
Review Article
Austin J Pulm Respir Med. 2023; 10(1): 1095.
Broader Function of Ficolins in Infectious Diseases
Wu X*
Department of Respiratory Medicine, Hunan Provincial People’s Hospital (The First Affiliated Hospital of Hunan Normal University), Changsha, China
*Corresponding author: Xu Wu*Hunan Provincial People’s Hospital (The First Affiliated Hospital of Hunan Normal University), NO. 61 Jiefang Road, Furong District, Changsha, Hunan Province, China
Received: February 10, 2023; Accepted: March 21, 2023; Published: March 28, 2023
Abstract
In the innate immune system, a diverse of pattern recognition molecules are important to prevent infection and maintain endogenous homeostasis. Ficolins are novel soluble recognition molecule, which sense Pathogen-Associated Molecular Patterns (PAMP) on microbes and abnormal structures on self-cells. Ficolins have been widely identified in animals from invertebrates to mammals. Although detailed comprehension about each ficolin is obscure, current information suggests that ficolins have a crucial role in host defense and are linked with many diseases. Ficolins function within innate immunity via the recognition of Pathogen-Associated Molecular Patterns (PAMPs) on microbial pathogens via two main mechanisms: (i) assembly of the Membrane Attack Complex (MAC), consisting of C5b-C9 proteins, which can directly lyse the bacterial membrane, and (ii) function as opsonins, potentiating the functions of immune cells. Complement activation may initiate positive responses against infections, but on the other hand, could exacerbate tissue damage or contribute to adverse side effects. Notably, there is mounting evidence implicating ficolins in the recognition and removal of numerous bacterial, viral, fungal, and parasitic pathogens in recent years. Additionally, there has been expanding evidence highlighting that cross-talk within these key complement immune proteins during the different infectious stages, enhance susceptibility to colonization by pathogens and dysfunctional immune responses. This review provides an overview of our up-to-date knowledge about ficolins, especially a broader perspective of the human ficolins and their mouse homologues.
Keywords: Human ficolins; Murine ficolins; MBL; Complement activation; Infection; Interaction
Introduction
Comprising more than 60 plasma and cell membrane receptor/regulator proteins, the complement system can be rapidly activated by pathogens via these pathways to generate enzymes that facilitate cleavage of its components, enabling rapid amplification of the complement cascade. Indeed, this cascade results in the release of peptides/proteins that facilitate not only chemotaxis/vasodilation (e.g., C3a, C5a) but also opsonization (e.g., C3b) and formation of the Membrane Attack Complex (MAC), which perturbs the cell membrane and potentiates cell lysis and death. Activation of the lectin pathway is initiated by the binding of several pattern-recognition molecules PRMs to glycan residues on the pathogen’s surface, complexed with a set of serine proteases named mannose-binding lectin-associated serine proteases MASP. These PRM are classified into two lectin families: collectins (mannose-binding lectin, MBL; collectin-10, CL-10 and collectin-11, CL-11) and ficolins (Ficolin-1 [or M-ficolin], Ficolin-2 [or L-ficolin], and Ficolin-3 [or H-ficolin]). They all share the common feature of a collagen- like triple helix structure coupled to a recognition structure. The latter is a globular Carbohydrate-Recognition Domain (CRD) (in collectins) or a fibrinogen-like (FBG) domain (in ficolins) responsible for ligand recognition and binding [1-3]. Ficolins were originally discovered from porcine uterus membrane that extracts as transforming growth factor-β-binding proteins [4]. To date, three and two ficolins have been identified in humans (M-ficolin, L-ficolin and H-ficolin) and mice [ficolin A (FcnA) and ficolin B (FcnB)], respectively (Table 1). Besides, other ficolin homologs have been identified in various lower vertebrates as well as in higher invertebrates and phylogenetic analysis suggests that ficolins are of ancient origin [5]. Domain and oligomeric structure of three human ficolins have been illustrated in detail [3]. Ficolin- M, ficolin- L, and ficolin- H are composed of 326, 313, and 299 aa polypeptide chains, respectively. Ficolin-M and ficolin-L shared with 70-80% primary amino acid identity of the fibrinogen-like domain each other. But ficolin-H showed only 50% amino acid identity in total, 48/54% in the collagen-like domain and 52/53% in the fibrinogen-like domain with M/L-ficolin respectively. More specifically, human M-ficolin or ficolin-1 (M-FCN, FCN-1) is the ortholog of murine ficolin B (FcnB). Human L-ficolin or ficolin-2 (L-FCN, FCN-2) is closely related to murine ficolin A (FcnA), although the genes encoding these ficolins are suggested to have evolved independently in each murine and primate lineage [6].
