients without a clinical mass on physical examination or mammography will have Ductal Carcinoma In Situ (DCIS) alone [1,5]. Prognosis is determined primarily according to the presence or absence of an invasive component [1,2,5], and recommendations for systemic therapy are suggested accordingly.
According to the Japanese General Rules for Clinical and Pathological Recording of Breast Cancer, PD with invasive ductal carcinoma is classified as a pagetoid disease and is differentiated from PD with ductal carcinoma in situ [6].
Fibroadenoma (FA) is the most common breast tumor in women. Development of FA may be related to reproductive hormone levels. FA occurs more often during the reproductive years, can grow during pregnancy or use of hormone therapy, and may shrink after menopause, when hormone levels decrease. FA is a common benign breast tumor in which carcinoma rarely arises. Based on Magnetic Resonance Imaging (MRI) findings and pathological diagnosis, invasive ductal carcinoma may originate from the FA epithelium. Herein, we report a case of pagetoid carcinoma with a coexisting FA of the breast.
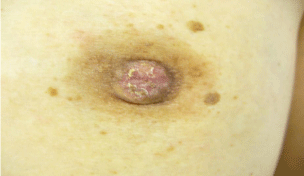
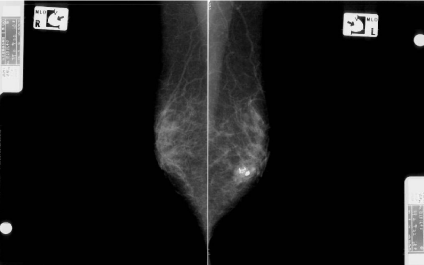
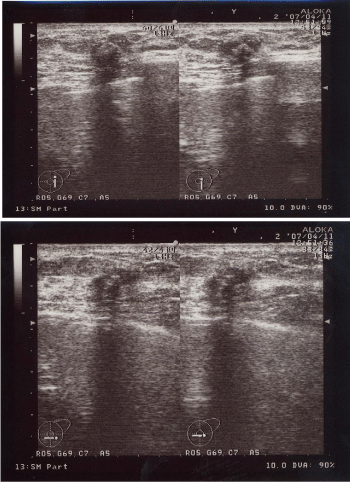
A 60-year-old woman is presented to our hospital with a principal complaint of erosion of the left nipple. A Further investigation into the patient’s history revealed that she had a history of Cholecystectomy at the age of 40 years. The patient had been long diagnosed with FA by screening Mammography (MMG) during breast cancer screening. Erosion of the left nipple was first observed in 200X and had been difficult to cure. Hence, the patient visited to hospital in 200X. Clinical finding shows the appearance of the eroded left nipple at the first visit (Figure 1). A mass was palpable at the lateral caudal area. The left axial lymph node was not palpable. Therefore, she underwent medical examination with MMG, Ultrasonography (US), MRI, fine-needle aspiration cytology, and left nipple biopsy. A mass lesion was suspected as FA on MMG (Figure 2). However, the mass lesion was irregularly shaped on US and had coarse calcification (Figure 3). Fine-needle aspiration cytology was performed on the mass lesion. The result showed cluster of atypical cells without myoepithelial cells (Figure 4).
In the MRI of the breast, the mass lesion presented with early-phase enhancement and late-phase plateau, and an extensive ductal component connecting it to the nipple (Figure 5-1 and 5-2). These MRI findings correspond to malignant lesions.
The erosion of the left nipple was examined through biopsy. The biopsy result showed atypical cells in the epidermal cells of the left nipple (Figure 6). According to the medical examination result, the mass lesion was diagnosed as invasive ductal carcinoma with FA. The erosion of the left nipple involved atypical cells. Therefore, in 200X+1, mastectomy with axial lymph node dissection was performed for the PD of the left breast with underlying invasive ductal carcinoma and FA.
A mastectomy specimen was divided into tissue sections A–F. The tissues were fixed with 10% formalin, embedded in paraffin, and cut with 4-μm thickness. The tissue specimens were stained with hematoxylin and eosin.
