
Case Report
J Stem Cell Res Transplant. 2023; 10(1): 1044.
Case Report of Cellgram® Autologous Mesenchymal Stem Cell Treatment for Relapsing-Remitting Multiple Sclerosis
Won Woo Choi1; Sang Yeol Yong1; Hyun Soo Kim2*; Jeonghwan Lee3#; Ju Eun Hwang3#; Chang Ho Jeong4#; Gawhan Lee4#
1Department of Rehabilitation Medicine, Ilsan-ro 20, Wonju-si, Kangwon-do, 26426, Republic of Korea
2R&D Center, Pharmicell Co. Ltd., Seongnam 13229, Korea
3Pharmicell, Pharmicell Co. Ltd., Seongnam 13229, Korea
4Dr. Kim’s Stem Cell Clinic, 874 Eonju-ro Gangnam, Seoul,06027, Republic of Korea
*Corresponding author: Hyun Soo Kim R&D Center, Pharmicell Co. Ltd., Seongnam 13229, Korea. Email: khsmd@pharmicell.com
#These authors have contributed equally to this article.
Received: July 17, 2023 Accepted: August 15, 2023 Published: August 22, 2023
Abstract
Multiple sclerosis is an autoimmune disease of the central nervous system that is increasing worldwide but difficult to diagnose and treat. Previous studies have revealed that the autologous Mesenchymal Stem Cells (MSC) for MS may serve as a possible effective tool for cell-based therapy of inflammatory, immune-mediated, and degenerative diseases due to their immunomodulatory, immunosuppressive, and regenerative potentials. This case report presents a patient diagnosed with Relapsing-Remitting Multiple Sclerosis (RRMS) who was treated with an autologous mesenchymal stem cell drug (Cellgram®). We administered two doses of Cellgram® to a 54-year-old Southwest Asian male with a 28-year history of RRMS. Changes were also observed in manual muscle strength test, range of motion, and lower extremity stiffness, but the biggest change was in functional performance. Before treatment, the patient was unable to stand without assistance even with an orthosis, but was able to stand up from a wheelchair using a walker on the 24th day after the first injection, and was able to stand up from the wheelchair without assistance on the 26th day. Thirty days after the first injection, and 3 days after the second injection, he was able to walk a few steps back and forth using a walker. Five months after the first injection, he was able to climb six steps. In conclusion, this case report illustrates that MSC therapy might serve as a potential therapeutic option for RRMS as it may be a safe and effective treatment for MS patients.
Keywords: Autologous mesenchymal stem cells therapy; Case report; Functional performance; Gait; Multiple sclerosis; Relapsing-remitting multiple sclerosis; Stem cell therapy
Introduction
Multiple Sclerosis (MS) is a disease of the central nervous system causing autoimmune-mediated neurodegeneration. The global incidence rate is 35.9 per 100,000 people, and the condition is most prominent in Caucasians. The MS prevalence has increased in all parts of the world, and the mean age of diagnosis is 32 years [1]. The diagnosis is often delayed due to the waxing and waning nature of the disease, hindering patients from seeking evaluation [2]. To make a definitive diagnosis of MS, clinicians often rely on the McDonald criteria, which relays a set of guidelines that help to diagnose MS through clinical and laboratory examinations as well as MRI. According to the criteria, evidence of dissemination in space and time of the disease must be present for the diagnosis of MS [3].
Upon the onset of the disease, inflammatory demyelination can lead to physical disability, cognitive impairment, and decreased quality of life [4,5]. Most of the current therapies available for MS aim to reduce inflammation but fail to repair the existing damage, resulting in the disappointing outcome of MS [6]. The treatments are costly and while they aim to reduce symptoms and inflammation, there are limitations due to their non-curative nature. Furthermore, the immunomodulatory and immunosuppressive properties of the medications may enhance autoimmune reactions, leading to the possible development of chronic progressive disease with a poor prognosis [7].
Treatment with autologous Mesenchymal Stem Cells (MSC) for MS may serve as a possible treatment as studies have revealed that MSC are an effective tool for cell-based therapy of inflammatory, immune-mediated, and degenerative diseases due to their immunomodulatory, immunosuppressive, and regenerative potentials [8]. MSC are highly proliferative and their ability to home to sites of injury, differentiate to necessary cell types, secrete stimulatory and inhibitory molecules, and act as immunomodulatory agents make them a highly valuable treatment option for treating various diseases, such as graft-versus-host, cardiovascular, bone/cartilage, diabetes, and neurological diseases [9].
