
Research Article
Sarcoma Res Int. 2025; 10(1): 1053.
Sunitinib in Advanced Gastrointestinal Stromal Tumors: A Systematic Review of Real-World and Randomized Controlled Trials
Tala Asha¹*, Khader Al-Habash² and Abeer A. Al-Rabaiah²
1Center for Drug Policy and Technology Assessment, King Hussein Cancer Center, Queen Rania Street, Amman 11855, Jordan
2Head, Center for Drug Policy and Technology Assessment, King Hussein Cancer Center, Queen Rania Street, Amman 11855, Jordan
*Corresponding author: Tala Asha, Center for Drug Policy and Technology Assessment, King Hussein Cancer Center, Queen Rania Street, Amman 11855, Jordan Email: ta.15832@khcc.jo
Received: March 13, 2025 Accepted: April 01, 2025 Published: April 04, 2025
Abstract
Sunitinib is indicated for second-line treatment of metastatic gastrointestinal stromal tumors (GISTs) based on a pivotal randomized controlled trial (RCT). However, integrating RCT results with real-world evidence is essential to fully understand its clinical impact. This systematic review evaluated the efficacy, effectiveness, and safety of sunitinib in advanced GISTs using both RCTs and observational studies. Following PRISMA guidelines, a comprehensive literature search was conducted in PubMed, Cochrane Library, and Embase for studies on adult patients treated with first-line imatinib followed by second-line sunitinib. Two reviewers performed screening and full-text review, with a third resolving disagreements. Risk of bias was assessed using appropriate tools, and data on study characteristics and outcomes were extracted.
Of 192 screened publications, 19 studies were included: one RCT, three comparative observational studies, and 15 real-world studies. The RCT showed a significant improvement in median progression-free survival (PFS) with sunitinib compared to placebo (HR 0.35, 95% CI: 0.25-0.48, p-value =<0.001), though median overall survival (OS) was not statistically significant after adjusting for cross-over. Observational studies reported inconsistent PFS results compared to dose-escalated imatinib, but median OS was consistently better with sunitinib. Single-arm studies reported median PFS ranging from 5.1 to 19.4 months and OS from 5.6 to 27 months. The initial dosing regimen (IDR, 50 mg/day, 4 weeks on, 2 weeks off) was used in 95% of studies. Continuous daily dosing (CDD, 37.5 mg/day) in one study showed a higher response rate (30% vs. 19%) and longer PFS (4 vs. 1.4 months) compared to IDR, with similar OS. Adverse drug events (ADEs) occurred in 35% of RCT patients, leading to discontinuation in 20% and dose reductions in 28%. Common grade =3 ADEs included fatigue, hypertension, thrombocytopenia, and hand-foot syndrome. This review highlights the clinical benefits of sunitinib, supported by real-world evidence, though safety and cost considerations remain.
Keywords: Sunitinib; Gastrointestinal stromal tumors; Health technology assessment; Imatinib failure; Advanced GIST
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common soft tissue sarcomas, originating from Cajal cells or their progenitors along the gastrointestinal tract, primarily affecting the stomach (60%) and small intestine (25%). Other less common sites include the rectum (5%), esophagus (2%), and locations such as the appendix, gallbladder, and pancreas [1-3].
Approximately 80-85% of GISTs have activating KIT gene mutations, mainly in exons 11, 9, 13, or 17. Some tumors exhibit PDGFRA gene mutations in exon 18 or 12, while a smaller subset is wild type for both KIT and PDGFRA. GISTs affect all genders equally and typically occur between teenage years and the 90s, peaking around age 60 [1]. Surgical resection remains the primary treatment for low to intermediate-risk GISTs. For advanced, inoperable, or metastatic GISTs, imatinib is the first-line therapy, offering longlasting clinical benefits. However, resistance or intolerance to imatinib occurs in some patients, necessitating alternative treatments. In a trial, 5% exhibited primary resistance, 14% developed early resistance, and 21% experienced severe adverse events (grade 3/4), particularly gastrointestinal bleeding [1,4].
Despite Imatinib being effective for the management of GISTs, resistance or intolerance to Imatinib in patients has been reported [5]. In a randomized-controlled-trial of Imatinib in advanced GISTs, 5% of patients showed primary resistance to Imatinib and another 14% developed early resistance [5]. Furthermore, intolerance to Imatinib due to serious adverse events (grade 3 or 4) occurred in 21.1% of patients leading to the urge of treatment discontinuation [5].
Patients diagnosed with Imatinib-sensitive tumors typically start treatment with a daily dosage of 400mg [6]. In cases where secondary resistance to Imatinib is observed, dose escalation to daily dosage of 800mg may be considered [6]. However, for patients experiencing primary or early resistance, a switch to another therapeutic agent is necessary. Those with exon 9 mutations initiate therapy at the maximum daily dose of 800mg, with a shift to an alternative treatment regimen becoming required upon disease progression [6].
The necessity for an alternative treatment for patients with GISTs who either exhibit resistance to Imatinib or are unable to tolerate it arose. Sunitinib is an oral multi-targeted tyrosine kinase inhibitor which also blocks signaling by KIT [4]. Sunitinib garnered multinational approval as a second-line treatment for GISTs following the failure of Imatinib, supported by findings from a Phase III, doubleblind, placebo-controlled randomized-controlled trial [7,8].
With only a solitary randomized controlled trial (RCT) available for evaluating Sunitinib in GISTs, the importance of integrating realworld data becomes paramount. Real-world data offer invaluable supportive evidence with greater generalizability compared to RCTs. By combining real-world evidence with RCT data, we can achieve a more comprehensive understanding of Sunitinib's effectiveness and safety profile in real-world clinical settings. This integration enhances the reliability and applicability of our findings.
Therefore, we aim to consolidate the generated results from randomized clinical trials and real-world observational studies to synthesize the available evidence on the efficacy/effectiveness and safety of Sunitinib in treating adult GISTs patients following the discontinuation of Imatinib.
This systematic review, conducted by the Center of Drug Policy and Technology Assessment at our institution, serves as part of a broader Health Technology Assessment (HTA) report. The primary objective of this systematic review is to provide decisionmakers at our institution with comprehensive evidence to facilitate informed decision-making regarding the utilization of Sunitinib in the treatment of GIST patients, and to provide reference for future economic evaluation [9,10].