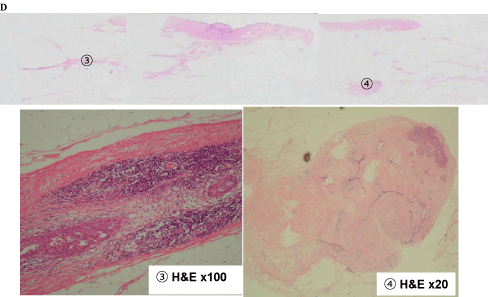
Figure 7-1 shows tissue specimens C, D, E, and F. Tissue C had extensive ductal components (1 and 2; Figure 7-2). Meanwhile, tissue D had an extensive intraductal component of breast cancer (3) and a finding of suggestive of FA (4; Figure 7-3).
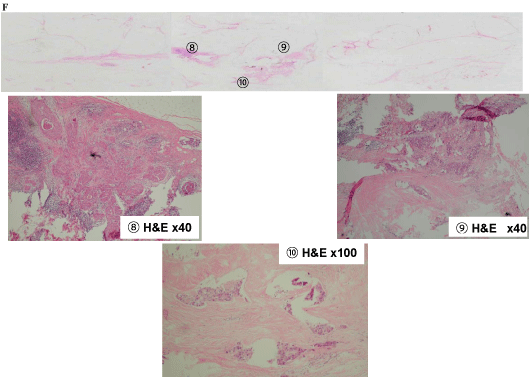
In tissue E, extensive intraductal components of breast cancer (5 and 6) and part of the FA (7) were found (Figure 7-4). Intraductal components were found from the invasive ductal carcinoma to the nipple (8), as well as the invasive ductal carcinoma that was connected to the FA (9 and 10; Figure 7-5).
Epidermal involvement of the nipple was connected to the invasive ductal carcinoma and FA via 2, 5, and 8. This resulted in a scirrhous carcinoma with extensive ductal spreading and epidermal involvement of the nipple (pagetoid carcinoma), with coexistence of FA. In addition, results were negative for the estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2.
The Japanese General Rules for Clinical and Pathological Recording of Breast Cancer describes PD as intraepidermal atypical cells of the nipple underlining a ductal carcinoma in situ. PD underlining invasive carcinoma is called pagetoid carcinoma. However, PD was differentiated from pagetoid carcinoma [6].
All persistent unilateral nipple lesions should be viewed with suspicion, and PD of the breast should be considered. Breast imaging workup should include an examination for an underlying malignancy, which is present in more than 80% of cases and not infrequently multifocal. Preoperative MRI is useful if breast conservative surgery is contemplated because of the high rate of occult malignancy discovered through MMG and US [7].
In a previous report, patients with pagetoid carcinoma tended to have a greater chance of lymph node involvement, lower hormone receptor expression level, higher HER2 expression level, and worse survival than patients without PD. The subsequent matched study confirmed that the survival of patients with pagetoid carcinoma was reduced compared with patients with invasive breast cancer who had similar prognostic factors (stage and characteristics). This finding suggests that PD itself is an indicator of poor survival. Some patients were pathologically diagnosed with PD in the absence of clinical PD manifestation. They might have worse prognoses than patients with clinical PD. Pathologists should carefully examine the nipple after mastectomy to diagnose non-clinical, occult PD.
The erosion of the left nipple at the first visit.
Mammographic findings in the medial lateral oblique view. The image shows the mass lesion with coarse calcifications at the lower area of the left side and the fibroadenoma lesion that was followed up in the previous hospital.
Ultrasonographic image showing the irregularly shaped low echoic lesion with coarse calcifications and disconnection of the anterior borderline of the mammary gland.
Findings from the fine-needle aspiration cytology of tumor lesion in the left breast. Atypical cells without myoepithelial cells were found.

