
Research Article
Austin J Pulm Respir Med. 2023; (10)1: 1094.
Viruses in the Respiratory Tract in Elective Cardiac Surgery Patients
van Paassen J1*, Swets MC2, Groeneveld GH2, de Vries JJC3, de Jong Y4, ten Brinck RM5, Hiemstra PS6, Zwaginga JJ7, de Jonge E1 and Arbous MS1,8
1Department of Intensive Care, Leiden University Medical Center, The Netherlands
2Department of Infectious Disease, Leiden University Medical Center, The Netherlands
3Department of Medical Microbiology, Leiden University Medical Center, The Netherlands
4Department of Internal Medicine, Leiden University Medical Center, The Netherlands
5Department of Rheumatology, Leiden University Medical Center, The Netherlands
6Department of Pulmonology, Leiden University Medical Center, The Netherlands
7Department of Immunohematology and Blood transfusion, Leiden University Medical Center, The Netherlands
8Department of Clinical Epidemiology, Leiden University Medical Center, The Netherlands
*Corresponding author: van Paassen J Department of Intensive Care, Leiden University Medical Center, The Netherlands, Albinusdreef 2, 2333 ZA Leiden, The Netherlands
Received: January 01, 2023; Accepted: February 27, 2023; Published: March 06, 2023
Abstract
Objectives: Acute respiratory distress syndrome after cardiac surgery is a severe complication that is associated with high morbidity and mortality. The presence of viruses in the respiratory tract is postulated to be one of multiple factors attributing to development of Acute Respiratory Distress Syndrome (ARDS), but studies report conflicting results. Since this possible risk factor can potentially be influenced by screening or vaccination, we aimed to further investigate the role of viruses in the development of ARDS after cardiac surgery.
Methods: We conducted an explorative prospective cohort study in 49 randomly chosen asymptomatic adult elective cardiac surgery patients. On four different time points, non-fiberscopic mini-broncho alveolar lavages (miniBAL) were collected and analysed with multiplex PCR testing for 11 types of respiratory viruses.
Results and Conclusions: Various (merely low pathogenic) respiratory viruses were detected in 12% of our study population. Respiratory viruses were present both within and out of the influenza-like-illness- season. 19 (39%) of all patients developed acute respiratory distress syndrome. No relationship of viral presence with major pulmonary outcomes (PaO2/FiO2 ratio, development of acute respiratory distress syndrome or mechanical ventilation time) could be demonstrated, though events were too few to allow multivariate analyses.
Conclusion: Asymptomatic elective cardiac surgery patients do carry respiratory viruses, though not associated with development of respiratory complications. Further research is warranted, in particular research into the (more pathogenic) diverse subtypes of the respiratory viruses, the relevance of virus load (cycle threshold-values) and even into the diagnostic method (throat swab versus deeper material).
Keywords: Thoracic surgery; Upper respiratory tract infection; Human Influenza; Lung Injury
Abbreviations and Acronyms: aCCI: Adjusted Charlson Comorbidity Index; ARDS: Acute Respiratory Distress Syndrome; CT-value: Cycle Threshold value; CPB: Cardio Pulmonary Bypass; CMV: Cytomegalovirus; COPD: Chronic Obstructive Pulmonary Disease; DNA: Desoxyribo Nucleic Acid; hCoV: human Corona Viridae; hMPV: Human Metapneumovirus; HSV: Herpes Simplex Virus; ICU: Intensive Care Unit; ILI: Influenza Like Illnesses; IVA: Influenza Virus Type A; IVB: Influenza Virus type B; P/F ratio: PaO2/FiO2 ratio; PCR: Polymerase Chain Reaction; RSV: Respiratory Syncytial Virus; RV: Rhino Virus; RNA: Ribo Nucleic Acid; SD: Standard Deviation; SARS-CoV-2: Severe Acute Respiratory Syndrome CoronaVirus-2
Introduction
Development of mild to severe Acute Respiratory Distress Syndrome (ARDS) after cardiac surgery occurs in 0.4-20% of patients [1-4]. Severe ARDS is associated with a complicated postoperative course with e.g. prolonged mechanical ventilation time, Intensive Care Unit (ICU)-and hospital stay [2-8], and high mortality (50-90%) [4-8]. It is hypothesised that ARDS in these patients results from sequential events that lead to an inflammatory response with disruption of the alveolar wall (that comprises alveolar epithelial cells and capillary endothelial cells) and progressive alveolar oedema with disturbed gas exchange [9-11]. Endothelial injury by the systemic inflammatory response due to surgery itself, Cardiopulmonary Bypass [CPB] and blood transfusion [11], could be one of these factors, while the mode of mechanical ventilation [12], and collapse of the lung to facilitate the surgeon [13,14], could additionally also disrupt the epithelium of the alveolar wall.
