
Research Article
Austin J Reprod Med Infertil. 2016; 3(2): 1043.
Unravel the Role of Oxidative Stress on Fertility Potential of Type II Diabetes Mellitus in Men in South India
Malini SS*
Department of Studies in Zoology, University of Mysore, India
*Corresponding author: Malini SS, Department of Studies in zoology, University of Mysore, Manasagangothri, Mysore-06, India
Received: October 27, 2016; Accepted: December 12, 2016; Published: December 15, 2016
Abstract
Objective: Study was undertaken to disentangle the role of oxidative stress in Type 2 diabetes mellitus and its effect on fertility potential of the individuals.
Materials and Methods: The study was conducted at Molecular reproductive and human genetics lab, University of Mysore, Mysore. Confirmed 200 Type 2 DM male patients, 100 infertile and 100 control males with the age group between 25-45 years were evaluated through semen analysis and sperm function test according to WHO guidelines. Oxidative stress was studied by estimating reactive oxygen species and enzymatic antioxidants such as superoxide dismutase and catalase along with total antioxidant capacity, using seminal plasma and pellet. Estimation of hormones was carried out by ELISA.
Results: Spermiogram shows noticeable significant differences in the count, pH, motility, morphology and vitality in infertile individuals but between diabetic and non-diabetic individuals only vitality showed significant difference. Functional potentiality of spermatozoa of such as plasma membrane permeability, nuclear decondensation and enzymatic activities of acrosomes of infertile conditions was below the normal levels of WHO guidelines. But between type II diabetic and healthy men except acrosome tests other two tests were significantly varied indicating the probability of subfertility/infertility. In addition, variation in insulin level was evident in type II diabetic men. Even though ROS level is significantly differ between type II diabetic and healthy men, but total antioxidant capacity was not altered could be due to increased scavenging activity of SOD.
Conclusion: Reactive oxygen species in type II diabetic mellitus being reduced due to high scavenging activity of antioxidant enzyme SOD. SOD levels were more in compared to catalase and total antioxidant capacity. Varied semen parameters could be due to insulin variation in type II diabetic men.
Keywords: Diabetes mellitus; Oxidative stress; Antioxidants; Sub-fertility and infertility
Introduction
Diabetes mellitus (DM) is a common, potentially devastating, treatable but incurable lifelong disease [1]. According to the diabetic atlas of the International Diabetic Federation, 366 million people were affected by diabetes worldwide in 2011, and diabetes prevalence is expected to 522 million by 2030 [2]. Diabetes has been associated with reproductive impairment in both men and women [3,4], and its impact on reproduction can be profound, as seen by diminution in fertility and increase in reproductive losses [5-8]. About 90% of diabetic male patients have disturbances in sexual function, including a decrease in libido, impotence and infertility [9]. Semen analyses reveal some decrease in sperm motility, density, abnormal morphology and generally increased semen plasma abnormalities in diabetic men. Interestingly, a contradicting paper has shown that, even when diabetic men present normal semen parameters, there is a higher level of damage to both nuclear and mitochondrial sperm DNA, when compared to what is found in healthy controls [10].
Un controlled hyperglycemia is an important risk factor for the development of micro and macro vascular disease in patients with type 2 diabetes, has been shown in several observational studies. There was a reduction in the risk of complications in the intensive compared with standard glycemic control group [11,12]. A phenomenal work from Queen’s University has revealed that high level of blood sugar may affect sperm quality and therefore decreases male fertility potentials [10]. Even though the degree of correlation between glucose level and testicular damage a established factor [13], several reports indicate the production of oxygen radicals from glycated proteins under physiological conditions results type II diabetic mellitus T2DM [14,15]. Free radicals are known to stimulate advanced glycation end products (AGEs) production by autoxidation of sugars [16], hence enhances Oxidative Stress (OS). OS has been linked to diabetic complications and it has also been reported to be associated with severe changes in the structure and function of the testes, 2 weeks after the onset of diabetes [17,18]. OS is the excess formation and/or insufficient removal of highly reactive molecules such as Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) [19,20].