Methods
Inclusion And Exclusion Criteria
This systematic review was conducted according to the Preferred Reporting Initiative for Systematic Reviews and Meta-analyses (PRISMA) [11]. The entire process was guided by a predefined detailed protocol. This systematic review encompassed studies involving a specific adult patient population of those with advanced, metastatic, or unresectable GISTs who had previously undergone treatment with a first line Imatinib for advanced disease.
The intervention of interest was Sunitinib, regardless of dose and duration. The outcome measures included overall survival (OS) and progression-free survival (PFS), objective response rate (ORR) and Adverse Drug reactions (ADRs) with grade 3 or more. Studies that did not meet the inclusion criteria were excluded and only trials reporting at least one of these key outcomes were included in the review [32].
Search Strategy
A comprehensive literature search was conducted across three databases—PubMed, Cochrane Library, and Embase—for RCTs and observational studies. The search terms were: “Gastrointestinal Stromal Tumors”, “Sunitinib” and “Imatinib failure”. The search process covered possible trials from inception to October 2nd 2024, and there was restriction to studies published in English language. Additionally, a hand search was performed on the ClinicalTrials.gov website to identify ongoing studies and access unpublished data. To further enhance the completeness of our search, a snowballing approach was employed scrutinizing reviews and meta-analysis related to the topic to ensure that no publications were overlooked during the initial literature search. The protocol is attached in Supplementary File S1.
Screening
For data management, EndNote was utilized to organize and track references acquired during the literature search, aiding in deduplication and citation management. Additionally, Rayyan reference manager was employed to screen titles, abstracts, and full-text articles, facilitating the determination of their relevance and inclusion in the review. Regarding the selection process, initial screening of each study's title and abstract was conducted independently by the first and second reviewers based on predefined inclusion and exclusion criteria. Unclear cases were categorized as "maybe" and reevaluated during the full-text review. Full texts of potentially relevant articles were obtained and independently reviewed by the same two authors. Any discrepancies between reviewers were resolved through discussion, with involvement from the third author if needed, to reach a consensus.
Data Extraction
Following the agreement on the included studies, data was extracted utilizing a customized data extraction form. This form included the following data: the study name, publication year, sample size, funding source, geographic location, demographic information (age, gender), Sunitinib dosing regimen, and any comparator particulars if applicable.
The primary outcomes of interest encompassed: Median PFS, representing the duration until half of the participants experience disease progression or recurrence, along with hazard ratios and corresponding confidence intervals. Median Overall Survival (OS) defines the time until half of the participants pass away from any cause, along with hazard ratios and corresponding confidence intervals. The Objective Response Rate (ORR) indicates the proportion of participants experiencing partial or complete reduction in the size or extent of their disease. Additionally, the incidence of grade 3 or higher adverse events were collected as a secondary outcome [31].
The Risk of Bias
RCTs underwent assessment using the Cochrane Risk of Bias tool (ROB.2) [13]. Non-randomized comparative clinical trials were appraised utilizing the Risk of Bias in Non-Randomized Studies - of Interventions (ROBIN-I) tool [13], while single-arm observational studies, were assessed using the Newcastle-Ottawa Scale (NOS) [14].
Data Analysis
Only one RCT was included in our review. Moreover, the included real-world studies were heterogeneous single arm studies. Consequently, a qualitative approach was adopted for synthesizing the evidence [7,8].
Results
Search Results
Our search yielded 192 records, out of which 184 were found through database searching and 8 through other sources. After the duplicates were removed, 163 records were screened, and 128 records excluded based on their titles and abstracts. After checking the full texts, 8 records were excluded. 19 studies, reported in 21 separate publications, were included in the review (PRISMA flowchart is shown in Figure 1).
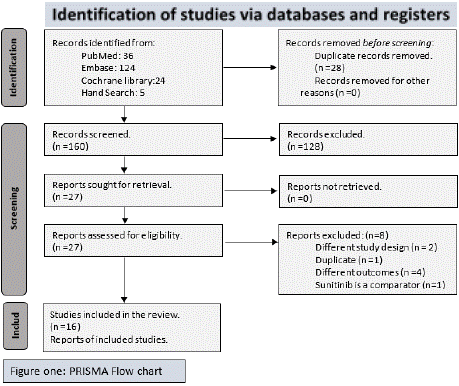
Figure 1: PRISMA Flow Chart depicting the identification, screening, and
inclusion process for the systematic review.
Study Characteristics
Most of the included studies were single arm observational studies (17 studies, 18 reports). Four comparative studies were identified (4 studies, 5 reports). A solitary RCT and its updated report were identified from the research findings. This RCT is a phase III, double-blind, placebo-controlled multi-national study. Patients were randomized to receive either Sunitinib or a placebo following the licensed initial dosing regimen (IDR) for Sunitinib of 50mg administered for 4 weeks, with a 2-week off period, repeated in 6-week cycles. The findings and outcomes from this trial played a pivotal role in securing the licensing of Sunitinib as a second-line treatment for GIST [7,8].
Two comparative studies of sunitinib and imatinib dose escalation were conducted, one in 2018 and the other in 2023. In both studies, patients who experienced failure with first line imatinib were classified into two groups: those who received dose-escalated imatinib (600- 800 mg) and those who received IDR or CDD sunitinib. Both studies included Chinese patients.
The other comparative study for Saski et al (2023) [25] was a retrospective observational trial that was conducted in Japan. This study compared the licensed IDR with the continuous daily dosing regimen (CDD); however, it included limited number of patients, and no information was reported on the previous imatinib use in terms of dose and duration. In both comparative studies, Sunitinib was used in the second line setting.