Ficolin
Gene
Protein
Locus
mRNA expression
Monomer(kDa)
Levels
Distribution
Human
M-ficolin/P35-related (FCN-1)
9q34
Bone marrow, promonocytic U937 cells, monocytes, neutrophils, macrophages, DC
34
60.5 ng/ml or 1.07ug/m lung and plasma
Peripheral monocytes
type II alveolar epithelial cells and human breast milk
L-ficolin/P35 (FCN-2)
9q34
Liver, adrenal gland,adipose tissue and pros
34
4 5.4ug/ml or 3.0 mg/ml Plasma H-
ficolin/Hakata antigen(FCN-1p35.3
Lung, liver and glioma cells
35
18.4 ug/ml or 25.5ug/m
Bronchus alveolus, bile and plasma
Mouse
Ficolin A (Fcna)
2A3
Liver, spleen and macrophage
36.298
ND
Plasma
Ficolin B (Fcnb)
2A3
Bone marrow, spleen and Drosophila S2 cell
32-34
ND
Peritoneal macrophage
intracellularH-ficolin
4D2
(pseudogene)
ND: not well defined.
Table 1: Characteristics of ficolins in humans and mice.
The human ficolins basal homotrimeric contained a short N-terminal region (24aa), a collagen-like triple helix (11Gly-Xaa-Yaarepeats) including hydroxyproline residues, a neck region (12aa) and a globular recognition domain comprised of three Fibrinogen-like domains (FBG) (207aa) and identical polypeptide (fibrinogen β and γ). Then oligomeric structure was assembled through two cysteines and interchain disulfide bonds [5,7,8]. FBG of ficolins is composed of a number of different binding sites that can work synergistically or alone in a complex interaction that allow ficolins to distinguish non-self structures from self. This allows ficolins to play an integral role in the opsonisation of various pathogens whereby they can recognize a vast number of ligands on the microbial cell-surface [9]. Five ficolins can activate the complement system, but ficolin-1 is weakest and ficolin-3 is the predominant player in complement activating capacity [10]. Moreover, ficolins can stimulate secretion of the inflammatory cytokines through macrophages, such as IL-6 and TNF, consequently orchestrating the adaptive immune response [11]. Besides general character of ficolins working as recognition molecules in innate immunity, some recently published data concerning the influence of ficolins on the infectious disease are summarized in this review.
Human Ficolins
Amongst human ficolins, ficolin-2 and 3 (Hakata antigen) are the plasma ficolins, synthesized by variety of cells (ficolin-2 by hepatocytes; ficolin-3 by hepatocytes, alveolar type II pneumocytes and ciliated bronchial cells). Ficolin-2 was also detected in adrenal gland, adipose tissue and prostate sequentially [3]. But the mechanism is rarely known. Of interest, ficolin-2 has been detected in the human lungs, which maybe deriving from bloodstream during infection [12].
Ficolin-1 was localized in secretory granules in the cytoplasm of neutrophils, monocytes, and type II alveolar epithelial cells in the lung and synthesized by neutrophils, monocytes and in bone marrow [3]. However, the expression of FCN-1 could is re-induced in silent macrophages when treated with LPS and Pam3Cys via TLRs [13]. Compared with macrophages, monocytes and neutrophils harbour a larger reservoir of ficolin-1 that can be secreted to serum upon stimulation with Formyl-Met-Leu-Phe(fMLP) or Porbolmyristate-Acetate (PMA), which proved a constituent of plasma protein and an intracellular associated protein [14,15]. These descriptions suggest that ficolin-1, may function as an acute protein temporarily stored in the secretory granules of the leukocytes. FCN-3 mRNA level in the liver was approximate three times higher than FCN-2 and 14 times higher than MBL-2, while FCN-1 almost was undetected in the liver [10]. It had been suggested that ficolin-3 is serum protein produced in hepatocytes resulting from decrease in patients with liver cirrhosis [8]. In the lung, ficolin-3 is generated by type II alveolar epithelial cells and ciliated brochial epithelial cells, then secreting into the bronchus and alveolus.In the liver, ficolin-3 was produced by bile duct epithelial cells and hepatocytes secreting into the bile duct [16].
The concentrations of ficolins in serum change with age and shows an inverted-U shape trend (highest concentrations in children, lowest in neonates, and adults that slightly lower compared with children). Three ficolin in childhood increased with ages, which were significantly correlated with gestational age, but not with gender [17]. Ficolin-1 reached its peak at 1–8yr. In adults, the serum concentration of human ficolin-1 was average 4.13mg/ml (1–7mg/ml). Studies also report that ficolin-1 detected in human plasma from healthy donors has a median concentration of 60.5ng/ml (45.7-100.4ng/ml) or 1.07ug/ml in Danish (0.28-4.05ug/ml). Ficolin-2 reached its peak at 1–4yr [17]. In adults, the average plasma concentrations of ficolin-2 in 214 Danish blood donors is about 5.4ug/ml (1.0–12.2ug/ml) or median 3.0mg/ml (0.72-6.0mg/ml) in Caucasians. Three polymorphisms in the promoter region (-986, -602 and -4) and one polymorphism at amino acid position 258 (A258S) or at position -986 in exon 8 all correlated with either a twofold decrease or increase in the ficolin-2 levels [18]. Ficolin-3 was first found in serum as Hakata antigen that reacted with an autoantibody with SLE (Systemic Lupus Erythematosus, SLE) from 10050 healthy Japanese donors, 751352 Japanese outpatients, and 41430 Swedish out patients. Therefore, the variable levels of ficolin-3 in SLE also indict early pathogenetic role of ficolin-3 in SLE [19]. It had been demonstrated that Hakata antigen is a novel thermolabile β2-macroglycoprotein, existing as a monomer of 35kD in reducing conditions and as a huge homopolymer of 650kD under non reducing conditions. Ficolin-3 was stable within a wide age range from infants to children up to 16yr. In adults, it is the most abundant ficolins in humans with average 18.4ug/ml (7-23ug/ml) in Japanese or average 25.5ug/ml in Caucasians (3-55ug/ml) [20].