Another factor that can influence alveolar integrity is perioperative airway colonisation with potentially pathogenic microorganisms, such as respiratory and herpes viruses. As example influenza, SARS-CoV-2, Cytomegalovirus (CMV), Herpes Simplex Virus (HSV) and Respiratory Syncytial Virus (RSV) are all known for their ability to cause disruption of the alveolar architecture by causing excessive inflammation [15]. In transthoracic esophagectomy- and lung cancer surgery patients, presence of viral RNA/DNA indeed was found to be associated with postoperative pulmonary complications [16-18]. Development of COVID-19 in the postoperative phase of cardiac surgery patients is also associated with adverse outcomes [19,20]. Moreover, cardiac surgery in the influenza season is followed by an increased risk of developing ARDS (9% versus 5%) and prolonged dependency of mechanical ventilation, ICU- and hospital stay [9,21,22]. Therefore, further knowledge on the role of respiratory viruses in development of ARDS after cardiac surgery is required, since, if established, it could lead to relevant preventive measures, such as rapid PCR screening and/or even targeted vaccination strategies in the pre-operative period.
In this prospective cohort study, we therefore investigated whether preoperative evidence of respiratory tract viral RNA/DNA in elective cardiac surgery patients is associated with adverse postoperative pulmonary outcomes.
Materials and Methods
Study Design and Study Population
From January 2014 to December 2018, a prospective cohort study was performed in a 26-bed ICU of the Leiden University Medical Centre at Leiden (LUMC), a tertiary referral hospital in the Netherlands. The study was approved by the Medical Ethical Committee (protocol P117-11) and conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments [23].
Eligible patients were adults (>18 years) undergoing elective cardiac surgery. Although approximately 1000 cardiac surgeries were performed each year, the number of inclusions was dependent on laboratory and staff availability. The exclusion criteria were inability to sign informed consent, emergency operations, and participation in another study. Written informed consent was obtained from all included patients on the day before surgery.
Perioperative Care
All patients visited the pre-operative outpatient clinic for pre-operative screening. At admittance, all patients were seen by an anaesthesiologist and a thoracic surgeon on the day before surgery. If there was the suspicion of an active infection on the basis of respiratory symptoms or elevated temperature, it was customary to postpone the operation. Peri-operative care for cardiac surgery patients is standardized in the LUMC and follows a pre-established care path. All details regarding the pre-operative, intra-operative and post-operative care at thoracic ward, operating room and the ICU are summarized in the supplementary information.
Data Collection
All pre-, intra- and postoperative data and clinical parameters were obtained from the Electronic Patient Database (EPD) system of the hospital. On the ICU, continuous hemodynamic and ventilation monitoring was recorded. Four times per day arterial blood gas analysis was performed and more frequently on indication. The age adjusted Charlson Comorbidity Index (aCCI), a prognostic score summing the weighted scores of 19 medical conditions, taking the seriousness and number of comorbid diseases into account, was calculated [24]. This score is developed to predict 1 year-mortality based on medical comorbid conditions and age, and has since been validated in many different populations [25,26].
Influenza like Illnesses (ILI) Season
Ever since 1970, the Dutch national knowledge institute for primary health care research keeps track of when the ILI and Influenza season starts and ends [27]. Predefined “index” general practices register the number of patients that report with ILI and standardised weekly tests on respiratory viruses are performed in these practices to define, by extrapolation, the number of new ILI cases/100.000 inhabitants. The ILI season is defined to last as long as this number of new patients is above 51/100.000 inhabitants per week.
Sample Collection
On four different time points, non-fiberscopic mini-broncho alveolar lavages (miniBAL) were performed after intubation and induction, on the first, third and fifth postoperative day, but only if patients were still intubated. Briefly, 10 ml NaCL 0, 9% was instilled via a Combicath® catheter and aspirated again. Samples were processed in the laboratory immediately. After mucolysis using Sputolysin Reagent [DTT] (Calbiochem, cat nr. 560000, Darmstadt, Germany and/or its affiliates), the cells and debris was separated by centrifugation. The remaining supernatant was collected and stored in 5 to 7 aliquots of 1 ml at minus 80 degrees Celsius.