Our systematic review includes 14 observational single arm studies. Seven out of the fourteen studies focused on evaluating the outcomes of Sunitinib treatment in diverse Asian populations in real life settings [16,19-24]. In addition, one study was conducted in East Europe (Poland) [28] and one in India [27]. On the other hand, two of the single arm studies were dose finding phase I/II trials, where patients received varying dosing regimens (25, 50, 75 mg) to establish a recommended sunitinib dosing schedule and evaluate efficacy, safety, pharmacokinetics, and pharmacodynamics of Sunitinib. One of these dose finding studies, assessed the impact of primary and secondary kinase genotypes on the activity of Sunitinib [18]. Table 1 and Table 2 display study and patient characteristics for RCTs and comparative observational studies, respectively, while Table 3 and Table 4 illustrate the same for single-arm studies.
Study
Funding
Region
Study
DesignPopulation
Line of treatment
Sunitinib Regimen
Control
Sample Size
End points
Demetri,2006 (7)
Demetri,2012 (8)
Pharmaceutical
Multiple countries
Phase III RCT
Adults with histologically proven GIST for whom prior Imatinib treatment had failed due to resistance or intolerance
2nd
(IDR)
50mg/day
4 weeks on, 2 weeks off
Placebo
243/118
Median PFS,
Median OS,
ORR,
Grade
= 3 ADRsYang, 2018 (33)
Non-funded
Single Country
(China)
Retrospective
comparative observational
studyPatients with GIST, aged 18 years or older, disease progression on Imatinib or intolerance
2nd
(IDR)
50mg/day
4 weeks on, 2 weeks off OR (CDD) 37.5mg/dayImatinib 600mg
29/11
Median PFS,
Median OS,
Grade
= 3 ADRSaski,2023 (25)
Non-Pharmaceutical
Single country (Japan)
Retrospective
comparative observational
study
Patients who underwent Sunitinib therapy for Imatinib resistant and/or intolerant unresectable and metastatic GISTs
2nd
(IDR)
50mg/day
4 weeks on, 2 weeks off
(CDD)
37.5mg/day
Imatinib 600mg (300mg twice daily) OR 800mg (400mg twice daily)
21/20
Median PFS,
Median OS,
ORR,
Grade
= 3 ADRsHuang,2023 (34)
Non-funded
Single Country
(China)
Retrospective
comparative observational
studyAdults Patients with histologically proven GISTs previously treated with 400mg Imatinib as first-line therapy
2nd
(IDR)
50mg/day
4 weeks on, 2 weeks off OR (CDD) 37.5mg/day103/100
Median PFS,
Median OS,
Grade
= 3 ADRsRCT: Randomized-control trial, IDR: Initial dosing regimen, CDD: continuous daily dosing, PFS: Progression-free survival, OS: Overall survival, ORR: Objective Response rate, ADRs: Adverse drug reactions.
Table 1: Studies Characteristics’: Rcts and Comparative Observational Studies.
Study
Treatment/
Sample Size
Median
Age Range
Gender
Maximum dose of previous Imatinib
Duration of previous Imatinib treatment (months)
Reasons of Imatinib discontinuation (Progression/
Intolerance)control
Age
(years)
(M/F)
Demetri,2006 (7)
IDR/ placebo
243/118
57/55
23-84
223/138
Median: 800mg
Range: (300-1600) mg24.57
344/17
Demetri,2012 (8)
Yang, 2018 (33)
SU/IM
29/11
56/59
37-78
25/15
400mg
NR
38/2
Saski,2023 (25)
IDR/ CDD
21/20
60/70.5
44-85
29/12
NR
NR
38/3
Huang,2023 (34)
SU/IM
103/100
52/52.2
50-54.3
136/67
400mg
NR
203/0
M: Male, F: Female, IDR: Initial Dosing Regimen, CDD: Continuous Daily Dosing, NR: Not Reported, SU: Sunitinib, IM: Imatinib.
Table 2: Baseline Patients Characteristics’: RCTs and comparative observational studies.
Study
Funding
Region
Study
Population
Line of treatment
Sunitinib Regimen
Sample Size
End points
Design
Demetri,2009 (17)
Pharmaceutical
NA
Open-label, dose ranging Phase I/II
Adults with histologically confirmed metastatic and/or unresectable GIST with documented Imatinib failure due to resistance or intolerance.
NA
(IDR)
50mg/day
4 weeks on, 2 weeks off
97
Median PFS,
Median OS,
ORR,
Grade
= 3 ADRsHeinrich, M,2008 (18)
Pharmaceutical
NA
Open-label, dose ranging Phase I/II
Biopsies of genotype analyses were obtained from patients who were adults who histologically confirmed metastatic/unresectable GIST and documented failure of Imatinib caused by resistance or intolerance.
NA
(IDR)
50mg/day
4 weeks on, 2 weeks off78
Median PFS,
Median OS,
ORRShiaro,2010 (16)
Pharmaceutical
Single country (Japan)
Open label,
Non-randomized multicenter,
Dose- escalation
Phase I/IIJapanese patients with histologically proven metastatic or unresectable malignant GIST and confirmed failure of prior Imatinib therapy or discontinuation of Imatinib due to toxicity.
2nd
(IDR)
50mg/day
4 weeks on, 2 weeks off
36
Median PFS,
Median OS,
ORR,
Grade
= 3 ADRsGeorge,2009 (9)
Pharmaceutical
NA
Open label,
Multicenter,
Phase II
Adults with histologically confirmed malignant GIST not amenable to standard therapy and with documented failure of Imatinib due to resistance or intolerance.
NA
(CDD)
37.5mg/day
60
Median PFS,
Median OS,
ORR,
Grade
= 3 ADRsLilshen,2017 (22)
Pharmaceutical
Single country (China)
Open label,
phase IV
Patients with diagnosis of GIST confirmed by histopathology and treatable by surgery, radiation, or combined modality therapy with curative intent. Patients had dimensionally measurable disease and prior Imatinib treatment failed.
NA
(IDR)
50mg/day
4 weeks on, 2 weeks off
59
Median PFS,
Median OS,
ORR,
Grade
= 3 ADRsLiJ,2012 (21)
Non-Funded
Single country (China)
Retrospective
Patients with metastatic GISTs that expressed CD117 or CD34 who were resistant or intolerant to prior Imatinib treatment, received Sunitinib for at least one cycle.