As for human ficolin, the protomer is similar to that of Tachylectin 5A(TL5A) characteristic of A, B, P domains [1,7]. The ligand binding site existed in P domain was near the Ca2+ binding site on the external part, which was named for S1 [21]. Based on the X- ray crystal structure of the recognition domains of the ficolins, it was revealed that ficolin- 2 contains four Carbohydrate Recognition Domain(CRD) sites (termed S1-S4), whereas only one site was detected in ficolin- 1 and ficolin- 3 [22]. The presence of multiple binding sites in ficolin- 2 may explain its extensive binding capacity. Binding to acetylated, in particular N-acetylglucosamine (GlcNAc) and N-acetylgalactosamine (GalNAc), is a common characteristic shared amongst the ficolins. Acetyl ligands binding for three human ficolins were calcium-dependent, such as acetylated albumins or LDL(20) [23]. Ficolins generally recognize polysaccharides on microbial pathogens(not necessarily of carbohydrate nature), including N-acetyl-D-glucosamine, N-acetyl-D-galactosamine (D-GalNAc), and D-galactose (D-Gal). Moreover, ficolins have also been observed to recognize specific microbial patterns such as sialic acid, lipopolysaccharides, bacterial peptidoglycan, and fungal 1,3-β-D-glucan [9]. Given that there are existing reviews on structural of the recognition properties of human Ficolins, this topic will not be discussed in detail here. Although it was initially believed that multimeric molecules of all complement-activating ficolins are built-up from identical polypeptides/subunits, it was demonstrated that ficolin-2 and ficolin-3 may form heterocomplexes, which are suspected to have additional biological relevance (i.e. broader spectrum of ligands) compared with their parent molecules [24].
Murine Ficolins
Ficolin-A (FcnA) is a functional equivalent of ficolin-2 and is present in serum as well as being expressed in the splenic macrophages and Kuppfer cells. Ficolin-B (FcnB) is a functional equivalent of ficolin-1 and has been described as being present both on cell membranes and in granules of neutrophils and monocytes, but not in the serum [25,26] Moreover, we find that both ficolin A and ficolin B are expressed in leukocytes from the bone marrow, peripheral blood, lung and spleen. Further analyses showed that macrophages and neutrophils are the main sources of FcnA and FcnB, and T and B cells also express a small amount of FcnB [27].
The major difference between FcnA and FCN-1 is that it has ten exons, and the two extra exon translated Gly-X-Y repeats and an additional neck sequence, respectively (Figure 1). Based on sequence analysis, gene FcnA contains 1002-base ORF translating 334 amino acids that were formed a subunit. The structure looks like a parachute clustered through 12 monomers (36.298kDa), which is similar to human ficolin and rat MBL-A.MASP binding site on ficolinA is a conserved motif which is lysine residue in the X position of the Gly-X-Y collagen repeat(Lys [56]) [26]. Slightly different from ficolin-1, the ligands of ficolin-A are a trisaccharide only containing a terminal a1-6 linked GlcNAc residue and a few sialated ligands [28].
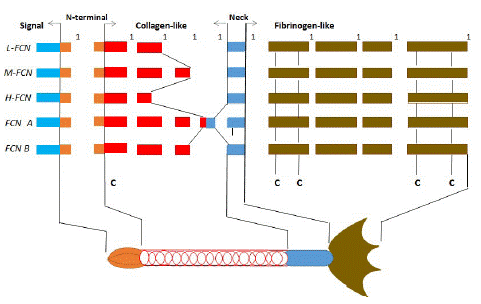
Figure 1: Exon organization of the genes encoding human and mouse ficolin and the exon-encoding domain structure of ficolin. The boxes depict the exons of the L-ficolin (L-FCN), M-ficolin (M-FCN), H-ficolin (H-FCN), ficolin A (FcnA) and ficolin B (FcnB) genes. Each domain encoded by the exons is represented by distinct patterns in the boxes and by a schematic representation of ficolin. The letters C denote the conserved cysteine residues in ficolin.