Reactive molecules such as superoxide (O2 -), Nitric Oxide (NO) and (peroxynitrite) ONOO- are the most widely studied species, and they play important roles in diabetic cardiovascular complications [21,22] Reduced antioxidant levels, as a result of increased free radical production in experimental diabetes, have been reported by Grankvist, et al. and Kanter, et al. [23,24]. As such the prevalence of type 1 diabetes mellitus is rare and research pertaining to its influence on fertility status in males is sparse. Hence, the main objectives of this study are to compare oxidative stress in control subjects, infertile and T2DM individuals to know whether oxidative stress has any role on semen profile and functional potentiality of spermatozoa among T2DM individuals by estimating reactive oxygen species and enzymatic antioxidants as well as total antioxidant capacity.
Materials and Methods
A total of 200 T2DM, 100 infertile individuals and 100 control male subjects aged between 25-45 years were recruited irrespective of caste and religion from different diabetic centers and clinics in and around Mysore, India. After excluding T2DM associated metabolic disorders such as obesity, thyroid, hypertension, testosterone replacement therapy within 6 months of randomization, hormonemodulating therapies or topical/systemic glucocorticoids within 3 months of randomization, or insulin therapy; a history of recurrent prostate infection, infertility along with smoking, alcohol and drug abuse, long term medication etc, 152 cases and 50 control subjects and 75 infertile cases were used for all the analysis. The study was approved by the institutional ethical clearance committee of University of Mysore and concerned hospitals (IHEC-UOM No.12/Res/2009-10). Informed written consent letter was taken from the participants and subjects were interviewed to collect information about family, medical, reproductive histories which includes the duration of active married life, premature ejaculation and psychological status of the subjects and life style factors.
Semen collection and preservation
The semen samples were collected after 3-5 days of ejaculatory abstinence according to WHO guidelines (2010). Physical examination such as volume, liquefaction time, colour, pH and viscosity were recorded after liquefaction. Microscopic examinations were carried out to record the count, density, motility and morphology of the spermatozoa. Single blinded semen analysis was carried out for both control and diabetic individuals and infertile individuals according to WHO guidelines.
Sperm function tests
Functional potentiality of spermatozoa was evaluated by conducting sperm function tests for both T2DM individuals and control subject after the completion of microscopic and macroscopic analysis of semen and spermatozoa.
Nuclear Chromatin Decondensation Test (NCD)
This test was carried out to check the ability of decondensation of nuclear chromatin in vitro in spermatozoa. Semen sample was centrifuged to separate plasma. The pellets were washed with 0.05M borate buffer. One volume of sample was mixed with nine volumes of EDTA-SDS mixture and incubated at 37° C for 60 min. An equal volume of glutaraldehyde borate buffer was added. A drop of this mixture was transferred on to clean glass slide and covered with cover slip and observed under microscope in 400X magnification. The number of condensed and decondensed heads was counted. If more than70% of spermatozoa shows decondensed nuclear chromatin then it was considered as normal.
Hypo-Osmotic Swelling Test (HOS)
Integrity of plasma membrane was performed using this test. Hypo-osmotic solution was prepared using fructose and sodium citrate in equal proportion. One ml of this solution was incubated at 37° C for 10 min and 100μl of semen sample was mixed. It was incubated at 37°C for 30 min. This mixture was dropped on precleaned glass slide, covered with cover slip and observed under microscope in 400X magnification. Percentage of coiled (curled) tail was recorded. If more than 60% of spermatozoa, shows coiled tail then it was considered as normal.
Acrosomal Intactness Test (AIT)
Quality of the acrosomal enzymes were analyzed using this test. Gelatin coated slides were prepared by spreading warm aqueous solution of gelatin on to a clean glass slide and kept horizontally at 40°C for 24 hours. These coated slides were immersed in PBSglutaraldehyde solution for 2 minutes and washed using distilled water and stored at 40° C. Semen was mixed with PBS- D-glucose in the ratio of 1:5 and incubated at 37° C for 10 min. Gelatin coated slides were allowed to come to room temperature. A drop of diluted semen samples was smeared over gelatin slide. This slide was placed on to a petri dish containing a moistened filter paper and incubated for two hours at 37°C. The slides were examined under phase contrast microscope in 400X magnification. The percentage spermatozoa with halos surrounding the head were recorded. Values more than 50% was considered as normal.