2nd
(IDR)
50mg/day
4 weeks on, 2 weeks off OR (CDD) 37.5mg/day55
Median PFS,
ORR,
Grade
= 3 ADRsYoon,2012 (24)
Pharmaceutical
Single country (Korea)
Retrospective
Patients with histologically proven metastatic or unresectable GIST with at least one measurable disease.
2nd
(IDR)
50mg/day
4 weeks on, 2 weeks off OR (CDD) 37.5mg/day88
Median OS,
ORR,
Grade
= 3 ADRsChen, YY,2011 (23)
Non-Funded
Single country (Taiwan)
Retrospective
Patients who had histologically confirmed, recurrent, unresectable, or metastatic GIST who failed prior Imatinib therapy as demonstrated by disease progression or toxicity.
2nd
(IDR)
50mg/day
4 weeks on, 2 weeks off OR (CDD) 37.5mg/day
23
Median PFS,
Median OS,
ORR,
Grade
= 3 ADRsMatsumoto,2011 (20)
Non-Funded
Single country (Japan)
Retrospective
Patients with advanced GIST who were resistant or intolerant to previous treatment with Imatinib.
NA
(IDR)
50mg/day
4 weeks on, 2 weeks off18
ORR,
Grade
= 3 ADRsKomatsu,2015 (19)
Pharmaceutical
Single country (Japan)
Prospective,
post-marketing
Patients with Imatinib-resistant/-intolerant GIST.
2nd
(IDR)
50mg/day
4 weeks on, 2 weeks off OR (CDD) 37.5mg/day
OR 25mg/day470
Median PFS,
Median OS,
ORR,
Grade
= 3 ADRsSahu,2015 (27)
Non-Funded
Single country (India)
Single Center
Prospective
Patients with advanced Imatinib resistant and/or intolerant.
NA
(IDR)
50mg/day
4 weeks on, 2 weeks off
15
Median PFS,
Median OS,
ORR,
Grade
= 3 ADRsRutkowski,2018 (26)
Non-Funded
Single country (Poland)
Prospective
Patients treated initially with Imatinib mesylate for inoperable and/or metastatic histologically confirmed CD117-positive GIST.
2nd
(IDR)
50mg/day
4 weeks on, 2 weeks off
232
Median PFS,
Median OS,
Grade
= 3 ADRs
Reichardt,2015 (28)
Reichardt,2016 (29)
Pharmaceutical
Multiple countries
Retrospective
Patients with histologically confirmed malignant GIST not amenable to standard therapy with curative intent after failure of prior Imatinib treatment.
NA
(IDR)
50mg/day
4 weeks on, 2 weeks off1124
Median PFS,
Median OS,
Grade
= 3 ADRsPrior, J,2009 (15)
Pharmaceutical
NA
Single arm
Patients who received Sunitinib salvage therapy who had
histologically prove GIST and had aborted previous Imatinib therapy because of recent tumor progression or unacceptable Imatinib toxicity.
NA
(IDR)
50mg/day
4 weeks on, 2 weeks off
23
Median PFS,
Median OS,
Grade
= 3 ADRs
Zhang,2021 (35)
Non-funded
Single country (China)
Retrospective
Patients with advanced or metastatic GIST after Imatinib failure who started sunitinib treatment.
2nd
(IDR)
50mg/day
4 weeks on, 2 weeks off OR (CDD) 37.5mg/day
OR 25mg/day107
Median PFS,
Median OS,
ORR,
Grade
= 3 ADRsIDR: Initial dosing regimen, CDD: continuous daily dosing, NA: Not available, PFS: Progression-free survival, OS: Overall survival, ORR: Objective Response rate, ADRs: Adverse drug reactions.
Table 3: Studies Characteristics’: Single-arm studies.
Study
Treatment
Sample Size
Median
Age
(years)Age Range
(year)Gender
(M/F)Median Maximum dose of previous Imatinib
Median Duration of previous Imatinib (months)/(Range)
Reason of Imatinib Cessation
(Resistance/Intoleranc)
Demetri,2009 (17)
IDR
97
55
26-76
64/33
Median :600
19
93/4
Range: (400-1000)
(2-38)
Heinrich, M,2008 (18)
IDR
78
55
26-76
53/25
Median :600
18.2
74/4
Range: (400-1000)
(2.3-35.2)
Shiaro,2010 (16)
IDR
36
56
33-54
24/12
NA
26
33/3
(2-46)
George,2009 (9)
CCD
60
59
24-84
28/32
Median: 800
25
57/3
Range: (200-1200)
(0.5-62.8)
Lilshen,2017 (22)
IDR
59
NA
29-82
39/20
NA
NA
57/2
LiJ,2012 (21)
IDR
OR CDD55
54
49.8-58.2
40/15
400mg:21
600mg:26
800mg:827
(22.5-31.5)53/2
Yoon,2012 (24)
IDR
OR CDD88
59
25-76
55/33
400mg:24
600mg:28
800mg:3631.1
(1.8-83.2)86/2
Chen, YY,2011 (23)
IDR
OR CDD23
59
24-83
16/7
NA
NA
22/1
Matsumoto,2011 (20)
IDR
18
58.7
26-77
13/5
NA
39
(1-65)16/2
Komatsu,2015 (19)
IDR OR CDD
470
64
17-88
296/174
NA
NA
392/53
13: progression and intoleranceSahu,2015 (27)
IDR
15
48
26-69
5-Oct
NA
29
(6-46)15/0
Rutkowski,2018 (26)
IDR
232
55
15-82
NA
NA
NA
Reichardt,2015 (28)
IDR
1124
59
Oct-92
672/452
Median: 600
NA
1024/99
1: unknownReichardt,2016 (29)
Range:(200-2400)
Prior, J,2009 (15)
IDR
23
53
24-76
16/7
NA
30
NA
(9.6-51)
Zhang,2021 (35)
IDR
OR CDD107
51.5
54.2-57.1
72/35
400mg:32
24
NA
600mg:57
800mg:18
M: Male, F: Female, IDR: Initial Dosing Regimen, CDD: Continuous Daily Dosing, NA: Not Available.
Table 4: Baseline Patients Characteristics’: Single-arm studies.