Ficolin-B shared 61% amino acid sequence semblance with ficolin-A, but ficolin B is 2502Da smaller than ficolin-A. The rapid postnatal decline of ficolin-B suggests different roles in distinctive stages; in the early stage, ficolin-B was response to the hematopoietic course. Ficolin-B exclusively bounding to sialyated structure, such as a2-3, a2-8 and β2-6 linkages, has a more broaden recognition than ficolin-A [28]. Its ability of complement activation is twofold lower than ficolin-A and its MASP-binding site could be blocked by a single amino acid mutation. However, it is noteworthy that ficolin-B produced in Drosophila S2 cells exhibited a strong activity of primitive opsonophagocytosis. Interestingly, ficolin-B was also found in peritoneal exudate macrophages and was coexisted with Lamp-1, a marker in the cytoplasm. When Lamp-1 is activated, the intracellular ficolin-B translation is up-regulated to act as a gatekeeper to remove debris inside the cell [29]. Actually, both mouse ficolins were reported to bind late apoptotic andabnormal cells which are of great physiological significance [26,30].
Comparisons between Ficolins and MBL
Previously, MBL was regarded as the prototypic initiator of the lectin pathway. However, it is now well established that also ficolins as well as the CL-10/11 utilize the MASPs to activate the complement system [3]. The ficolins recognize PAMP on the surface of microorganisms to form complexes interacting with a set of serine proteases named MASPs (MASP- 1, MASP- 2, and MASP- 3), in addition to two nonenzymatic fragments MAp19 [31] and MAp44 [32]. Ficolin-MASP complexes can then cleave C4 and C2 to form the C3 convertase C4bC2a (Figure 2). In contrast to other collagen-like defence molecules, such as MBL, pulmonary Surfactant-associated Protein (SP)-A and SP-D, the ficolins do not contain the typical a–helical neck region between the collagen-like region and the recognition domain. The distinctive difference is that FBG domains of ficolin replace a Ca2+-dependent CRD of collections, and the mechanism of trimerization do not need involvement of collagen-like region [33]. Accordingly, they provide additional functionality to be PRM drive lectin pathway activation on the surface of Streptococcus pneumonia [34], but not MBL. H-ficolin inhibited both PR-8 H1N1 and H1N1pdm09 strain whereas MBL did not, due to a sprinkle of glycan attachments on the hemagglutinin of these strains [34-36]. What is important, H-ficolin can evade some bacterial collagenase proteolytic destruction, which attacks helical collagen fibril, compared with collectins [35,36]. In addition, the average ficolins levels are higher than MBL in human serum without huge fluctuation [37]. Overall, this highlights the importance of MBL-independent lectin pathway activation in the host defense against pathogen.
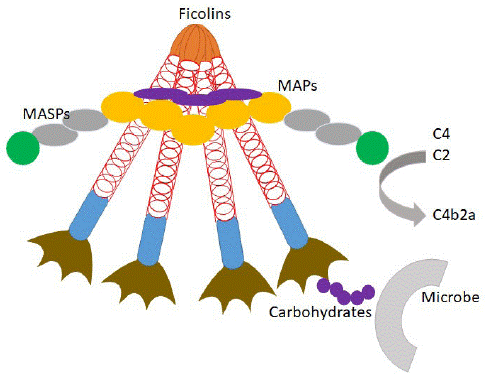
Figure 2: The presumed organization of the ficolins/MASPs/MAPs complex.
This complex might be designated precisely as the ficolins/MASPs/MAp19/MAp44 complex. Ficolins likely associate with MASPs, MAp19 and MAp44 through their collagen-like domains. Upon binding of ficolins to microbes, the MASPs convert into their active forms and activate complements C4 and C2. MAp19 and MAp44 are thought to attenuate the lectin pathway by competing with MASPs to bind with ficolins.
Ficolins and Infectious Disease
As a consequence of their widely recognition spectrum, ficolins have been suggested to play a critical role in innate immunity against pathogens infection. In recent years, there have been a number of studies implicating ficolins in the recognition and removal of numerous bacterial, viral, fungal, and parasitic pathogens, especially in pulmonary infection. It has been discovered that the FBG of ficolins is composed of a number of different binding sites synergistically or alone in a complex interaction to recognize a vast number of ligands on the microbial cell-surface (Table 2). Yet the binding specificity of the individual ficolins differs(including bacterial lipoteichoic acids, capsular polysaccharides, fungal 1,3-b-glucans, DNA and elastin).
Species
Ficolins
Ligands
Other Ligands
Function
Human
M
S1
GlcNAc, GalNAc, Sialic acid and gangliosides
Mitochondria, hCRP,polysaccharide, LPS and PTX3
1.Complement activation
L
S2
GlcNAc, CysNAc and galactose
Pneumolysin Mitochondria,DNA,fibrinogen,fibr
2.Opsonin
S3
N-acetylated, sulfate and phosphate compounds in, hCRP, elastin, steriod and LTA
3.Maintenance of homeostasis
S4
β-D-glucan and acetylchlline
H
S1
D-fucose, galactose and N-acetylated compound
PSA, DNA and hCRP
A
GlcNAc and sialic acid
Elastin,LPS and apoptotic cells
B
Sialyated structure
Apoptotic and necrotic cells
Table 2: Ligand-specificities of human/mouse ficolins and function.