Chemiluminescence’s assay for reactive oxygen species (ROS) measurement
Liquefied semen was collected from T2DM individuals and control subjects and centrifuged at 300x g for 7 minutes, and the seminal plasma was separated. The pellet was washed with Phosphate- Buffered Saline (PBS) and resuspended in the same washing media at a concentration of 20 x 106 sperm/ml. Four hundred microliter aliquot of the resulting suspensions was used to assess basal ROS levels. Ten microliter of luminol (5-amino-2,3-dihydro-1,4-phthalazinedione), prepared as 5mM stock in dimethyl sulfoxide (DMSO) was added to the mixture and serve as a probe. A negative control was prepared by adding 10μl of 5mM luminol to 400μl of PBS. Luminol is an extremely sensitive oxidizable substrate that has the capacity to react with a variety of ROS at neutral pH. The reaction of luminol with ROS results in production of a light signal that is read in luminometer as arbitrary light units.
Assay of Catalase in human seminal plasma
In the UV range, H2O2 shows a continual increase in absorption with decreasing wavelength. The decomposition of H2O2 can be followed directly by the decrease in absorbance at 240nm. The difference in absorbance per unit time is a measure of the Catalase activity. The substrate was diluted with phosphate buffer and its initial absorbance was recorded. 10μl of seminal plasma was added and the decrease in OD was noted at 240nm. The Catalase activity is expressed as k/ml and is calculated using the following relation: The Catalase activity is expressed as k/ml and is calculated using formula k/ml = ka, k = (2.3/t)*logA1/A2 where, t= time interval, A1 = Initial absorbance, A2 = final absorbance, a = dilution factor.
Superoxide Dismutase (SOD)
SOD levels for the study subjects and controls were estimated by Das, et al. (2000) method to infer the concentration of SOD being consumed for lipid peroxidation taking place in the cells of the subjects to reduce the oxidative stress. A reaction mixture was prepared using respective volumes of phosphate buffer, a Methionine, Triton X, HAC (Hydroxylamine Hydrochloride) and EDTA (Ethylenediamine Tetra Acetic acid). 139μl of the reaction mixture was added to 10μl of sample and incubated for 5 min at room temperature. Simultaneously a blank was also run which constituted distilled water instead of sample. 8μl of phosphate buffer and riboflavin each was then added to the blank and samples respectively. All these were incubated at room temperature for 10min and then 100μl of Griess reagent was added to them. OD was observed at 543nm. In order to express the SOD level protein levels of the respective subjects are required which is estimated by Lowry’s method.
Total Antioxidant Capacity (TAC)
TAC levels of the subjects and controls were estimated by Phosphomolybdenum method to infer the total amount of antioxidants present in the subjects. To 100μl of serum 100μl of 5% TCA was added and left undisturbed for precipitation and ten centrifuged at 3000 rpm for 5min. to 100μl of this supernatant 1ml of TAC reagent was added and incubated for 90min at 90°C. OD was checked at 695nm. A blank was also run simultaneously.
Analysis of the hormones
The blood samples were collected from the T2DM individuals and control subjects and serum was separated by centrifuging at 3000 rpm for 5 minutes at room temperature. The obtained serum was used to estimate the levels of FSH, LH, testosterone, prolactin and insulin using CALBIOTECH ELISA kit. The readings were taken under Thermo-fisher multimode microtiter plate reader.
Statistical analysis
The obtained data was expressed in mean and standard error. Independent- samples‘t’-test was used to find out whether the significant difference exist between case and control subjects using statistical program SPSS (version 14.0). P value less than 0.05 were considered as significant.
