
Review Article
J Stem Cell Res Transplant. 2021; 8(2): 1039.
Checkpoint Inhibitor Pneumonitis Induced by Programmed Death-1 Antibody: A Case Report and Literature Review
Zheng Y*, Li J, Chen D, Li Y, Dai L, Huang L, Wang M
Department of Oncology, Dalian Friendship Hospital, Liaoning Sheng, China
*Corresponding author: Yujun Zheng, Department of Oncology, Dalian Friendship Hospital, Dalian 116001, Liaoning Sheng, China
Received: May 20, 2021; Accepted: June 16, 2021; Published: June 23, 2021
Abstract
Immune Checkpoint Inhibitors (ICIs) are effective treatment therapies for majority advanced tumours. However, the immune-related Adverse Events (irAEs) triggered by ICIs may affect human organs, including skin, lungs, pituitary, thyroid, blood and digestive system, leading to immunotoxicity in these organs. Checkpoint Inhibitor Pneumonitis (CIP) is one of the irAEs that could cause mortality, thereby needs special attention from the clinical physicians. In the present manuscript, the CIP occurred in a unilateral lung in one patient case with advanced oesophageal cancer treated with anti-PD-1 was analysed. Furthermore, a literature review was conducted to investigate its pathogenesis, clinical features, associated risk factors, prevention and the correlation with ICI efficacy, providing valuable information for clinical physicians.
Keywords: Programmed death-1; Pulmonary toxicity; Side effect; Checkpoint inhibitor pneumonitis
Introduction
At present, immunotherapy has broken the bottleneck of the treatment in the treatment of advanced oesophageal cancer, providing effective treatment options for patients. The clinical usage of immunotherapy in treating advanced oesophageal cancer has been explored in the last decade, as first-line treatment, neoadjuvant and adjuvant therapy with surgery, as well as in real-world studies, showing its great potential. With the comprehensive clinical usage of the ICIs its irAEs appear to attract attention. According to a publication by Wang et al [1], irAEs occurred in 333 patient cases treated with ICIs such as anti-Programmed Death-1 (anti-PD-1) or anti–PD-1 ligand 1 (PD-L1) therapies. These irAEs included immunerelated pneumonitis (115 cases), hepatitis (74 cases), enteritis (58 cases), neuropathy (50 cases), heart disease (27 cases), myositis (22 cases) and blood system diseases (14 cases). Other rare irAEs included immune-related adrenal disease, nephritis, hypophysitis, thyroids, skin lesions and diabetes. Furthermore, two or more irAEs occurred in 51 patient cases. Of note, fatal irAEs happened 40 days (median time) after treatment initiation, with a mortality rate of 0.37%. The main fatal irAEs were immune-related pneumonitis and heart disease; therefore, the clinical physicians should be equipped with adequate information to improve the identification of clinical features, diagnosis and prevention of fatal irAEs. In the present manuscript, the mechanism, clinical features, prevention and risk factors of ICI-triggered Checkpoint Inhibitor Pneumonitis (CIP) were investigated, based on the one patient case with CIP caused by anti-PD-1, providing valuable information regarding the safety and effect of medications for clinical physicians.