Quality Assessment of Studies
We recognized that all single-arm studies were of ‘poor’ quality. Regarding the comparative studies, Demetri et al. (2006, 2012) [7,8] emerged as the only study with a low risk of bias while the other observational studies; Yang (2018), [33] Huang (2023), [34] and Saski (2023) [25] showed a notable risk of bias, particularly due to confounding factors. Additionally, George, 2009 [9] was deemed to have a high risk of bias due to its lack of blinding.
Clinical Efficacy/Effectiveness
In the solitary phase III RCT conducted by Demetri et al. (2006, 2012) [7,8], Sunitinib IDR exhibited a marked advantage over placebo, with an ORR of 6.58% compared to 0%, and a median PFS of 5.32 months versus 1.4 months (HR: 0.34, 95% CI 0.253-0.475; p<0.0001). The median OS was 16.9 months for the Sunitinib group and 9.1 months for the placebo group. However, there was no statistically significant difference in OS between the two groups (HR: 0.876, 95% CI 0.679-1.129; p=0.306) (Table 5).
Study
Treatment/
Median PFS (months)
PFS HR
Median OS
OS HR
Complete Response/
Partial Response/ Sample size
Incidence of Grade
Control
(95% CI)
(Months)
(95% CI)
Sample size
= 3 ADRs
Demetri,2006(7)
Demetri,2012(8)IDR
5.32
0.347
(0.253-0.475)
p-value =<0.00116.9
(14.3-19.3)0.876
(0.679-1.129) P-value =0.3060/243
16/243
140/1201
Saski,2023 (25)
Placebo
1.4
NR
9.1
(6.5-12.6)
0/118
0/118
13/202
IDR
1.4
13.7
IQR (7.5-22.9)NR
0/21
21-Apr
21/21
CDD
4
13.4
IQR (9.3-36.8)0/20
20-Jun
15/19
Yang,2018 (33)
SU
9
(1-74)NR
26
(1-82)
29-Jan
29-Sep
14/29
IM
13
(2-51)19
(5-64)
Huang,2023 (34)
SU
12
0.348 (0.251-0.482)
25
(21.9-28.1)NR
0/103
16/103
NR
(10.3-13.7)
IM
5
21.5
(18.9-24.1)2/100
11/100
NR
(3.6-6.4)
RCT: Randomized-Control Trial, IDR: Initial Dosing Regimen, CDD: Continuous Daily Dosing, PFS: Progression-Free Survival, OS: Overall Survival, ORR: Objective Response Rate, Adrs: Adverse Drug Reactions, SU: Sunitinib, IM: Imatinib, NR: NOT REPORTED.
Table 5: Efficacy and SAFETY: RCTs and comparative observational studies.
Study
Number of Cases
Median PFS (months)
Median OS
(Months)Complete Response/
Sample sizePartial Response/ Sample size
Incidence of Grade
= 3 ADRsDemetri,2009 (17)
97
7.8
19
0/97
Jul-97
82/570
Heinrich, M,2008 (18)
Exon 9 mutation
19
19.4
26.9
0/19
19-Jul
Shiaro,2010 (16)
Exon 11 mutation
44
5.1
12.3
0/44
Feb-44
NR
George,2009 (9)
Wildtype-genotype
9
19
30.5
NR
NR
Lilshen,2017 (22)
36
6.6
Not reached
0/36
13/36
62/381
LiJ,2012 (21)
60
7.93
24.97
0/60
Aug-60
62/381
Yoon,2012 (24)
59
10.8
26
0/59
Nov-59
40/58
Chen, YY,2011 (23)
55
8.16
Not reached
0/55
Jun-55
7/128
Matsumoto,2011 (20)
88
NR
17.6
0/88
Sep-88
200/743
Komatsu,2015 (19)
23
8.4
14.1
23-Feb
23-Apr
14/145
Sahu,2015 (27)
18
NR
NA
0/18
18-Jan
16/140
Rutkowski,2018 (26)
470
5.22
5.6
2/470
75/470
329/447
Reichardt,2015 (28)
15
15.5
18.7
0/15
15-Apr
12-Dec
Reichardt,2016 (29)
Prior, J,2009 (15)
232
10.3
22.9
NR
NR
53/433
Zhang,2021 (35)
1124
8.3
16.6
NR
NR
197/516
Demetri,2009 (17)
23
6.2
14
NR
NR
NR
Heinrich, M,2008 (18)
45
8
33
Jul-45
Apr-45
36/45
PFS: Progression-Free Survival, OS: Overall Survival, ORR: Objective Response Rate, Adrs: Adverse Drug Reactions, NR: Not Reported.
Table 5 off 1: Laboratory values at hospital admission.
The efficacy of CDD was evaluated in a phase II open-label nonrandomized clinical trial conducted by Demetri et al. as well (2009) [9], revealing an ORR of 13.3%, a median PFS of 7.9 months, and a median OS of 25 months. Table 5
These two regimens were compared in a subgroup analysis by Reichardt (2015) [28,29], with the median PFS for the IDR groups at 5.2 months versus 12.7 months for the CDD. Correspondingly, median OS values were 11.1 months versus 23.5 months, respectively.
Also, sunitinib was compared with dose-escalated imatinib after progression on first line imatinib in studies by Yang (2018) and Huang (2023). Huang (2023) showed a median PFS of 12 months for the sunitinib group compared to 5 months for the imatinib group. Sunitinib significantly reduced the hazard of tumor progression with an HR of 0.348 (95% CI: 0.251-0.482, p-value==<0.001) [34]. However, there was less difference in the median OS, which was 25 months for the sunitinib group versus 21.5 months for the doseescalated imatinib group. On the other hand, Yang (2018) reported equal median PFS of 4 months for both regimens and a median OS of 19 months for the imatinib escalation group compared to 9 months for the sunitinib group [33].
Recently, the IDR and CDD were compared in a double-armed observational study by Saski (2023) [25] where 41 patients were retrospectively analyzed, with 21 patients assigned to the IDR and 20 patients to the CCD regimen. The ORR was 19% in the IDR arm and 30% in the CDD group. Median PFS durations were 1.4 months for IDR and 4 months for CCD. Despite differences in ORR and median PFS, the discrepancy in median OS was slight, with values of 13.4 months and 13.7 months, respectively (Table 5).