Ficolin-2 is undoubtedly the most widely investigated ficolin, and ficolin-2 was first observed to enhance the opsonophagocytosis of Salmonella typhimurium leading to complement activation [7]. More binding sites expand the binding range of ficolin -2 to pathogens. Through four binding sites in its FBG domain (termed S1-S4), ficolin-2 can recognize a variety of respiratory pathogens, including Staphylococcus aureus, Streptococcus. pneumoniae (in both cases encapsulated strains), Haemophilus influenzae, Mycobacterium tuberculosis, Pseudomonas aeruginosa, Aspergillus fumigatus, and influenza A virus [9]. Interestingly, ficolin-A (functional equivalent of ficolin-2) was found not able to bind to Group B streptococci strains. It was moreover suspected that the low invasiveness of Str. pneumoniae 11 Aserotype is related to recognition of its capsular polysaccharide by ficolin-2 [38]. Chalmers et al. noticed an association of very low ficolin-2 serum levels (<1,200 ng/ml) with higher risk of 30-day mortality in Community Acquired Pneumonia (CAP) patients [39].
Based on those data, it was supposed that ficolin-2 may be protective against pathogens exacerbating allergic inflammation in the lung. These various ability of recognition and bond between ficolin-2 and pathogens need to be considered while optimally developing therapeutics to control specific pathogen infection and mitigate tissue injury. Furthermore, through investigating 158patients with leprosy and 210 healthy control subjects from Brazil, it was found that functional FCN-2haplotypes associated with normal ficolin-2 levels have a protective effect against leprosy [40].
In China, genetic variants of FCN-2(rs3811140 and rs7851696) representing low FCN-2 transcriptional activity conferred leprosy susceptibility and clinical outcomes in Han Chinese; Similarly, the variant homozyous genotype of -557A>G, -64 A>C and +6424 G>T SNP were correlated with the pathogenesis and protection of pulmonary TB [41]. Moreover, the heterozygous of FCN-2(-986A and -4G alleles) were observed to be a risk factor for susceptibility to schistosomiasis, whereas the homozygous genotypes were suggested to shield against schistosomiasis [42].
The acute malaria severity was no associations in FCN-2 promoter haplotypes in African children, but FCN-2 levels decreased significantly after malaria treatment [43]. These data suggest that ficolin-2 genotyping should be a valuable additional tool for future studies of infectious disorders.
In addition, ficolin-1 can bind to certain respiratory pathogens, as Staph. aureus, Str. pneumoniae, M. tuberculosis, Ps.aeruginosa, and A. fumigates [9]. With regard of viruses, Ebola virus was concerned recently. The Glycoprotein (GP) of EBOV plays a crucial role in EBOV infection by mediating its cellular attachment and entry into host cells. Astonishingly, ficolin-1-GP interaction was shown to enhance EBOV infection on human macrophages through common cellular receptor with MBL, which facilitate membrane fusion as an agent, thereby contributing to viral subversion of the host immunity [44]. In any case, the role of ficolin-1 in EBOV remains to be deciphered.
However, few microbial targets ficolin-3 have been reported. Moreover, other factors than polymorphisms in the FCN genes might give rise to altered ficolins levels; overall, it appears that infectious and inflammatory diseases are associated with low ficolins levels, whereas autoimmune disorders are associated with high levels [45].
Ficolin-3 shares characteristics with ficolin-2 such as the common cis conformation of the Asp282 and Cys283 peptide bond. Significantly, the role of ficolins in host defense was evaluated by infection with S. pneumonia, and all three strains of ficolin- deficient mice showed reduced survival rates compared to wild type mice, suggesting that ficolins play a crucial role in immunity against pneumococcal infection [46]. Compared with MBL, the focus of ficolin on lung disease is limited, but increasing.
Regarding the role of ficolins in pulmonary infection, the ficolin knockout mouse model was established to study the expressions and roles of local ficolins in LPS-induced pulmonary inflammation and injury. The severity of the lung injury and local inflammation of Fcna-/- mice was increased by the induction of extracellular complement activation. The recovery of LPS-induced local lung inflammation and injury was delayed in Fcnb-/- mice [27]. Besides, to explore the dynamic changes of ficolins during pH1N1 infection-induced severe pneumonia, they initially measured the mRNA and protein levels of ficolins in the lung. The results demonstrated that pulmonary myeloid-derived ficolin A may be linked to proinflammatory mediators [47].