PCR Testing
After completing miniBAL sample collection and processing, the stored supernatants of all patients were tested at the same time, with the same batch for viral RNA/DNA. A multiplex real-time PCR (qPCR) developed in our laboratory was used, consisting of four types human corona viridae (hCoV) (229E, HKU1, NL63, and OC43), Influenza Virus type A (IVA), Influenza Virus type B (IVB), Human metapneumovirus (hMPV), Parainfluenza (PIV) 1–4, Respiratory Syncytial Virus (RSV), Rhinovirus (RV), adenovirus, bocavirus, Cytomegalovirus (CMV), herpes simplex virus (HSV) 1-2 [27-30]. Total nucleic acids were extracted directly from 200μl clinical samples, using the Total Nucleic Acid extraction kit on the MagnaPure LC system (Roche Diagnostics, Almere, the Netherlands) with 100 μL output eluate. Nucleic acid amplification and detection by real-time PCR was performed on a BioRad CFX96 thermocycler, using primers, probes and conditions as described previously [28,30]. The Cycle Threshold (CT) value is the number of cycles of amplification required for a positive signal, measured as a curve above a threshold. CT-values were normalised using a fixed baseline fluorescence threshold. To prevent false positive results due to technical laboratory issues, such as artefact fluorescention or contamination, CT-values >40 were considered negative.
End Points
The primary endpoint is the presence of respiratory viruses in adult elective asymptomatic cardiac surgery patients. The secondary endpoints were to assess whether perioperative presence of viral RNA/DNA in the respiratory tract is associated with clinical outcomes, such as PaO2/FIO2 ratio (P/F ratio: formula in supplementary information), Alveolar-arterial gradient (A-a gradient: formula in supplementary information), development of ARDS according to Berlin criteria [31]. (definition summary in supplementary information), and duration of mechanical ventilation and length of ICU stay.
Statistical Analyses
Normal distribution of the data was tested according to Shapiro-Wilk. Mean and SD were used to describe normally distributed variables. Median and Interquartile Range (IQR) were used to describe non normally distributed variables. For the comparison of normally distributed variables, a Welch two sample t-test was used for two group comparison, and an analysis of variance (AnOVa) test for more than two groups. Non normally distributed variables were compared using the Mann-Whitney U test for two-group comparisons and the Kruskal Wallis test for more than two groups. Categorical variables were described as a count and a percentage. Given the small sample size, especially in the viral RNA/DNA group, we used Fisher’s exact test rather than the Chi-squared test to compare categorical variables. Statistical analysis was performed using R Statistical Software (version 4.0.5).
Results
Patient Characteristics
From January 2014 to December 2018, 49 random patients were included in this study, six of whom had postoperative evidence of respiratory viral RNA/DNA at the day of surgery. In two patients it took more than 40 PCR cycles to detect viral genetic material, and they were considered virus negative. The patient characteristics are shown in (Table 1). There were more male patients (83 versus 54%), and less comorbidity (aCCI of 2.7 (SD 1.5) versus 3.7 (SD 1.6) in the virus versus the non-virus patient group. In both groups surgery took place in the ILI season in the majority of patients (virus: 67% versus non-virus: 60%). Furthermore, there were less patients with heart failure, defined as a left ventricular ejection fraction of <40% (virus: 0 (0%) versus non-virus: 5 (11%). COPD (GOLD 1 and 2) was more frequent in the non-virus group as well. In general, a high risk population was included, as evidenced by the high NYHA score, the pre-surgery risk score (Euroscore [32]), and the limited number of solely Coronary Artery Bypass Grafting (CABG) surgery. Surgery was more complex with more combined interventions in the non-virus group, but Euroscore, CPB time, and surgery time were not significantly different.