Patients with exon 9 mutations exhibited a superior response to Sunitinib, as demonstrated by Heinrich in 2008 [18]. This cohort showed an ORR of 37%, with a median PFS of 19.4 months and a median OS of 26.9 months, compared to 5.1 months and 12.3 months, respectively, in patients with exon 11 mutations. These results are consistent with a subgroup analysis conducted by Reichardt in 2015 [28,29], which revealed a median PFS of 12.3 months and a median OS of 26.3 months among patients with KIT exon 9 mutations and a median PFS of 7 months and a median OS of 16.3 months in patients with exon 11 mutations.
Rutkowski (2018) [26] aimed to analyze treatment outcomes in older patients. The median PFS for patients aged <70 and those aged >70 (10.3 vs. 9.7 months, respectively), and the median OS was (22.9 vs. 21.5 months, respectively).
In single-armed observational studies conducted in Asian countries, varied results were demonstrated. ORR ranged from 10.2% to 36.1%, with median PFS ranging from 5.22 to 10.8 months, and median OS ranging from 5.6 to 26 months (Table 5).
Safety
When the IDR was contrasted with placebo (Demetri, 2012) [8] the predominant hematological ADRs of grade 3 and above were thrombocytopenia (4%), neutropenia (12%), lymphocytopenia (12%), anemia (11%), and leukopenia (3%). The most common severe (grade 3 or 4) non-hematological adverse events associated with treatment included fatigue (10%), hypertension (8%), and hand-foot syndrome, asthenia, and diarrhea (5% each) [8,9].
A comparison of hematological ADRs revealed a higher incidence with CDD compared to IDR specifically, thrombocytopenia occurred in 4% of patients on CDD versus 3% on intermittent dosing, lymphocytopenia in 12% versus 25%, neutropenia in 12% versus 13%, leukopenia in 3% versus 12%, and anemia in 5% versus 7%, respectively.
Additionally, two observational studies; (Saski, 2023) [28] and (Li, J, 2012) [21] investigated the incidence of ADRs in patients receiving IDR and CDD reported different results. IDR group had a higher incidence of thrombocytopenia compared to CDD (24.5% vs. 12.8%). Rates of neutropenia were similar between the groups (26.3% vs. 25.6%). Anemia was reported in 10.5% of patients with IDR versus 7.7% with CDD. Hand-foot syndrome incidences were comparable (5.2% vs. 5.12%), while diarrhea was more prevalent in the IDR group (3.5% vs. 0%).
In single-arm observational studies, ADRs of grade 3 or higher were commonly reported regardless of the dosage administered. Hematological adverse effects were notably common, with thrombocytopenia being the most prevalent, followed by neutropenia and anemia. Leukopenia was also observed. Additionally, handfoot syndrome, hypertension, fatigue, and diarrhea were reported. Changes in complete blood count parameters and abnormal liver function tests were noted in some patients. Hypothyroidism and increased lipase levels or pancreatitis were also reported in a smaller proportion of cases.
Table 5 illustrates the efficacy/effectiveness and safety data from RCTs and comparative observational studies and Table 5 presents the efficacy/effectiveness and safety data of single armed studies.
Discussion
Our systematic review results showed that since Sunitinib's FDA approval for the treatment of GISTs in June 2006 [30], only one RCT, led by Demetri, has been conducted [7,8]. However, our review found increased interest in generating real word evidence using observational study designs.
In the sole multinational RCT, Sunitinib (50mg/day for four weeks with a two-week break) was compared with a placebo. The Sunitinib group had a higher confirmed ORR (7% vs 0%). Demetri et al. showed Sunitinib prolonged PFS to 5.32 months versus 1.5 months with placebo. Additionally, Sunitinib nearly doubled the median OS (16.9 months vs 9.6 months) and halved the risk of death compared to placebo [7,8].
A CDD administration of Sunitinib at a dosage of 37.5 mg/ day was explored as an alternative to the standard IDR in a phase II clinical trial [9]. Results revealed an ORR of 13.3%, along with a median PFS of 7.9 months and a median OS of 25 months. Despite these promising findings, further investigation of this regimen was not pursued beyond this phase II clinical trial, thereby relegating it to off-label use [9].
Direct comparison between these two regimens in an observational study conducted by Saski [23] revealed higher ORR in the IDR arm compared to the CDD (30% vs 19%), and a notable difference in median PFS with the IDR arm showing a PFS of 1.4 months compared to 4 months in the CDD. However, the median OS was quite similar between the two arms, with 13.4 months in the 50mg/day arm and 13.7 months in the 37.5mg/day arm [23]. CDD appears to offer no efficacy or safety advantage [23].
Building upon the findings of the clinical trials, our systematic review delved into single-armed observational studies, further illuminating sunitinib's performance across a diverse array of patient populations. Our research uncovered eleven single-armed observational studies, with seven focusing on Asian populations, including Japanese, [16,19,20] Korean, [23] Taiwanese, [23] and Chinese [21,22] populations. Other studies were conducted in India, [27] Poland, [26] and across multiple countries [15,17,18,28,29]. These studies unveiled median PFS durations ranging from 5.1 to 19.4 months, median OS durations spanning from 5.6 to 27 months, and ORR ranging between 6% and 36%.
Overall, efficacy measures in these studies were of high diversity, non-randomized studies showed higher median PFS and median OS, probably due to confounding factors, such as ethnicity, while certain studies involving Asian populations suggested better efficacy outcomes compared to Western populations, Asians and specifically Japanese patients appear to be more sensitive to sunitinib, resulting in higher incidence of grade 3-5 ADRs [16,19,20]. In general, Sunitinib presents a manageable toxicity profile, which can often be addressed by dosage adjustments, supportive measures, or temporary pauses in treatment [7,8]. Lowering the dose below the standard 50-mg 4/2 dosing schedule notably reduces the incidence of AEs, with discontinuation being contemplated for more severe cases [7,8].
Another confounding factor might be considered is tumor genotype. The results also demonstrated that patients with primary KIT exon 9 mutations or a wild-type genotype achieved an ORR, median PFS and median OS are considerably higher compared to those with exon 11 mutations [18].