Results
Comparison of semen parameters between T2DM, and control subjects was statistically depicted in table 1. In the present investigation vitality (the percentage of living, healthy sperm in the semen) showed significant difference between control and diabetic group whereas other semen parameters were not affected. This result is not consistent with previous findings. Hence hyperglycaemic condition and wired management of insulin level has strong impact on vitality which is important for spermatozoa. The functional potential of the spermatozoa through NCD and HOS tests scores significantly different in T2DM and control group indicating failure in the decondensation of nuclear chromatin seems to be a vital factor for healthy spermatozoa. Similarly permeability of plasma membrane also highly compromised could be due to hyperglycemic condition in T2DM. But AIT showed no variation indicating acrosomal enzymes were not affected (Table 1).
condition
N
Mean± Std. Error Mean
t-valve
p-value
Volume
DM
152
2.05±0.08
1.231
0.220
Control
33
2.30±0.16
pH
DM
152
7.9±0.03
1.407
0.161
Control
33
7.81±0.05
Count
DM
152
47.97±2.69
0.912
0.363
Control
33
53.63±4.73
Motility
DM
152
49.67±1.22
1.818
0.071
Control
33
54.54±1.07
Morphology
DM
152
14.25±0.79
1.250
0.213
Control
33
16.57±1.60
Vitality
DM
152
58.86±1.49
4.780
0.001*
Control
33
74.27±1.16
NCD
DM
152
67.26±1.22
3.487
0.001*
Control
33
76.63±1.47
HOS
DM
152
66.17±1.08
3.607
0.001*
Control
33
74.78±1.38
AIT
DM
152
56.40±1.02
1.176
0.242
Control
33
52.89±3.46
Table 1: Analysis of semen parameters and sperm function tests between T2DM and control subjects.
The comparison of oxidative stress markers, ROS and total antioxidant capacity in T2DM and control indicated in table 2. ROS levels showed significant difference between control and T2DM (Table 2) and evident in Figure 1 (ANOVA; P = 0.001, F-value = 14.342), wherein it was high in infertile individuals followed by control and T2DM subjects. Even though SOD levels was statistically not significant when compared between T2DM and control subject (Table 2). Even total antioxidant capacity and catalase levels remain also same without any variations in controls and T2DM. But the levels of SOD was more in T2DM when compared between infertile, control and T2DM individuals (Figure 2 ANOVA; P = 0.001, F-value = 0.84). Hence the level of ROS was low in T2DM when compared between control and infertile groups. Increased level of SOD was able to combat with free radicals, hence the healthy balance made between free radicals and antioxidants remains same.
Condition
N
Mean± Std. Error Mean
t-valve
p-value
TAC
DM
152
74.55±1.14
0.590
0.556
Control
33
76.13±2.27
Catalase
DM
152
2.66±0.13
0.614
0.540
Control
33
2.86±0.27
ROS
DM
152
798.5±70.62
3.372
0.001*
Control
33
1022.86±71.39
SOD
DM
152
0.98±0.20
0.540
0.590
Control
33
0.74±0.06
(TAC: Total Antioxidant Capacity; ROS: Reactive Oxygen Species; SOD: Superoxide Dismutase; N: Number of individuals )
Table 2: Comparison of Oxidative stress markers, ROS and total antioxidants capacity between T2DM and control subjects.
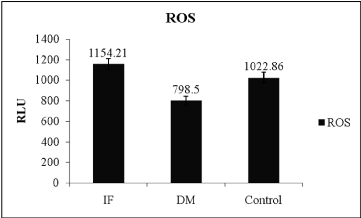
Figure 1: Level of reactive oxygen species between different condition, (IFInfertile,
DM – Type 2 Diabetes mellitus, ROS- Reactive oxygen species,
ANOVA; P=0.001, F-value=14.342).
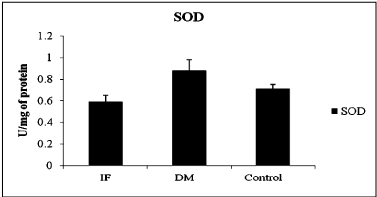
Figure 2: Activity of Super oxide dismutase between different condition,
(IF- Infertile, DM –Type 2 Diabetes mellitus, SOD- Superoxide dismutase,
ANOVA; P=0.001, F-value=0.84).