Patient presentation
A 68-year old man with a body weight of 60 kg was admitted. In October 2012, he was diagnosed with space-occupying lesions in the upper esophagus and muscularis propria in the lower esophagus by the gastrointestinal biopsy showed differentiated squamous cell carcinoma. Subsequently, he underwent curative surgery. Postoperatively, moderately differentiated squamous cell carcinoma occurred in the esophagus, 3 cm from the upper resection edge, also invading submucosa and nerves. At 10 cm from the upper resection edge in the esophagus, invasive growth of poorly differentiated carcinoma was found in submucosal lymph nodes, expanding out of which and invading the esophageal squamous epithelium. The tumor cells were positive for P63, CK (AE1/AE3), and negative for CK7 and LCA. This patient was not treated with chemotherapy following surgery. In March 2020, the abdominal CT showed an upper-right abdominal lesion next to the stomach, with subsequent multiple enlarged lymph nodes at peritoneum. MRI scans confirmed multiple small nodules and lesion in soft tissues around the bottom of the stomach and peritoneum, indicating tumor reoccurrence. The patient was treated with sintilimab (200 mg, d1 ivgtt, q3w) in combination with albumin nab-paclitaxel (200 mg d1, 8 ivgtt) and cisplatin (40 mg d1, 8 ivgtt) for one week between 3rd April 2020 and 10th April 2020. Following the chemotherapy, this patient experienced intolerable grade III nausea and vomiting. Between 20th April 2020 and 21st May 2020, the patient was treated with sintilimab (200 mg, d1 ivgtt, q3w) in combination with albumin nab-paclitaxel (300 mg d1, ivgtt) for two weeks. Two weeks after the chemotherapy, there was a confirmed Complete Response (CR: disappearance of all lesions). The patient was under observation until 1st July 2021 (3 months after anti-PD-1 treatment), and showed symptoms of dry cough. His chest CT showed patchy shadow (Figure 1A), indicating CIP. Following treatment with 30 mg prednisone acetate once daily orally, the symptoms were relieved three days after treatment initiation. The drug dosage was consequently reduced to one tablet every five days until stopping the treatment. The chest CT showed that majority of the patchy shadow disappeared after more than ten days of treatment (Figure 1B). However, grade II rash occurred. Between 13th July 2020 and 21st August 2020, due to the increasing level of tumor markers, the patient was consequently treated with oral chemotherapy drug–tiggo (60 mg capsule, taken twice-daily d1-14, q3wk) for two weeks. Between 15th September 2020 and 29th October 2020, due to the continuously increasing level of tumor markers, this patient was treated with oxaliplatin (200 mg d1 ivgtt) combined with tiggo (60 mg capsule, taken twice-daily d1-14, q3wk) for two weeks. Over time, three events of CIPs occurred. The second, third and fourth events of CIP occurred on 19th August 2020 (4.5 months after immunotherapy initiation), 25th October 2020 (6.5 months after immunotherapy initiation) and 25th November 2020 (7.5 months after immunotherapy initiation). Which are shown in Figure 2, 3 and 4, respectively. The symptoms of all these CIP events were relieved following treatment of prednisone acetate using the same dosage as the first time. After the patient stopped the anti-PD-1 treatment, the tumor maker–CEA increased gradually, with no improvement in videography. The patient was in general good condition with no major adverse event reported. The progression-free survival achieved seven months in February 2021 (Figure 5).
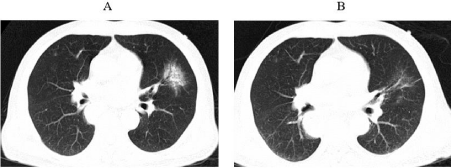
Figure 1: The first occurrence of immune-related pneumonia at A. three
months after initiation immunotherapy (1st July 2020) B. ten days after
initiating prednisone.
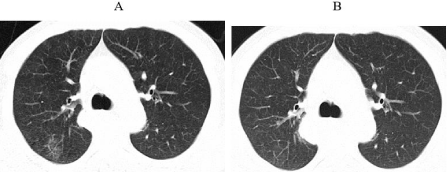
Figure 2: The second occurrence of immune-related pneumonia at A. 4.5
months after initiation immunotherapy (19th August 2020) B. ten days after
initiating prednisone.
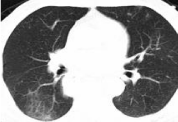
Figure 3: The third occurrence of immune-related pneumonia at A. 6.5 months
after initiation immunotherapy (25th October 2020), no after prednisone.