Considering escalated imatinib dose as a comparator for sunitinib remains controversial. While direct head-to-head RCTs comparing these interventions are lacking, comparative observational studies and systematic reviews suggest that patients with wild-type KIT and exon 9 mutations are more likely to respond to escalated doses of imatinib compared to those with exon 11 mutations [30,31,32].
Results of this systematic review align with what is followed in guidelines [6], as regarding Imatinib dosing, patients with Imatinibsensitive tumors typically commence treatment at a daily dosage of 400mg. In cases where secondary resistance to Imatinib occurs, dose escalation to a daily dosage of 800mg may be considered. However, for patients encountering primary or early resistance, switching to an alternative therapeutic agent becomes necessary [6]. Those with exon 9 mutations typically initiate therapy at the maximum daily dose of 800mg, with a transition to an alternative treatment regimen required upon disease progression [6,30].
This aligns with the understanding that Sunitinib is typically viewed as a second-line agent following both standard and high doses of Imatinib [6]. However, there's a growing recognition in clinical practice of the potential for mutational status to guide the customization of a patient's initial Imatinib dose [30]. HTA agencies relied on the RCT for economic evaluation, which served as the basis for recommendations from the National Institute for Health and Care Excellence (NICE) and the Canadian Agency for Drugs and Technologies in Health (CADTH). According to this trial, about 22% of patients completed the treatment after disease progression [7,8]. It was possible that patients receiving sunitinib post-progression experienced additional benefits, and some may have had tumor flares on sudden withdrawal of sunitinib [36]. For the economic evaluation, NICE included treatment costs beyond disease progression, and the treatment met the criteria for being a life-extending, end-of-life treatment, making it cost-effective [36]. CADTH's report resulted in a higher incremental cost-effectiveness ratio (ICER), primarily due to the greater acquisition cost of sunitinib and the calculation of effects only until disease progression [37].
To our knowledge, this systematic review is the first to incorporate data from both RCTs and real-world data (RWD). By addressing this gap in the literature, we aim to assess the suitability of the target population for this intervention and evaluate its efficacy and safety for resource allocation decisions. Integrating data from both RCTs and RWD ensures a more comprehensive understanding of the benefits and risks of the intervention, aiming to provide a more thorough HTA report.
However, the limitations and challenges of this systematic review include the exclusion of non-English studies, which may result in missing relevant data, and the reliance on published studies only, potentially overestimating sunitinib's effectiveness and safety. Interpreting the findings within the broader clinical context requires careful consideration of potential biases and challenges present in the included studies.
Additionally, the small number of RCTs assessing the efficacy of Sunitinib and the reliance on non-randomized, observational data poses limitations to our systematic review. These studies are susceptible to heterogeneity and various biases, such as confounding, and are often characterized by small sample sizes. Ideally, enhancing the existing evidence base with new RCTs and comparative observational trials would be advantageous. However, the feasibility of conducting such trials may be hindered by the nature of the disease and its low incidence rates.
Conclusion
In conclusion, the findings from the double-blind RCT emphasize sunitinib's effectiveness, evidenced by a notable improvement in time to tumor progression, which was four times longer with sunitinib compared to placebo. Additionally, the median PFS and OS demonstrated notable results. The treatment exhibited good tolerability with an acceptable safety profile, indicating the potential suitability of sunitinib for our population. This was supported by realworld evidence from observational studies across diverse populations. However, addressing existing uncertainties and providing a more definitive basis for decision-making requires conducting more randomized trials, which is crucial for better consideration in resource allocation decisions. At our institution, there is currently no available choice for patients with GIST who have developed progression on imatinib or were unable to tolerate it.
Acknowledgements
The authors thank the Center of Drug Policy and Technology Assessment (CDPTA) at King Hussein Cancer Center for their support in conducting this review. We also extend our gratitude to our colleagues for their valuable input and assistance throughout the research process.
Authors' Contributions
Tala Asha designed the study, developed the search strategy, and conducted the literature search, screening, and data extraction. Khader Al-Habash screened the studies, performed data extraction, and contributed to the analysis. Dr. Abeer A. Al-Rabaiah resolved disagreements, ensured the review's integrity, and supervised the process. All authors contributed to drafting and revising the manuscript. All authors have read and approved the final manuscript.
Use of Artificial Intelligence Tools
ChatGPT was used to assist in reviewing the grammer and the clarity of sentences and all content was reviewed and edited after its use.
References
- Corless CL, Fletcher JA and Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004; 22: 3813-3825.
- Miettinen M and Lasota J: Gastrointestinal stromal tumors: Pathology and prognosis at different sites. Semin Diagn Pathol. 2006; 23: 70-83.
- Søreide K, Sandvik OM, Søreide JA, Giljaca V, Jureckova A and Bulusu VR: Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 2016; 40: 39- 46.
- DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM and Brennan MF: Two hundred gastrointestinal stromal tumors: Recurrence patterns and prognostic factors for survival. Ann Surg. 2020; 231: 51-58.
- Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002; 347: 472-480.
- ESMO Interactive Guidelines.
- Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. The Lancet. 2006; 368: 1329–1338.
- Demetri GD, Garrett CR, Schöffski P, Shah MH, Verweij J, Leyvraz S, et al: Complete longitudinal analyses of the randomized, placebo-controlled, phase III trial of sunitinib in patients with gastrointestinal stromal tumor following imatinib failure. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research [Internet]. 2012; 18: 3170–3179.
- S George, JY Blay, PG Casali, A Le Cesne, P Stephenson, SE DePrimo, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. 2009; 45: 1959–1968.
- Sunitinib, HIGHLIGHTS OF PRESCRIBING INFORMATION.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al: The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst Rev. 2021; 10: 89.
- Cochrane. RoB 2: A revised Cochrane risk-of-bias tool for randomized trials [Internet]. Cochrane.org. 2011.
- ROBINS-I tool | Cochrane Methods [Internet]. methods.cochrane.org.
- https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Prior JO, Montemurro M, Orcurto M-V, Michielin O, Luthi F, Benhattar J, et al. Early Prediction of Response to Sunitinib After Imatinib Failure by 18F-Fluorodeoxyglucose Positron Emission Tomography in Patients With Gastrointestinal Stromal Tumor. Journal of Clinical Oncology. 2008; 27: 439–445.