MSC for stem cell therapy have recently increased in their usage and are now viewed as a safe and effective treatment option for different diseases, including MS [10]. The properties of MSC have resulted in improved MS clinical outcomes in experimental autoimmune encephalomyelitis models [11]. Thus, stem cell therapy may serve as hope for MS patients, who are often left without viable treatment options.
This study presents a patient diagnosed with Relapsing-Remitting Multiple Sclerosis (RRMS) who was treated with Cellgram®, an autologous mesenchymal stem cell drug. The patient previously received various MS treatments with no avail and specifically sought out Cellgram® as the clinic administers stem cell therapy without the use of chemotherapy. Cellgram® is the world’s first commercialized stem cell therapy and has been approved by the governmental regulatory institute (Korea Food and Drug Administration, KFDA) [12]. This report is first to study Cellgram®’s effect in treating a patient from West Asia.
Materials and Methods
Twenty to twenty-five milliliters (mean±SD: 23.1±11.5 mL) of Bone Marrow (BM) aspirates were obtained under local anesthesia from the posterior iliac crest in the MSC group on 3.8±1.5 days after admission, and following testing negativity for HBV, HCV and HIV. All manufacturing and product testing procedures for the generation of clinical-grade autologous MSC were carried out under good manufacturing practice (FCB-Pharmicell Company Limited, Seongnam, Korea). Mononuclear cells were separated from the BM by density gradient centrifugation (HISTOPAQUE-1077; Sigma-Aldrich, St. Louis, MO, USA) and washed with Phosphate-Buffered Saline (PBS). Cells were resuspended in Dulbecco's modified Eagle's medium-low glucose (DMEM; Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (Gibco), 100 U/mL penicillin/100 μg/mL and streptomycin (Gibco). They were plated at 2-3×105 cells/cm² into 75 cm² flasks. Cultures were maintained at 37o in a humidified atmosphere containing 5% CO2. After 5-7 days, non-adherent cells were removed by replacing the medium; adherent cells were cultured for another 2-3 days. When the cultures were near confluence (70%-80%), adherent cells were detached by using trypsin containing ethylene diamine tetra-acetic acid (EDTA; Gibco) and replated at 4-5×103 cells/cm² in 175 cm² flasks. Cells were serially subcultured up to passage 4 or passage 5 for infusion (mean±SD: 4.4±0.5 passages).
On the day of administration, MSC were harvested using trypsin and EDTA, washed twice with PBS and once with saline solution, and resuspended to a final concentration of 1×106 cells/kg. The criteria for the release of MSC for clinical use included viability >80%, absence of microbial contamination (bacteria, fungus, virus, and mycoplasma) if undertaken 3-4 days before administration, and expression of CD73 and CD105 by >90% of cells and absence of CD14, CD34, and CD45 by <3% of cells as assessed by flow cytometry (data not shown). Also, the in vitro osteogenic and cardiomyogenic differentiation potential of MSC in passage 0 or 1 was tested before release as a potency test. Alkaline phosphatase staining was used to demonstrate the osteogenic differentiation. Immunostaining with a-sarcomeric actin and troponin T was used to demonstrate the cardiomyogenic differentiation. Qualitative analysis showed well differentiation potential of all MSC [13]. This process follows in accordance with our patented technology in Good Manufacturing Practices compliant laboratory at our facility (Patent-WO 2014/132129A2 PCT/IB 2014 Published 20 November 2014).
The treatment consisted of three rounds of Cellgram® therapy. The patient was administered 18mL (9x10^7cells) Cellgram® on 1 July 2022, 29 July 2022, and 26 August 2022 via an intravenous route to allow cells to reach the site of injury for the purpose of motor function recovery through repair and regeneration. The patient was compliant with 3 stem cell injections and treatment during the follow-up period. Adverse events, including skin redness and anaphylaxis, were not observed during the treatment period and follow-up period.