Estimation of hormones was made both in T2DM and control subjects and the results were depicted in table 3. Hormone profile of diabetic and non-diabetic remains same except insulin levels implying that altered metabolism has no impact on hormones like FSH, LH, prolactin and testosterone when considered as independent entity. Altered insulin level would cause hyperglycaemic condition in turn affect sperm quality in T2DM due to impairment in spermatogenesis, increased germ cell depletion, and Sertoli cell vacuolization, was evident in both diabetic men and knockout mice.
HORMONES
Condition
N
MEAN
INDEPENDENT SAMPLES TEST
t-value
pvalue
TESTOSTERONE
(3-10ng/ml)
DM
70
4.30±0.1
0.685
0.521
Control
40
4.54±0.3
FSH
(2-15 mlU/ml)
DM
70
6.17±0.8
1.409
0.162
Control
40
4.49±0.5
LH
(1.5-9.3mlU/ml)
DM
70
4.56±0.3
0.433
0.666
Control
40
4.31±0.4
PROLACTIN
(2-17ng/ml)
DM
70
12.07±0.9
1.145
0.255
Control
40
10.40±0.9
INSULIN
(μlU/ml)
DM
70
44.11±2.2
2.588
0.011*
Control
40
34.77±2.6
(FSH: Follicle Stimulating Hormone; LH: Leutinizing Hormone; N: Number of individuals)
Table 3: Analysis of hormone profile between T2DM and control subjects.
Discussion
Diabetes mellitus is a chronic metabolic condition characterized by disorder of glucose homeostasis. Numerous experimental and clinical observations have indicated that hyperglycemia may directly contribute to excess formation of free radicals [25,26] and decreased activity of antioxidant defense systems [27]. Increased formation of free radicals in T1DM and T2DM has been suggested to be a contributory risk factor in complications of the disease [28]. It occurs as a result of two processes: viz (i) decreased activity of the body antioxidant systems [29], and (ii) auto-oxidation of reducing saccharides and formation of adducts with proteins [27,28]. Chapple [30] and Bonnefont-Rousselot, et al. [31] have differentiated antioxidants into three: (i) Preventative antioxidants that prevent the formation of new ROS as caeruloplasmin, metallothioniene, albumin, myoglobin, ferritin and transferring. (ii); scavenging antioxidants which remove ROS once formed, thus preventing radical chain reactions - these include reduced GSH, vitamin E, vitamin C, a-carotene, uric acid and bilirubin, and (iii) enzyme antioxidants that function by catalizing the oxidation of other molecules. This group includes SOD that produces hydrogen peroxide from superoxide radicals, glutathione reductase, glutathione peroxidase and catalase which decompose hydrogen peroxide [32]. It has been suggested that oxidative stress plays a role in the development of diabetes and diabetic complications [33].
In the present study, T2DM males exhibited a normal semen parameters such as pH, count, motility, morphology but vitality of the spermatozoa was highly compromised. Even strong supportive evidence was obtained by sperm function tests wherein HOS and NCD scores were not comply with the WHO reference values. But AIT consequently confirms a negative association between acrosome enzyme activity and fertility potential of spermatozoa in T2DM cases. For successful fertilization and formation of pronucleus sperm nuclear chromatin decondensation ability in the oocyte is a very important and crucial process. The failure of sperm decondensation in the oocyte due to sperm abnormalities is unrecognizable by conventional semen analysis [34,35] showed that a subset 5%-15% of infertility in men. Consequently NCD of spermatozoa and subsequent male pronucleus formation are essential for fertilization and normal embryonic development but these processes were not in healthy status in T2DM individuals. HOS test evaluates the integrity of the sperm membrane and can be used as routine clinical investigations. The plasma membrane may be physically intact but chemically nonfunctional which may impair the ability of spermatozoa to undergo capacitation, acrosome reaction and bind to the zona pellucida. HOS results in free passage of fluids into the cell through the intact sperm membrane results in osmotic equilibrium. Since the plasma membrane around the sperm tail fibre is more loosely attached than that around other parts, the sperm tail is particularly susceptible to hypo-osmotic exposure and responds by coiling [36,37]. These tests are also useful for detection of damage during cryopreservation, ROS, toxic effect of drugs and chemicals to assess the quality of spermatozoa. In the present study,T2DM cases showed very poor response for HOS with lesser value from the normal range. The abnormality of plasma membrane of sperm was further evidenced by vitality, the only variation reported in the present study. This indicates the abnormality in the membrane intactness and the damages maybe caused by different factors like environmental toxicants, ROS etc, but in the present study a major factor which could compromise semen parameters is hyper glycaemic condition due to varied insulin, because ROS were reduced due to higher activity of SOD enzymes,