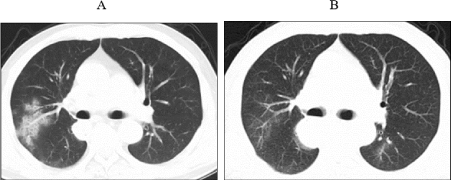
Figure 4: The fourth occurrence of immune-related pneumonia at A. 7.5
months after initiation immunotherapy (25th November 2020) B. ten days after
initiating prednisone.
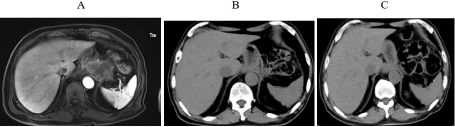
Figure 5: A. MRI scans confirmed multiple small nodules and lesion in soft
tissues around the bottom of the stomach and peritoneum in March 2020, prior
to immunotherapy; B. No obvious lump observed using upper-abdominal CT
at three months after immunotherapy initiation (3rd July 2020); C. 7.5 months
after immunotherapy initiation (25th November 2020).
Discussion
Mechanism of immune-related pneumonitis
ICIs can enhance the cellular immunity in not only tumor cells, but also normal cells, leading to immune dysregulation, thereby resulting in irAEs. Although the underlying pathophysiological mechanism remains unclear, the suggested potential mechanisms include: (1) T cell activities against cross-antigens expressed in tumor and normal tissues are increased; (2) levels of pre-existing autoantibodies are increased continuously; (3) levels of inflammatory cytokines are also increased continuously.
Clinical features of immune-related pneumonitis
In clinical studies, the overall adverse event rate associated with ICI is 60% - 80%, with a severe adverse event rate of 10-15 % [2- 4]. It was found that different types of tumors may present various features associated with adverse events, such as skin toxicity appeared more often in patients with melanoma and pneumonitis occurred more often in patients with lung cancer [5]. There is a lack of typical clinical symptoms in patients with CIP, out of which, 1/3 display no symptoms. Symptoms associated with CIP include severe dyspnea, cough, chest pain, fever and fatigue, etc [6]. In the retrospective studies on patients treated with anti-PD-1 and anti-PD-L1, the most common clinical symptoms of CIP are dyspnea (53%) and cough (15%). There is no specific sign for CIP, but patients appear to have frequent breathing, lip cyanosis, and wet bibasilar crackers or Velcro crackles in lungs. If patients with pre-existing lung diseases, such as chronic obstructive pulmonary disease and pulmonary fibrosis, display symptoms and physical signs that are more severe than their previous ones with respiratory system, it is highly likely that they are developing CIP. Chest CT scan is recommended to be the main imaging methodology as it can identify CIP more efficiently than chest X-Ray. The videography associated with CIP varies, and can be presented as new or progressive scattered or diffuse grand glass shadow, patchy consolidation, interlobular septal thickening, gridlike shadows, traction bronchiectasis, and fiber streaks in one or both lungs [7]. In addition to the typical symptoms of pneumonia, immune-related lung injury may lead to pleural effusion and pulmonary sarcoid-like granulomatosis [8,9]. The changes in lung dysfunction associated with CIP commonly include the decrease of Diffusing Capacity of the Lung for Carbon Monoxide (DLCO) and restrictive ventilatory dysfunction. Bronchoalveolar lavage and biopsy in lungs are often utilized for a definite diagnosis, which may be mainly presented as increased level of lymphocytes, and occasional increased level of eosinophils, in bronchoalveolar lavage fluid [10]. The pathology in the lung tissues is dominated by T-cell infiltration [11]. However, the mechanism of different event rates of CIP among various types of tumors is still unclear. Possible mechanisms include different tumor microenvironment, immune infiltration, adaptive tumor responses and neoantigen presentation that may be affected by histology [12].