Case Presentation
A 54-year-old male patient was referred to our clinic from Cleveland Clinic Abu Dhabi for motor function recovery through stem cell treatment using Cellgram®. The patient has a 28-year history of RRMS, and his initial symptoms began with muscle spasms and numbness in the lower limbs, which occurred once a year. After neurological examinations, the patient underwent an MRI and was formally diagnosed with MS in 1994. Prior to diagnosis, the patient served in the UAE military and maintained a very active lifestyle by frequently participating in sports such as horseback riding, mountain biking, and other vigorous exercises. However, the patient received a medical discharge from the military in 2010 due to the slow but steady deterioration of his condition. In the summer of 2016, the patient developed sudden lower limb weakness suggestive of relapse in conjunction with gradual secondary progression. In November 2016, the patient underwent plasma exchange therapy due to severe weakness in gait and reported minimal improvement. The patient continued to take interferon beta-1a (Anovex®), which aims to reduce neuron inflammation, until November 2018 and changed to cladribine (Mavenclad®), which targets lymphocytes to prevent the attack against the brain and spinal cord. Despite medication, the patient progressively experienced gradual deterioration of function in the lower limbs, resulting in immobility without assistance. Although the patient was advised to take ocrelizumab (Ocrevus®), a drug that targets and destroys B cells from damaging the myelin, the patient instead sought stem cell therapy.
On 03 June 2022, the patient presented to our clinic, and a thorough physical examination was performed to assess his mobility and symptoms. After acquiring informed consent as contained in the supplement, three bone marrow harvests and intravenous injections were carried out in one-month intervals. The patient’s stem cell treatment was accompanied by neurodevelopmental treatment three times a week to decrease muscle tone and spasticity, including stretching exercises to relax and lengthen the muscles that stiffened over the years.
Outcome
Three stem cell therapy injections were performed on 1 July 2022, 29 July 2022, and 26 August 2022, and the following is a summary of the patient’s progress with respect to his ability to stand and walk:
● 25 July 2022: Stand up from wheelchair using a walker
● 27 July 2022: Stand up from wheelchair without assistance
● 01 August 2022: Take a few steps forward and backward using a walker
● 22 December 2022: Walk up the stairs 6 steps using a walker
Through the series of carefully monitored physical therapy, the patient’s improvement was also recorded as below:
Other aspects of the patient’s progress are also noteworthy. Previously, the patient was prescribed solifenacin (Vesicare®), a competitive cholinergic receptor antagonist that is selective for the M3 receptor subtype to treat neurogenic detrusor overactivity. However, after Cellgram® treatment, it was deemed more appropriate to prescribe the patient tamsulosin (Flomax®), a drug to relieve urination, due to the recovery of detrusor activity and minor incomplete emptying due to obstructive symptoms. The new medication aimed to improve urine flow and emptying of the bladder. Consequently, while the patient previously used Clean Intermittent Catheterization (CIC) six times a day to remove about 200 mL of residual urine per CIC, the patient’s use of CIC decreased to three times a day post-medication change, and the residual urine also decreased to about 50 mL. As the medication change coincided with stem cell therapy, the potential benefit of stem cell therapy in improving bladder function cannot be excluded.
Furthermore, for several years, the patient had been taking amantadine hydrochloride (Gocovri®), a medication that relieves fatigue in MS patients [14]. However, post-stem cell therapy, the patient ceased to take the drug due to increased energy and continues to report significant improvement in mood and vigor compared to the persistent fatigue he has previously suffered. In addition, the frequency of medication the patient needed to consume for pain and constipation has decreased noticeably post-stem cell treatment.