Oxidative stress seems to be implicated in the pathophysiology of diabetes mellitus with an increased production of free radicals and/or a sharp reduction of antioxidant defenses appearing to be determinant in the process [38]. In our study, we evaluated the involvement of free radicals in T2 DM individuals on testicular changes by measuring oxidative stress and antioxidant enzyme activities. The result of the present study showed an increase in the level of SOD hence there is reduction in ROS levels. These results indicated that increased indigenous antioxidants might have inactivated free radicals and reduced testicular damages. The vascular and multi-organ complications in diabetes and obesity are causally associated with hyperglycemia-induced overproduction of ROS [39]. In our study all other metabolic disorders were excluded along with smoking, alcohol and drug abuse hence the effect of these factors did not count the free radicals. Compromised semen parameters in the present study could be due to insulin variations, perse insulin action in motility and other parameters of human spermatozoa is not well defined, but defects in insulin secretion may change testicular and accessory sexual glands function. Hyperglycemia resulting from uncontrolled glucose regulation is widely recognized as the causal link between diabetes and associated complications. Brief episodes of hyperglycemia cause tissue damage by mechanisms involving repeated acute changes in cellular metabolism. However, exposure to high glucose also causes cumulative changes in long-lived macromolecules, which persist despite restoration of euglycemia. A large amount of data emphasizes some key metabolic pathways as being major contributors to hyperglycemia induced cell damage: (a) Increased glycolysis;(b) glucose autoxidation; (c) increased polyol pathway flux; (d) increased Advanced Glycation End product (AGE) formation; (e) activation of Protein Kinase C (PKC) isoforms; and (f) increased hexosamine pathway flux [38,40].
Until recently there was no unifying hypothesis linking these four mechanisms. However, it has been shown that hyperglycemiainduced overproduction of superoxide anions by mitochondria is the trigger that drives each of these pathways. Hyperglycemia-induced overproduction of superoxide significantly inhibits glyceraldehyde-3- phosphate dehydrogenase activity. In turn, this inhibition will activate all the pathways of hyperglycemic damage by diverting upstream glycolytic metabolites to these pathways [38]. As such, there is a direct interaction of insulin with the testes and sperm cells, as both the testes and sperms themselves produce insulin, thus providing an autocrine regulation of glucose metabolism according to their energetic needs independent of systemic insulin [7,41]. Variation in the insulin expression in the testes also seems to be affected by diabetes [41]. It was evident by many research findings wherein both diabetic men and knockout mice had notably impaired spermatogenesis, increased germ cell depletion, and Sertoli cell vacuolization, suggesting that insulin may have an important role in spermatogenesis [6,42]. Even in the present study varied semen parameters and functional potential of spermatozoa could be due to hyperglycemia and varied insulin levels not due to oxidative stress.
Conclusion
T2DM is a growing public health problem, affecting more and younger people every year. Nearly 35% of T2DM patients suffer from infertility. Thus, it urges to study possible ways to ameliorate the effect of T2DM in male infertility. As T2DM grows to epidemic proportion it is important to find a ways to counter its negative effects. In the present study there is negative impact of oxidative stress on Type II, hence the insulin levels play a foremost role in spermatogenesis and maturation of sperm for healthy fertility status in individuals rather than any other factors.
Acknowledgement
We thank the patients and volunteers for their participation in this study. This study was supported by funds from ICMR, New Delhi, India. Author is thankful to Dr. Sreenivasa G, Dr. Mahadeshwar Prasad for their support during manuscript preparation.