- Kuniaki Shirao, Toshirou Nishida, Toshihiko Doi, Yoshito Komatsu, Kei Muro, Yinhua Li, et al. Phase I/II study of sunitinib malate in Japanese patients with gastrointestinal stromal tumor after failure of prior treatment with imatinib mesylate. 2010; 28: 866–875.
- Demetri GD, Heinrich MC, Fletcher JA, Christopher D. M. Fletcher, Van, Corless CL, et al. Molecular Target Modulation, Imaging, and Clinical Evaluation of Gastrointestinal Stromal Tumor Patients Treated with Sunitinib Malate after Imatinib Failure. Clinical Cancer Research. 2009; 15: 5902– 5909.
- Heinrich MC, Maki RG, Corless CL, Antonescu CR, Harlow A, Griffith D, et al. Primary and Secondary Kinase Genotypes Correlate With the Biological and Clinical Activity of Sunitinib in Imatinib-Resistant Gastrointestinal Stromal Tumor. Journal of Clinical Oncology [Internet]. 2008; 26: 5352–5359.
- Komatsu Y, Ohki E, Ueno N, Yoshida A, Toyoshima Y, Ueda E, et al: Safety, efficacy and prognostic analyses of sunitinib in the post-marketing surveillance study of Japanese patients with gastrointestinal stromal tumor. Komatsu, 2015.
- Matsumoto K: Akira Sawaki, Mizuno N, Hara K, Susumu Hijioka, Niwa Y, et al. Clinical Efficacy and Safety of Sunitinib After Imatinib Failure in Japanese Patients with Gastrointestinal Stromal Tumor. Japanese Journal of Clinical Oncology [Internet]. 2010; 41: 57–62.
- Li Jian, Gao Jing, Hong Jinlin, Shen Lin. Efficacy and safety of sunitinib in Chinese patients with imatinib-resistant or -intolerant gastrointestinal stromal tumors. Future Oncology, (Li, J, 2012).
- Shen L, Sun Y, Xu JM, Linn C, Wang Q, Yang LQ, et al. Phase IV Study of Sunitinib in Chinese Patients with Imatinib-Resistant or Imatinib-Intolerant Gastrointestinal Stromal Tumors. Oncology and Therapy. 2017; 5: 171–180.
- Chen YY. Sunitinib for Taiwanese patients with gastrointestinal stromal tumor after imatinib treatment failure or intolerance. World Journal of Gastroenterology. 2011; 17: 2113.
- Dok Hyun Yoon, Min-Hee Ryu, Baek-Yeol Ryoo, Moyeol Beck, Dae Ro Choi, Yoojin Cho, et al. Sunitinib as a second-line therapy for advanced GISTs after failure of imatinib: relationship between efficacy and tumor genotype in Korean patients. 2012.
- Sasaki K, Kanda T, Matsumoto Y, Ishikawa T, Hirota S, Saijo Y. Sunitinib therapy for imatinib-resistant and/or intolerant gastrointestinal stromal tumors: comparison of safety and efficacy between standard and reduced dosage regimens. Japanese Journal of Clinical Oncology. 2023.
- Rutkowski Piotr, Bylina Elzbieta, Lugowska Iwona, Teterycz Pawel, Klimczak Anna, Streb Joanna, et al. Treatment outcomes in older patients with advanced gastrointestinal stromal tumor (GIST). Journal of Geriatric Oncology. 2018; 520-525.
- Gupta S, Sahu A, Godbole S, Jain P, Ghosh J, Shrikhande S, et al. Sunitinib in patients with imatinib-resistant gastrointestinal stromal tumor: A single center experiencep-190 chronic myelogenous leukemia presenting as extramedullary blast crisis. Indian Journal of Cancer. 2015; 52: 320.
- Reichardt P, Kang YK, Rutkowski P, Schuette J, Rosen LS, Seddon B, et al. Clinical outcomes of patients with advanced gastrointestinal stromal tumors: Safety and efficacy in a worldwide treatment-use trial of sunitinib. Cancer. 2015; 121: 1405–1413.
- Reichardt P, Demetri GD, Gelderblom H, Rutkowski P, Im SA, Gupta S, et al. Correlation of KIT and PDGFRA mutational status with clinical benefit in patients with gastrointestinal stromal tumor treated with sunitinib in a worldwide treatment-use trial. BMC Cancer. 2016; 16.
- Research C for DE and: FDA approves sunitinib malate for adjuvant treatment of renal cell carcinoma. FDA [Internet]. 2019.
- Hislop J, Mowatt G, Sharma P, Fraser C, Elders A, Jenkinson D, Vale L and Petty R. Systematic review of escalated imatinib doses compared with sunitinib or best supportive care, for the treatment of people with unresectable/ metastatic gastrointestinal stromal tumours whose disease has progressed on the standard imatinib dose. J Gastrointest Cancer. 2012; 43: 168-176.
- Meta Static Trial Talk. OS vs PFS: What You Need to Know [Internet]. Metastatic Breast Cancer Trial Talk. 2019.
- Yang W, Li K, Yu J, Shou C, Zhang Q, Hong Y, et al. Clinical outcomes of imatinib dose escalation versus sunitinib in first-line imatinib-failure gastrointestinal stromal tumour. Scandinavian journal of gastroenterology. 2018; 53: 1328–1334.
- Huang S, Liu X, Guo X, Wu H, Lu H, Pan Z, et al. Sunitinib versus imatinib dose escalation after failure of imatinib standard dose in patients with advanced Gastrointestinal stromal tumors – a real-world multi-center study. Translational Oncology [Internet]. 2023; 30: 101641.
- Zhang C, Zhang C, Zhang T, Liu H, Zhong J, Wang Z, et al. Second-line sunitinib for Chinese patients with advanced gastrointestinal stromal tumor: 37.5 mg schedule outperformed 50 mg schedule in adherence and prognosis. Translational Cancer Research. 2021; 10: 3206–3217.
- https://www.nice.org.uk/guidance/ta179/chapter/4-Consideration-of-theevidence
- Common Drug Review [Internet]. 2024.