In order to obtain a diagnostic evaluation of the patient’s progress, the patient underwent MRI scans of the brain and spinal cord following the second stem cell therapy. The finding of the most recent MRI, referred to as year 2022, was compared with MRI scans performed in years 2020 and 2021. The 2022 MRI brain scans were performed using Turbo Spin Echo (TSE), Fluid-Attenuated Inversion Recovery (FLAIR), and Gadolinium-Contrast Enhancement (GdCE) in T2-weighted sequences. In comparing 2020 and 2021 brain scans, several stable small ovoid high signal intensity lesions in T2 weighted images of callososeptal, callosomarginal, periventricular, juxtacortical, and scattered white matter legions. However, no definite new or abnormal active lesions were present. When scans from 2021 and 2022 were compared, multifocal T2 high signal in both the frontal-parietal juxtacortical and periventricular white matter were present with no definite enhancement, abnormal enhancing lesion in the brain parenchyma, or other remarkable findings (ischemic lesion, hemorrhage, mass, ventriculomegaly or atrophy) in the parenchyma of cerebrum, cerebellum, brain stem, and CSF space. The 2022 MRI spinal cord scans were performed using sagittal T2-weighted DIXON Turbo Spin Echo (TSE), axial T2-weighted and T1-weighted, and coronal T2-weighted sequences. With respect to the cervical spine contrast MRI scans of 2020 and 2021, T2 hyperintense signal foci was most noticeable at the cervicomedullary junction, and C2-3C3, C4-C5, and C7-T1 level remained stable. No abnormal enhancement was noted in the spinal cord. The thoracic cord indicated normal convincing signal intensity, size, and contours. There was no abnormal enhancement and no definite demyelinating lesions in the thoracic cord. In comparing 2021 and 2022 MRI scans of the spinal cord, multifocal subtle high signal intensity at mainly posterior and posterolateral spinal was noted. The images included in this report are organized to show changes that occurred before (2020 and 2021) and after stem cell therapy (2022) in the C3 vertebral body, C5 vertebral body, and C5-6 intervertebral disc level (Figure 1). Though there was scarring present due to MS, previously noted definite lesions were difficult to find. Other findings in the MRI scans include degenerative disc disease at C-spine, which is listed as follows: bilateral facet Osteoarthritis (OA) in C2-3; central protrusion and bilateral facet OA causing mild central canal stenosis in C3-4; central protrusion and bilateral facet OA causing mild central canal stenosis in C4-5; right central protrusion and bilateral facet OA causing mild central canal stenosis in C5-6; central protrusion and left uncovertebral arthrosis in C6-7; atlantoaxial OA. In the whole spine survey image, compression at L1 and anterolisthesis at L3-4 were noted.
Pre-Intervention
Post-Intervention
Left upper limb
0
0
Right upper limb
0
0
Left lower limb
3
1
Right lower limb
3
2
Table 1: Modified Ashworth Scale.
Pre-Intervention
Post-Intervention
Light touch (Left: L4, L5, S1)
0
2
Pinprick
2
2
Proprioception
2
2
Table 2: Sensory test result.
Pre-Intervention
Post-Intervention
Muscle groups
Right
Left
Right
Left
Elbow flexors
N
N
N
N
Wrist extensors
N
N
N
N
Finger flexors
N
N
N
N
Finger abductors
N
N
N
N
Hip flexors
P-
P-
P+
P+
Knee extensors
P-
P-
F-
F-
Ankle dorsiflexors
T
T
T
T
Long toe extensors
T
T
T
T
Ankle plantarflexors
T
T
T
T
*N: normal; G: good; F: fair; P: poor; T: trace grade
Table 3: Manual Muscle Test (MMT) result.
Pre-Intervention
Post-Intervention
Muscle groups (normal range in degree)
Right
Left
Right
Left
Upper limbs
Full
Full
Full
Full
Hip flexion (100)
90
90
100
100
Hip extension (30)
0
0
10
10
Hip abduction (40)
20
20
30
30
Hip ER (50)
30
30
40
40
Hip IR (40)
20
20
30
30
Knee flexion (150)
100
100
110
110
Knee extension (0)
0
0
0
0
Ankle DF (20)
-5
-10
0
0
Ankle PF (40)
40
40
40
40
Ankle inversion (30)
30
30
30
30
Ankle eversion (20)
0
-5
5
5
*ER: external rotation; IR: internal rotation; DF: dorsiflexion; PF: plantar flexion
**Values reported in degrees
Table 4: Range Of Motion (ROM) result.
Pre-Intervention
Post-Intervention
Function
Right
Left
Right
Left
Side rolling
3
3
5
5
Sitting balance
4
4
5
5
Standing balance
2
2
3
3
WC to mat transfer
3
3
5
5
Sit to standing
2
2
4
4
Gait
1
1
2
2
Stair
NT
NT
NT
NT
*WC: wheelchair; NT: not tested
**Functional ability tested with a grading scale that measures the level of independency is described as the following (percentage indicates the level of assistance required): 5 independent (0%), 4 minimum assistance (20%), 3 moderate assistance (50%), 2 maximum assistance (80%), 1 full assistance (100%)
Table 5: Functional ability result.