References
- Atef M, Al-Attar, Talal AZ. Influences of crude extract of tea leaves, Camellia sinensis, on streptozotocin diabetic male albino mice. Saudi J Biol Sci. 2010; 17: 295-301.
- Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011; 94: 311-321.
- Baccetti B, La Marca A, Piomboni P, Capitani1 S, Bruni1 E, Petraglia F, et al. Insulin-dependent diabetes in men is associated with hypothalamo-pituitary derangement and with impairment in semen quality. Hum Reprod. 2002; 10: 2673-2677.
- Seethalakshmi L, Menon M, Diamond D. The effect of streptozotocin induced diabetes on the neuroendocrine-male reproductive tract axis of the adult rat. J Urol. 1987; 138: 190-194.
- Ricci G, Catizone A, Esposito R, Pisanti FA, Vietri MT, Galdieri M. Diabetic rat testes: Morphological and functional alterations. Andrologia. 2009; 41: 361-368.
- Mallidis C, Agbaje I, McClure N, Kliesch S. The influence of diabetes mellitus on male reproductive function: A poorly investigated aspect of male infertility. Urologe A. 2011; 50: 33-37.
- Schoeller EL, Schon S, Moley KH. The effects of type 1 diabetes on the hypothalamic, pituitary and testes axis. Cell Tissue Res. 2012; 349: 839-847.
- La Vignera S, Condorelli R, Vicari E, D’Agata R, Calogero AE. Diabetes mellitus and sperm parameters. J Androl. 2012; 33: 145-153.
- Jiang GY. Practical Diabetes. In Beijing People's Health Publishing House.1996; 295.
- Agbaje IM, Rogers DA, McVicar CM, McClure N, Atkinson AB, Mallidis C, et al. Insulin dependant diabetesmellitus: implications for male reproductive function. Hum Reprod. 2007; 22: 1871-1877.
- Klein R, Klein BE, Moss SE, Cruickshanks KJ. Relationship of hyperglycemia to the long-term incidence and progression of diabetic retinopathy. Arch Intern Med. 1994; 154: 2169-2178.
- Bash LD, Selvin E, Steffes M, Coresh J, Astor BC. Poor glycemic control in diabetes and the risk of incident chronic kidney disease even in the absence of albuminuria and retinopathy: Atherosclerosis Risk in Communities (ARIC) Study. Arch Intern Med. 2008; 168: 2440-2447.
- Sudha S, Valli G, Mary Julie P, Arunakaran J, Govindarajulu P, Balasubramanian K. Influence of streptozotocin-induced diabetes and insulin treatment on the pituitary-testicular axis during sexual maturation in rats. Endocrinol Diabetes. 1999; 107: 14-20.
- Sakurai T, Tsuchiya S. Superoxide production from non-enzymatically-glycated protein. FEBS Lett. 1998; 236: 406–410.
- Hunt JV, Smith CC, Wolff SP. Autoxidative glycosylation and possible involvement of peroxides and free radicals in LDL modification by glucose. Diabetes. 1990; 39: 1420-1424.
- Baynes JW. Role of oxidative stress in the development of complications in diabetes. Diabetes. 1991; 40:405-412.
- Maiorino M and Ursini F. Oxidative stress, spermatogenesis and fertility. Biol Chem. 2002; 383: 591-597.
- Foresta C, Flohe L, Garolla A, Roveri A, Ursini F, Maiorino M. Male fertility is linked to the selenoprotein phospholipids hydroperoxide glutathione peroxidase. Biolof reprod. 2002; 67: 967-971.
- Turko IV, Marconges S, Murad F. Diabetes-associated nitration of tyrosine and inactivation of succinyl-CoA:3-oxoacid CoA-transferase. Am J Physiol. 2001; 281: H2289-2294.
- Maritim AC, Sanfers RA, Watkins JB. Diabetes, Oxidative stress, and antioxidants: A review. J Biochem Mol Toxicol. 2003; 17: 24-38.
- Evans, JL, Goldfine ID, Maddux B.A.; Grodsky GM. Oxidative stress and stress activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002; 23: 599-622.