Risk factors of immune-related pneumonitis
The risk factors associated with CIP are complex. According to the meta-analyses, there were significant higher rates of CIP (6.6%, 1.6%, p<0.001) and severe CIP (1.5%, 0.2%, p=0.001) with anti-PD-1 in combination with anti-CTLA-4, compared with a single treatment [13,14]. The combination of immunotherapy and targeted molecular therapy can also increase the event rate of CIP. In a phase Ib research on the efficacy and safety of durvalumab in combination with osimertinib in patients with Non-Small-Cell Lung Carcinoma (NSCLC), 38% of patients had CIP, and 15% had severe CIP; consequently this research had to stopped prematurely [15]. In a pacific research, there was an increased risk of treatment-related pneumonia associated with durvalumab, compared with placebo (33.9% and 24.5%, respectively), in patients with phase III, unresectable NSCLC, following combined radiotherapy and chemotherapy. Based on a meta-analysis of ICIs in combination with chemotherapy, the Relative Risk (RR) of combination therapy (ICIs and chemotherapy) versus placebo was 2.37 (95% CI 1.27–4.32, p=.0007), indicating that immunotherapy combined with chemotherapy increased the risk of CIP [17]. While immunotherapy combined with radiotherapy and chemotherapy, targeted therapy and double immunotherapy provide benefits to patients’ survival, the risk of CIP is also increased, which needs clinical physicians’ attention. Multiple retrospective analyses showed that advanced age (age ≥ 70 years), Asian race, previous or current smoking, prior lung diseases, impaired lung function and prior combination therapy are the potential risk factors for CIP [18- 22]. Identification of risk factors for CIP can aid screening patients who are high risk of CIP, thereby leading to its early diagnosis, timely treatment and close monitoring, avoiding serious consequences.
Prevention and management of immune-related pneumonitis
Prior to ICIs are initiated, the lung function of patients should be evaluated in terms of underlying lung diseases, such as interstitial fibrosis and chronic obstructive pulmonary disease, lung lesions and previous treatment history including history of EGFR-TKI and chest radiotherapy. Following diagnosis of immune-related pneumonitis, glucocorticoid treatment should be initiated. The symptoms of the previously described patient case were relieved following timely treatment of glucocorticoid. The management of irAEs should be in accordance with the National Comprehensive Cancer Network (NCCN) Guidelines for the management of immunotherapy-related toxicity, 1st edition 2020 and the Chinese Society of Clinical Oncology (CSCO) 2020 guidelines.
According to the NCCN and CSCO guidelines, immune-related pneumonitis is graded based on the toxicity and side effects in various systems in patients, the clinical physicians should adopt the continue or discontinue treatment based on these grades. Glucocorticoid is the first-line treatment for irAEs, and its dosage should be adjusted based on the severity of toxicity and side effects, involved organs, whether urgent intervention is needed and potential risks etc. Generally speaking, the initial glucocorticoid treatment can start with prednisone 0.5 mg/kg/day, taken orally or via intravenous injection. The treatment and management of immune-related pneumonitis should be differentiated based on the graded severity. For the patients with grades 3-4 (severe clinical symptoms, limiting activities of daily living, oxygen indicated, life-threatening difficulty in breathing and emergency tracheal intubation required), ICI treatment should be permanently discontinued, bronchoscopy or lung puncture should be performed, after excluding infection and lung metastasis, medium to high dose of intravenous glucocorticoid, such as methylprednisolone 1-4 mg/kg/day, should be given immediately. If no improvement after 48 hours, immunosuppressants, such as infliximab, cyclophosphamide, mycophenolate mofetil or gamma globulin, should be added. For patients with relieved symptoms, the dosage of glucocorticoid treatment should be reduced to 1 mg/kg/day, and gradually stop within two months. Additionally, whether the ICI treatment should be continued will need to be re-evaluated. For fatal irAEs, the treatments inhibiting inflammatory components in the pathophysiology of irAEs , blocking the acute immune inflammatory toxicity, and inhibiting tumor development that is promoted by cytokines, such as IL-1 and IL-6, should be considered [23]. Until present, there is still a lack of effective predictive markers for irAEs.