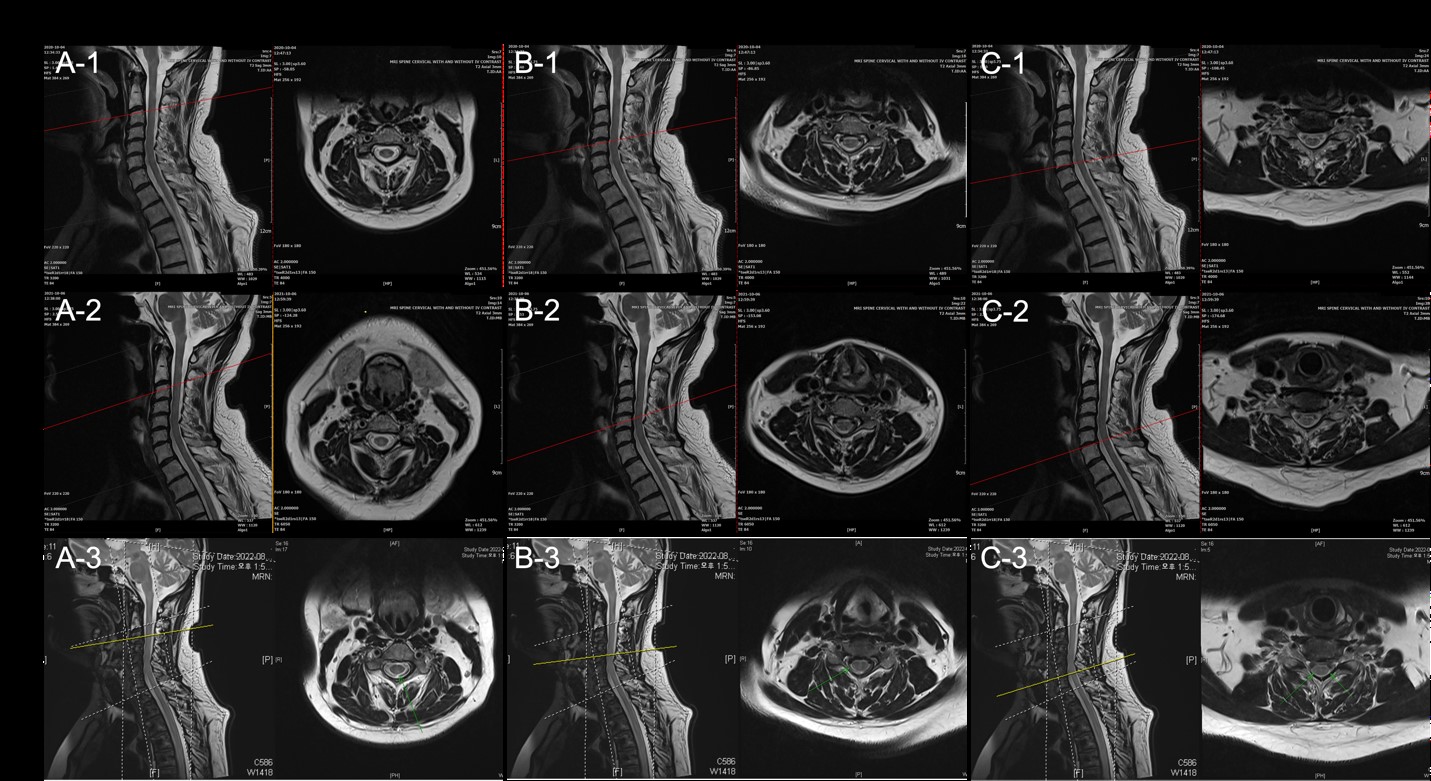
Figure 1: T2 image of C3 vertebral body (A1-3), C5 vertebral body (B1-3), C5-6 intervertebral disc (C1-3) ordered vertically according to year taken: 2020, 2021, and 2022. (A1-3) In C3 vertebral body, there is no evidence of active lesions of newly developed lesions, although the area indicated by the arrow in the 2022 image (A-3) shows slight changes in lesion size from year to year (A1-2).