- Liu Y, Terata K, Chai Q, Li H, Kleinman LH, Gutterman DD. Peroxynitrite inhibits Ca2+-activated K+ channel activity in smooth muscle of human coronary arterioles. Circ Res .2002; 91:1070-1076.
- Grankvist K. Marklund SL, Taljedal LB. CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem. J. 1981; 199: 393–398.
- Kanter M, Meral I, Dede S, Gunduz H, Cemek M, Ozbek H, et al. Effects of Nigalla sativa Linn. and Utrica sativa Linn. on lipid (2003a): peroxidation, antioxidant enzymes systems and some liver enzymes in CCl4-treated rats. J. Vet. Med. Physiol. Pathol. Clin. Med. 2003a; 50: 264-268.
- Feillet-Coudray C, Rock E, Coudray C, Grzelkowska K, Azais-Braesco V, Dardevet D, et al. Lipid peroxidation and antioxidant status in experimental diabetes. Clin Chim Acta.1999; 284: 31-34.
- Ceriello A. New insights on oxidative stress and diabetic complications may lead to a “causal” antioxidant therapy. Diabetes Care. 2003; 26: 1589-1596.
- Durackova Z. Oxidative stress. In: Free radicals and antioxidants in Medicine (II). Durackova Z, Bergendi L, Carsky J. (eds.), Bratislava: Slovak Academic Press: 1999.
- Soon YY, Tan BKH. Evaluation of the hypoglycemic and antioxidant activities of Morinda officinalis in streptozotocin-induced Diabetic Rats Singapore. Med J. 2002; 43: 77-85.
- Muchova J, Liptakova A, Orszaghova Z, Garaiova I, Tison P, Carsky J, et al. Antioxidant systems in polymorphonuclear leucocytes of type 2 diabetes mellitus. Diabet Med .1999; 16: 74-78.
- Chapple IL. Reactive oxygen species and antioxidants in inflammatory diseases. J Clinical Endocrinol. 1997; 24: 287-296.
- Bonnefont-Rousselot D, Bastard JP, Jaudon MC, Delattre J. Consequences of diabetic status on the oxidant/antioxidant balance, Diabetes and metabolism (Paris). 2000; 26: 163-176.
- Trocino RA, Akazawa S, Ishibashi M, Matsumotok MH, Yamamoto H, Goto S, et al. Significance of glutathione depletion and oxidative stress in embryogenesis in glucose-induced rat embryo culture, Diabetes. 1995; 44: 992-998.
- King GL, Brownlee M. The cellular mechanisms of diabetic complications. Endocrinol. Metab. Clin North Am. 1996; 25: 255-270.
- Caglar GS, Hammadeh M, Asimakopoulos B, Nikolettos N, Diedrich K, Al-Hassani S. In Vivo and In Vitro Decondensation of Human Sperm and Assisted Reproduction Technologies. In vivo. 2005; 19: 623-630.
- Ch°C, jung-ha H, willis WD, Goulding Eh, stein P, xu Z, et al. Protamine 2 deficiency leads to sperm DNA damage and embryo death in mice. Biolo reprod. 2003; 69: 211-217.
- Pal D, Chakraborthy P, Ray HN, Pal BC, Mitra D, Kabir SN. Acaciaside-B-enriched fraction of Acacia auriculiformis is a prospective spermicide with no mutagenic property. Reproduction. 2009; 138: 453-462.
- Misro MM and Chaki SP. Development of a rapid, sensitive, and reproducible laboratory test kit for the assessment of plasma membrane integrity of human sperm. Fertile Steril. 2008; 89: 223-227.
- Ahmed RG. The physiological and biochemical effects of diabetes on the balance between oxidative stress and antioxidant defense system. Med J Islamic World Acad Sci. 2005; 15: 31-42.
- Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci USA. 2006; 103: 2653-2658.
- Rolo AP, Palmeira CM. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006; 212: 167-178.
- JainGC, Jangir RN. Modulation of diabetes-mellitus-induced male reproductive dysfunctions in experimental animal models with medicinal plants. Pharmacogn Rev. 2014; 8: 113-121.
- Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000; 289: 2122-2215.