Correlation between the occurrence of irAEs and the efficacy of ICI treatment
It was found that the overall survival time for patients with ICI treatment over three months and irAEs lasting more than 3 months was the longest, indicating that the occurrence of irAEs may indicate a better response to ICI treatment; however, further researches with larger samples size are needed [24]. In another study, 39 patients with lung cancer treated with ICIs had irAEs [25]. Following improvement in these patients with glucocorticoid treatment, ICI treatment continued, and subsequently 10 patients had re-occurrence of previous irAEs, 9 patients had new irAEs, and 17 patients had re-occurrence of previous irAEs or new irAEs, all of which were controlled using glucocorticoid treatment [25]. This also indicated that for patients who responded to ICI treatment prior to incidence of irAEs, re-using or discontinuing ICI treatment do not affect the overall ICI efficacy, and treatment with glucocorticoid for irAEs do not decrease the overall ICI efficacy [26]. In another study, out of 54 patients treated with ICIs, 26 patients were treatment responders (CR+PR+SD), and vitiligo occurred in 10 responders but none in the 28 non-responders. The event rate of thyroid dysfunction was 65.4% in treatment responders, and 21.4% in non-responders [27]. A Χ² test was conducted to analyze the relationship between the side effects and efficacy [27]. Vitiligo (p=0.001) and thyroid dysfunction (p=0.007) might be two predictors of ICI treatment efficacy, suggesting that the occurrence of these two side effects indicated good prognosis [27]. While enhancing the immune response to treat cancer, ICIs can trigger irAEs; therefore the incidence of irAEs is considered to be associated with treatment efficacy, thereby indicating a good prognosis. Downey et al found that in patients treated with ipilimumab, the objective reaction rates with and without side effects were 26% and 2%, respectively.
Esophageal cancer has a poor prognosis, with a 5-year survival rate of only 10-20 % prior to the usage of immunotherapy [29]. Immunotherapy has been an effective treatment associated with prolonged overall survival time for patients with esophageal cancer. In addition, immunotherapy has a long-lasting treatment effects, as the described case had a progression-free survival of seven months. In the recent phase 3 KEYNOTE-590 study published at the annual meeting of European Society for Medical Oncology (ESMO) 2020, pembrolizumab plus chemotherapy was compared with chemotherapy as first-line therapy in patients with advanced esophageal cancer. The combination therapy (pembrolizumab plus chemotherapy) provided superior overall survival compared with chemotherapy (12.4 vs 9.8 months; hazard ratio 0.73, p<0.0001). The combination therapy was associated with a higher overall rate (45.0% vs 29.3%), and duration of response (8.3 vs 6.0 months, p<0.0001), versus chemotherapy. While achieving curative effect, incidence of irAEs also increases. The time of CIP occurrence in the previously described patient case is in alignment with available research data in China and abroad. Of note, in the above patient case, between three and eight months after initiating ICI treatment, unilateral grade II CIP occurred at various parts four times, all of which were treated with thyroid and improved, however with grade II skin adverse reactions. To summarize, most CIP can be controlled by ICI treatment discontinuation and using glucocorticoid and majority CIP can be reversed; furthermore, treatment with glucocorticoid does not affect the efficacy of immunotherapy [31,32]. The key of CIP management is early diagnosis and early intervention.
References
- Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018; 4: 1721-1728.
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012; 366: 2455- 2465.
- Robert C, Schachter J, Long G, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015; 372: 2521–2532.
- Eggermont A, Blank C, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage melanoma. N Engl J Med. 2018; 378: 1789–1801.
- Khoja L, Day D, Wei-Wu Chen T, et al. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017; 28: 2377-2385.
- Rashdan S, Minna JD, Gerber DE. Diagnosis and management of pulmonary toxicity associated with cancer immunotherapy. Lancet Respir Med. 2018; 6: 472-478.
- Nishino M, Sholl LM, Hodi FS, et al. Anti-PD-1 related pneumonitis during cancer immunotherapy. N Engl J Med. 2015; 373: 288-290.