Cross-Talk with Other Molecules in the Complement System against Infection
As is well known, ficolin is a part of network in complementary system. In terms of lectin pathway; its activation is induced directly by MBL, ficolins, and collectins, and can also be mediated by MBL or ficolins interacting with Long pentraxin 3 (PTX3), C- Reactive Protein (CRP), or Serum Amyloid P component (SAP) [3]. As well as cross-talk between lectin and alternative pathways and with the coagulation and contact systems, ficolins were demonstrated to interact with long and/or short pentraxins contributing to the enhancement or regulation of the early immune response [3,48]. Moreover, Ma YJ et al. observed that ficolin- 2 and PTX3 are able to recruit each other to Aspergillus fumigatus and thereby enhance complement activation on the fungus, when they studied the interaction between ficolin-2 and PTX3. The further more evidenced that collectin-11 and ficolin-2 (but not MBL) were shown to activate complement upon recognition of surface structures of Streptococcus pneumoniae. As lectin pathway-associated PRM (ficolin-1,2,3) are constitutively present in the respiratory system or are transferred from the bloodstream to the infected sites [ficolin-2 detected in Bronchoalveolar Lavage Fluid (BALF) from patients suffering from pneumonia or invasive aspergillosis] [49], they may contribute to excessive inflammation and its detrimental effects. These results suggest that ficolins, in addition to their important role in recognizing invading pathogens, also function as scavenger- molecules participating in the removal of endogenous debris and maintenance of tissue homeostasis.
Conclusion
The ongoing pandemic of coronavirus disease 2019 (COVID-19) have been causing more deaths than previously expected due to the etiological agent of which is a betacoronavirus called the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Epidemiological data show that up to 10% of all COVID-19 patients will become critically ill with multiorgan failure and require admission to the Intensive Care Unit (ICU) for Acute Respiratory Distress Syndrome (ARDS). Complement activation may contribute to the evolution of pathogenesis, and treatment options available for ARDS [50]. Therefore, investigation of the protective and harmful associations of complement factors in pulmonary diseases is crucial for understanding pathogenic mechanisms and establishing therapeutic strategies.In brief, numerous studies have investigated the role of ficolins in susceptibility to clinical diseases (Figure 3), our objective in this review was to explore the role of ficolins in infectious diseases, especially pulmonary infection, which are known to participate in the systemic host-response to infection but the exact function is still incompletely understood. Future research could therefore focus on exploration of the role of ficolins within infection immunity and expanding our knowledge on the roles of ficolin within infectious diseases.
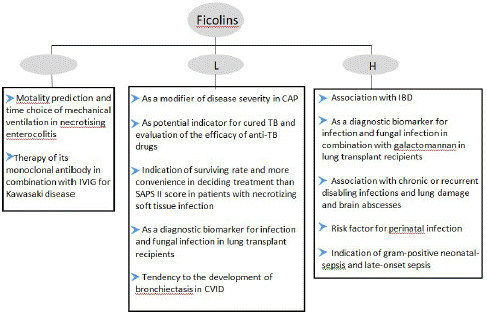
Figure 3: The correlation between ficolins and diseases.
Declaration of Competing Interest
The authors had declared no conflict of interests.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (82200017), and Natural Science Foundation of Hunan Province, China (2022JJ40227), and the Project funded by China Postdoctoral Science Foundation (2022M711124).
Author Contributions
X W participated in the whole review writing.
References
- Howard M, Farrar CA, Sacks SH. Structural and functional diversity of collectins and ficolins and their relationship to disease. Seminars in Immunopathology. 2018; 40: 75-85.
- Swierzko AS, Cedzyński M. The Influence of the Lectin Pathway of Complement Activation on Infections of the Respiratory System. Frontiers in Immunology. 2020; 11: 585243.
- Garred P, Genster N, Pilely K, Bayarri-Olmos R, Rosbjerg A, et al. A journey through the lectin pathway of complement—MBL and beyond. Immunological Reviews. Blackwell Publishing Ltd. 2016; 274: 74-97.
- Ichijo H, Hellman U, Wernstedt C, Gonez LJ, Claesson-Welsh L, et al. Molecular cloning and characterization of ficolin, a multimeric protein with fibrinogen- and collagen-like domains. Journal of Biological Chemistry. 1993; 268: 14505-13.
- Garred P, Honoré C, Ma YJ, Rørvig S, Cowland J, et al. The Genet ics of Ficolins. Journal of Innate Immunity. 2010; 2: 3-16.
- Endo Y, Liu Y, Kanno K, Takahashi M, Matsushita M, et al. Identification of the mouse H-ficolin gene as a pseudogene and orthology between mouse ficolins A/B and human L-/M-ficolins. Genomics. 2004; 84: 737-44.
- Garlatti V, Martin L, Lacroix M, Gout E, Arlaud GJ, et al. Structural Insights into the Recognition Properties of Human Ficolins. Journal of Innate Immunity. 2010; 2: 17-23.
- Endo Y, Matsushita M, Fujita T. Role of ficolin in innate immunity and its molecular basis. Immunobiology. 2007; 212: 371-9.
- Bidula S, Sexton DW, Schelenz S. Ficolins and the Recognition of Pathogenic Microorganisms: An Overview of the Innate Immune Response and Contribution of Single Nucleotide Polymorphisms. Journal of Immunology Research. 2019; 2019: 3205072.
- Hummelshoj T, Fog LM, Madsen HO, Sim RB, et al. Comparative study of the human ficolins reveals unique features of Ficolin-3 (Hakata antigen). Molecular Immunology. 2008; 45: 1623-32.
- Ren Y, Ding Q, Zhang X. Ficolins and infectious diseases. Virologica Sinica. 2014; 29: 25-32.