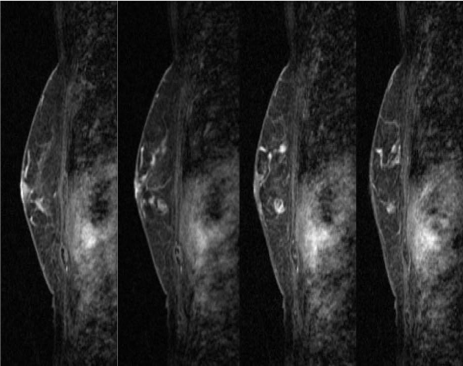
Magnetic resonance image (MRI) of the breast showing early enhancement and late phase washout or plateau, suggesting malignancy. Early-phase MRI of the left breast. Gadolinium-enhanced MRI showing earlyphase findings of the left breast. The enhanced mass lesion showed coarse calcifications and an extensive intraductal component that were connected from the enhanced mass lesion to the nipple at the early phase.
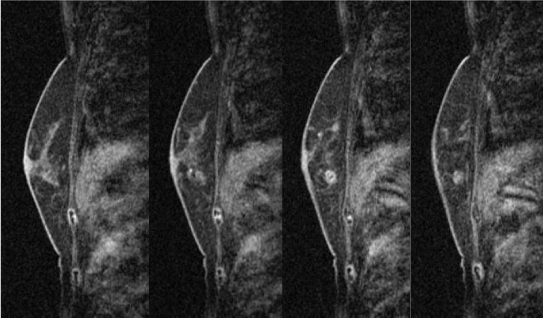
Late-phase MRI of the left breast. Gadolinium-enhanced MRI showing the late-phase findings of the left breast. The enhanced mass lesion had coarse calcifications and an extensive intraductal component that was connected from the enhanced mass lesion to the nipple at the late phase.
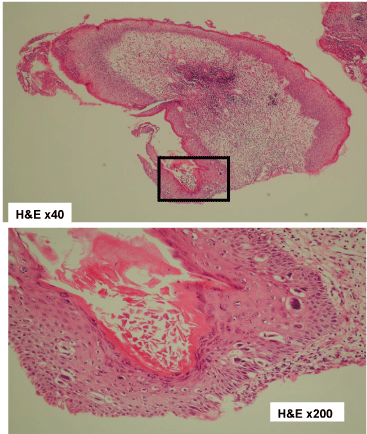
Biopsy findings of the left nipple. Atypical cells at the left nipple epithelium were found. H&E, hematoxylin and eosin.
The left mastectomy specimen. The mastectomy specimen divided into tissue sections C–F. The tissues were fixed in 10% formalin, embedded in paraffin, cut into 4-μm-thick sections, and stained with hematoxylin and eosin (H&E).

Tissue C findings of (1), (2) Extensive intraductal component of the breast cancer below the nipple.
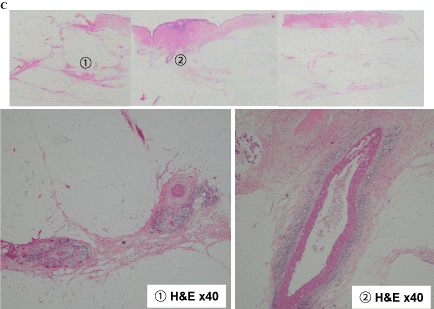
Tissue D findings of (3) Extensive intraductal component of the breast cancer, (4) Fibroadenoma.
Tissue E findings of (5), (6) Extensive intraductal component of the breast cancer, (7) Part of the fibroadenoma.
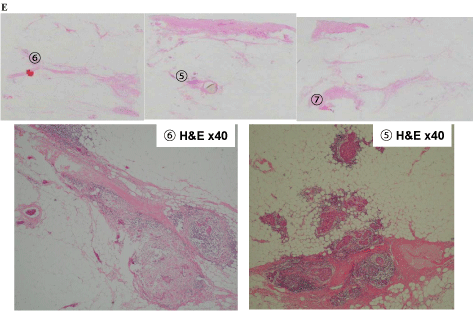
Tissue F findings of (8) Intraductal component from the invasive ductal carcinoma to the nipple. (9), (10) The invasive ductal carcinoma that originated from the fibroadenoma epithelium.