- Possick JD. Pulmonary Toxicities from checkpoint immunotherapy for malignancy. Clin Chest Med. 2017; 38: 223-232.
- Murphy KP, Kennedy MP, Barry JE, et al. New onset mediastinal and central nervous system sarcoidosis in a patient with metastatic melanoma undergoing CTLA4 monoclonal antibody treatment. Oncol Res Treat. 2014; 37: 351-353.
- Delaunay M, Cadranel J, Lusque A, et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients.Eur Respir J. 2017; 50: 1700050.
- Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with antiprogrammed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017; 35: 709-717.
- Khoja L, Day D, Wei-Wu CT, et al. Tumour- and class-specific pattern of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017; 28: 2377-2385.
- Wu J, Hong D, Zhang X, et al. PD-1 inhibitors increase the incidence and risk of pneumonitis in cancer patients in a dose-independent manner: a metaanalysis. SciRep. 2017; 7: 44173.
- Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with antiprogrammed death-1/programmed death Ligand 1 therapy. J Clin Oncol. 2017; 35: 709-717.
- Ahn MJ, Yang J, Yu H, et al. 136O: Osimertinib combined with durvalumab in EGFR-mutantnon-small cell lung cancer: Results from the TATTON phase-b trial. J Thorac Oncol. 2016; 11: S115.
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage Non-Small-Cell Lung Cancer. N Engl J Med. 2017; 377: 1919-1929.
- Huang Y, Fan H, Li N, et al. Risk of immune-related pneumonitis for PD1/ PD-L1 inhibitors:Systematic review and network meta-analysis. Cancer Med. 2019; 8: 2664-2674.
- Ma K, Lu Y, Jiang S, et al. The relative risk and incidence of immune checkpoint inhibitors related pneumonitis in patients with advanced cancer: a meta-analysis. Front Pharmacol. 2018; 9: 1430.
- Nishino M, Giobbie-Hurder A, Hatabu H, et al. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol. 2016; 2: 1607-1616.
- Khunger M, Rakshit S, Pasupuleti V, et al. Incidence of pneumonitis with use of PD-1 and PD-L1 inhibitors in non-small cell lung cancer: a systematic review and meta analysis of trials. Chest. 2017; 152: 271-281.
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015; 372: 2006-2017.
- Owen DH, Wei L, Bertino EM, et al. Incidence, risk factors, and effect on survival of immune-related adverse events in patients with non-small-cell lung cancer. Clin Lung Cancer. 2018; 19: e893-e900.
- Dong S, Hu S, Cai Q. Mechanism of fatal toxic effect associated with immune checkpoint inhibitors and its individualized management. China Cancer. 2019; 28: 440-444.
- Owen DH, Wei L, Bertino EM, et al. Incidence, Risk factors, and effect on survival of immune-related adverse events in patients with non-small-cell lung cancer. Clin Lung Cancer. 2018; 19: 893-900.
- Faje AT, Lawrence D, Flaherty K, et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer. 2018; 124: 3706-3714.
- Santini FC, Rizvi H, Wilkins O, et al. Safety of retreatment with immunotherapy after immunerelated toxicity in patients with lung cancers treated with anti- PD(L)-1therapy. J Clin Oncol. 2017; 35: 9012.
- Yang Xiaoling, Si Lu, Mao Lili, et al. Adverse events of pembrolizumab in patients with advanced melanoma and correlation analysis. China Oncology. 2020; 30: 362-368.
- Downey SG, Klapper JA, Smith FO, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007; 13: 6681-6688.
- Pennathur MK, Jobe BA, Luketich JD. Oesophageal carcinoma, Lancet. 2013; 381: 400–412.
- Kato K, Sun J, Shah M, et al. Pembrolizumab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced esophageal cancer: The phase 3 KEYNOTE-590 study. ESMO. 2020.
- Champiat S, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2016; 27: 559-574.
- Horvat TZ, et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015; 33: 3193-3198.