- Bidula S, Sexton DW, Abdolrasouli A, Shah A, Reed A, et al. The Serum Opsonin L-ficolin Is Detected in Lungs of Human Transplant Recipients Following Fungal Infections and Modulates Inflammation and Killing of Aspergillus fumigatus. Journal of Infectious Diseases. 2015; 212: 234-46.
- Frankenberger M, Schwaeble W, Ziegler-Heitbrock L. Expression of M-Ficolin in human monocytes and macrophages. Molecular Immunology. 2008; 45: 1424-30.
- Rørvig S, Honore C, Larsson L-I, Ohlsson S, Pedersen CC, et al. Ficolin-1 is present in a highly mobilizable subset of human neutrophil granules and associates with the cell surface after stimulation with fMLP. Journal of Leukocyte Biology. 2009; 86: 1439-49.
- Honoré C, Rørvig S, Munthe-Fog L, Hummelshøj T, Madsen HO, et al. The innate pattern recognition molecule Ficolin-1 is secreted by monocytes/macrophages and is circulating in human plasma. Molecular Immunology. 2008; 45: 2782–9.
- Akaiwa M, Yae Y, Sugimoto R, Suzuki SO, Iwaki T, et al. Hakata Antigen, a New Member of the Ficolin/Opsonin p35 Family, Is a Novel Human Lectin Secreted into Bronchus/Alveolus and Bile. Journal of Histochemistry & Cytochemistry. 1999; 47: 777–85.
- Sallenbach S, Thiel S, Aebi C, Otth M, Bigler S, et al. Serum concentrations of lectin-pathway components in healthy neonates, children and adults: mannan-binding lectin (MBL), M-, L-, and H-ficolin, and MBL-associated serine protease-2 (MASP-2). Pediatric Allergy and Immunology. 2011; 22: 424-30.
- Munthe-Fog L, Hummelshøj T, Hansen BE, Koch C, Madsen HO, et al. The impact of FCN2 polymorphisms and haplotypes on the Ficolin-2 serum levels. Scandinavian Journal of Immunology. 2007; 65: 383-92.
- Andersen T, Munthe-Fog L, Garred P, Jacobsen S. Serum Levels of Ficolin-3 (Hakata Antigen) in Patients with Systemic Lupus Erythematosus. The Journal of Rheumatology. 2009; 36: 757-9.
- Zacho RM, Jensen L, Terp R, Jensenius JC, Thiel S. Studies of the Pattern Recognition Molecule H-ficolin. Journal of Biological Chemistry. 2012; 287: 8071-81.
- Kairies N, Beisel H-G, Fuentes-Prior P, Tsuda R, Muta T, et al. The 2.0-A crystal structure of tachylectin 5A provides evidence for the common origin of the innate immunity and the blood coagulation systems. Proceedings of the National Academy of Sciences. 2001; 98: 13519-24.
- Garlatti V, Belloy N, Martin L, Lacroix M, Matsushita M, et al. Structural insights into the innate immune recognition specificities of L- and H-ficolins. EMBO Journal. 2007; 26: 623-33.
- Krarup A, Thiel S, Hansen A, Fujita T, Jensenius JC. L-ficolin is a pattern recognition molecule specific for acetyl groups. Journal of Biological Chemistry. 2004; 279: 47513-9.
- Jarlhelt I, Pilely K, Clausen JB, Skjoedt M-O, Bayarri-Olmos R, et al. Circulating Ficolin-2 and Ficolin-3 Form Heterocomplexes. The Journal of Immunology. 2020; 204: 1919-28.
- Hunold K, Weber-Steffens D, Runza VL, Jensenius JC, Männel DN. Functional analysis of mouse ficolin-B and detection in neutrophils. Immunobiology. 2012; 217: 982-5.
- Liu Y, Endo Y, Homma S, Kanno K, Yaginuma H, et al. Ficolin A and ficolin B are expressed in distinct ontogenic patterns and cell types in the mouse. Molecular Immunology. 2005; 42: 1265-73.
- Wu X, Yao D, Bao L, Liu D, Xu X, et al. Ficolin A derived from local macrophages and neutrophils protects against lipopolysaccharide-induced acute lung injury by activating complement. Immunology & Cell Biology. 2020; 98: 595-606.
- Girija UV, Mitchell DA, Roscher S, Wallis R. Carbohydrate recognition and complement activation by rat ficolin-B. European Journal of Immunology. 2011; 41: 214-23.
- Runza VL, Hehlgans T, Echtenacher B, Zähringer U, Schwaeble WJ, et al. Localization of the mouse defense lectin ficolin B in lysosomes of activated macrophages. Journal of Endotoxin Research. 2006; 12: 120-6.
- Schmid M, Hunold K, Weber-Steffens D, Männel DN. Ficolin-B marks apoptotic and necrotic cells. Immunobiology. 2012; 217: 610-5.
- Michelow IC, Dong M, Mungall BA, Yantosca LM, Lear C, et al. A novel L-ficolin/mannose-binding lectin chimeric molecule with enhanced activity against Ebola virus. Journal of Biological Chemistry. 2010; 285: 24729-39.