Discussion
The use of Cellgram®, autologous MSC drug therapy, resulted in significant improvement in the patient, whose long history of RRMS had not responded to previous treatments. As the patient’s autologous mesenchymal stem cells were isolated from the patient’s bone marrow and cultured in-house, the patient was void of side effects and not subject to graft-vs-host disease. In assessing the patient’s improvement, it was deemed not appropriate to use the American Spinal Injury Association (ASIA) impairment scale but rather the sensory test given the patient’s sensory and motor function deterioration were localized to the lower limbs. Furthermore, because MS affected the patient in both the brain and spine as detected by MRI findings, ASIA impairment scale was not used. Although the nature of development, changes, and disappearance of lesions is unpredictable in the disease of MS, and thus, MRI changes noted in this study may not be significant, it is noteworthy that the patient’s condition in his ability to function has markedly improved post-stem cell therapy. Even more, this case report stands out in that it showed a therapeutic effect even in non-optimal patients over 50 years of age and with a long duration of disease (>10 years), which deviated from the optimal conditions presented in previous studies [15]. The clinical and radiologic improvements in the patient illustrate the potential of MSC therapy for MS. While multiple case reports signify the feasibility of MSC therapy for MS, randomized and blinded clinical trials are required to fully determine the safety and efficacy of the treatment.
In conclusion, this case report illustrates that MSC therapy might serve as a potential therapeutic option for RRMS as it may be a safe and effective treatment for MS patients.
References
- Walton CKR, Rechtman L, et al. Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS. 3rd ed. Mult Scler J. 2020; 26: 1816-21.
- Maroney M, Hunter SF. Implications for multiple sclerosis in the era of the Affordable Care Act: a clinical overview. Am J Manag Care. 2014; 20: S220-7.
- Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983; 13: 227-31.
- Yamout BI, Alroughani R. Multiple sclerosis. Semin Neurol. 2018; 38: 212-25.
- McGinley MP, Goldschmidt CH, Rae-Grant AD. Diagnosis and treatment of multiple sclerosis: a review. JAMA. 2021; 325: 765-79.
- Pluchino S, Zanotti L, Brini E, Ferrari S, Martino G. Regeneration and repair in multiple sclerosis: the role of cell transplantation. Neurosci Lett. 2009; 456: 101-6.
- Alanazi A, Alassiri M, Jawdat D, Almalik Y. Mesenchymal stem cell therapy: a review of clinical trials for multiple sclerosis. Regen Ther. 2022; 21: 201-9.
- Saeedi P, Halabian R, Imani Fooladi AA. A revealing review of mesenchymal stem cells therapy, clinical perspectives and Modification strategies. Stem Cell Investig. 2019; 6: 34.
- Wang S, Qu X, Zhao RC. Clinical applications of mesenchymal stem cells. J Hematol Oncol. 2012; 5: 19.
- Xiao J, Yang R, Biswas S, Qin X, Zhang M, Deng W. Mesenchymal stem cells and induced pluripotent stem cells as therapies for multiple sclerosis. Int J Mol Sci. 2015; 16: 9283-302.
- Bowles AC, Scruggs BA. Bunnell B. Mesenchymal stem cell-based therapy in a mouse model of experimental autoimmune encephalomyelitis (EAE). Methods Mol Biol. 2014; 1213: 303-319.
- Yang H. South Korea’s stem cell approval. Nat Biotechnol. 2011; 29: 857.
- Lee J-W, Lee S, Youn Y, Ahn M, Kim J, Yoo B et al. A randomized, open-label, multicenter trial for the safety and efficacy of adult mesenchymal stem cells after acute myocardial infarction. J Korean Med Sci. 2014; 29: 23-31.
- Bowman MA, M, Michielutte R, Preisser JS. Use of amantadine for chronic fatigue syndrome. Arch Intern Med. 1997; 157: 1264-5.
- Miller AE, Chitnis T, Cohen BA, Costello K, Sicotte NL, Stacom R, et al. Autologous hematopoietic stem cell transplant in multiple sclerosis: recommendations of the National Multiple Sclerosis Society. JAMA Neurol. 2021; 78: 241-6.