- Degn SE, Hansen AG, Steffensen R, Jacobsen C, Jensenius JC, et al. MAp44, a Human Protein Associated with Pattern Recognition Molecules of the Complement System and Regulating the Lectin Pathway of Complement Activation. The Journal of Immunology. 2009; 183: 7371-8.
- Tanio M, Kondo S, Sugio S, Kohno T. Trivalent Recognition Unit of Innate Immunity System. Journal of Biological Chemistry. 2007; 282: 3889-95.
- Ali YM, Lynch NJ, Haleem KS, Fujita T, Endo Y, et al. The lectin pathway of complement activation is a critical component of the innate immune response to pneumococcal infection. PLoS Pathogens. 2012; 8: e1002793.
- Verma A, White M, Vathipadiekal V, Tripathi S, Mbianda J, et al. Human H-Ficolin Inhibits Replication of Seasonal and Pandemic Influenza A Viruses. The Journal of Immunology. 2012; 189: 2478-87.
- White MR, Tripathi S, Verma A, Kingma P, Takahashi K, et al. Collectins, H-ficolin and LL-37 reduce influence viral replication in human monocytes and modulate virus-induced cytokine production. Innate Immunity. 2017; 23: 77-88.
- Thiel S. Complement activating soluble pattern recognition molecules with collagen-like regions, mannan-binding lectin, ficolins and associated proteins. Molecular Immunology. 2007; 44: 3875-88.
- Brady AM, Calix JJ, Yu J, Geno KA, Cutter GR, et al. Low Invasiveness of Pneumococcal Serotype 11A Is Linked to Ficolin-2 Recognition of O-acetylated Capsule Epitopes and Lectin Complement Pathway Activation. The Journal of Infectious Diseases. 2014; 210: 1155-65.
- Chalmers JD, Fleming GB, Rutherford J, Matsushita M, Kilpatrick DC, et al. Serum Ficolin-2 in Hospitalised Patients with Community-Acquired Pneumonia. Inflammation. 2014; 37: 1635-41.
- de Messias-Reason I, Kremsner PG, Kun JFJ. Functional Haplotypes That Produce Normal Ficolin-2 Levels Protect against Clinical Leprosy. The Journal of Infectious Diseases. 2009; 199: 801-4.
- Zhang D-F, Huang X-Q, Wang D, Li Y-Y, Yao Y-G. Genetic variants of complement genes Ficolin-2, Mannose-binding lectin and Complement factor H are associated with leprosy in Han Chinese from Southwest China. Human Genetics. 2013; 132: 629-40.
- Ouf EA, Ojurongbe O, Akindele AA, Sina-Agbaje OR, van Tong H, et al. Ficolin-2 Levels and FCN2 Genetic Polymorphisms as a Susceptibility Factor in Schistosomiasis. Journal of Infectious Diseases. 2012; 206: 562-70.
- Faik I, Oyedeji SI, Idris Z, de Messias-Reason IJ, Lell B, et al. Ficolin-2 levels and genetic polymorphisms of FCN2 in malaria. Human Immunology. 2011; 72: 74-9.
- Favier A-L, Gout E, Reynard O, Ferraris O, Kleman J-P, et al. Enhancement of Ebola Virus Infection via Ficolin-1 Interaction with the Mucin Domain of GP Glycoprotein. Journal of Virology. 2016; 90: 5256-69.
- Endo Y, Matsushita M, Fujita T. New Insights into the Role of Ficolins in the Lectin Pathway of Innate Immunity. Int Rev Cell Mol Biol. 2015; 316: 49-110.
- Endo Y, Takahashi M, Iwaki D, Ishida Y, Nakazawa N, et al. Mice Deficient in Ficolin, a Lectin Complement Pathway Recognition Molecule, Are Susceptible to Streptococcus pneumoniae Infection. The Journal of Immunology. 2012; 189: 5860-6.
- Wu X, Bao L, Hu Z, Yao D, Li F, et al. Ficolin A exacerbates severe H1N1 influenza virus infection-induced acute lung immunopathological injury via excessive complement activation. Cellular and Molecular Immunology. 2021; 18: 2278-80.
- Ma YJ, Lee BL, Garred P. An overview of the synergy and crosstalk between pentraxins and collectins/ficolins: their functional relevance in complement activation. Experimental & Molecular Medicine. 2017; 49: e320-e320.
- Bidula S, Sexton DW, Abdolrasouli A, Shah A, Reed A, et al. The Serum Opsonin L-ficolin Is Detected in Lungs of Human Transplant Recipients Following Fungal Infections and Modulates Inflammation and Killing of Aspergillus fumigatus. Journal of Infectious Diseases. 2015; 212: 234-46.
- Alosaimi B, Mubarak A, Hamed ME, Almutairi AZ, Alrashed AA, et al. Complement Anaphylatoxins and Inflammatory Cytokines as Prognostic Markers for COVID-19 Severity and In-Hospital Mortality. Frontiers in Immunology. 2021; 12: 668725.