Respiratory virus present in miniBAL
NO (n=43)
YES (n=6)
p value
Demographic characteristics
Gender – Male
n (%)
23 (53)
5 (83)
0.22
Age (years)
Median (IQR)
68 (61 to 77)
66 (57 to 67)
0.15
BMI (kg/m2)
Mean (SD)
27 (5)
26 (3)
0.83
Surgery in influenza-like illness season
n (%)
26 (60)
4 (67)
1.00
Pulmonary condition
Smoking status
Current smoker
n (%)
5 (12)
0 (0)
1.00
Former smoker
n (%)
21 (49)
4 (67)
Never
n (%)
15 (35)
2 (33)
Packyears (years)
Median (IQR)
27 (14 to 39)
45 (38 to 53)
0.18
COPD
NO
n (%)
36 (84)
6 (100)
1.00
GOLD 1
n (%)
4 (9)
0 (0)
GOLD 2
n (%)
2 (5)
0 (0)
GOLD 3
n (%)
0 (0)
0 (0)
GOLD 4
n (%)
0 (0)
0 (0)
Cardiac condition
Clinical signs of decompensation
n (%)
2 (5)
0 (0)
1.00
Myocardial infarction in history
n (%)
8 (19)
2 (33)
0.59
PCI in history
n (%)
12 (28)
2 (33)
1.00
Left ventricle systolic function
Good (EF > 55%)
n (%)
27 (63)
2 (33)
0.31
Moderate (EF 40% - 55%)
n (%)
11 (26)
4 (67)
Impaired (EF 25% - 40%)
n (%)
3 (7)
0 (0)
Poor (EF <25%)
n (%)
2 (5)
0 (0.0)
NYHAg class
Class I
n (%)
8 (19)
0 (0.0)
1.00
Class II
n (%)
7 (16)
1 (17)
Class III
n (%)
14 (33)
1 (17)
Class IV
n (%)
2 (5)
0 (0)
Other comorbidities
Diabetes Mellitus
n (%)
12 (28)
0 (0)
0.31
Hypertension
n (%)
23 (53)
3 (50)
1.00
Hypercholesterolaemia
n (%)
17 (40)
0 (0)
0.13
Malignancy
n (%)
0 (0)
4 (67)
1.00
Chronic liver disease
n (%)
1 (2)
0 (0)
1.00
Chronic kidney disease
n (%)
4 (9)
0 (0)
1.00
Preoperative creatinine (umol/L)
Median (IQR)
81 (67 to 96)
82 (78 to 92)
0.47
Age adjusted CCI
Mean (SD)
3.7 (1.6)
2.7 (1.5)
0.15
Surgical characteristics
Euro SCORE 2
Mean (SD)
5.9 (2.8)
6.5 (1.5)
0.59
Type of surgery performed
CABG
n (%)
17 (40)
2 (33)
0.85
CABG + single valve
n (%)
1 (2)
0 (0)
CABG + multiple valve
n (%)
4 (9)
0 (0)
Single valve
n (%)
2 (5)
0 (0)
Multiple Valve
n (%)
4 (9)
0 (0)
Thoracic Aorta Surgery + CABG + valve
n (%)
6 (14)
1 (17)
Heart failure surgery
n (%)
9 (21)
3 (50.0)
Total duration on CPB (minutes)
Mean (SD)
188 (89)
175 (51)
0.73
Total duration of surgery (minutes)
Mean (SD)
403 (128)
441 (111)
0.50
Intra-aortic balloon pump
n (%)
1 (2)
0 (0)
1.00
Erythrocyte transfusion (ml)
Median (IQR)
0 (0 to 0)
250 (63 to 625)
0.02
Fresh Frozen plasma transfusion (ml)
Median (IQR)
0 (0 to 0)
300 (0 to 600)
0.10
IQR: Interquartile Range; BMI: Body Mass Index; SD: Standard Deviation; COPD: Chronic Obstructive Pulmonary Disease; PCI: Percutaneous Coronary Intervention; EF: Ejection Fraction; NYHA: New York Heart Association; CCI: Charlson Comorbidity Index; CABG: Coronary Artery Bypass Grafting; CPB: Cardiopulmonary Bypass
Table 1: Patient characteristics.
Viral Characteristics
In six out of 49 patients (12%) respiratory viruses could be detected (Table 2). All patients were positive on the day of surgery itself. In one patient, two types of viral DNA were present (IAV and PIV type 2), and this patient was the only one to be still intubated on the first postoperative day. In this patient the CT value of IAV was lower in the second sample (decrease from 39.3 to 2 24.2), indicative of an increasing viral load as seen in an active infection. Mean CT values in the samples from the six positive patients were 37.2 (SD 2.1) in the ILI season showing no difference with 36.7 (SD 3.2) outside the ILI season (p = 0.84). Presence of viral RNA/DNA was similar in the ILI and outside the ILI season (4/30 in versus 2/19 outside the season) (p=1.00).
Patient
Lowest CT value
Virus
Surgery in ILIl season
Timepoint virus positivity
1
24.15;
influenza A virus (IAV)
yes
Surgery day
39.06
parainfluenza virus (PIV-2)
and Day 1
2
38.28
parainfluenza virus (PIV-1)
yes
Surgery day
3
39.03
parainfluenza virus (PIV-2)
no
Surgery day
4
36.46
parainfluenza virus (PIV-3)
no
Surgery day
5
34.12
human Corona Virus (HCoV-HKU1)
yes
Surgery day
6
37.43
human Corona Virus (HCoV-HKU1)
yes
Surgery day
CT cycle threshold, ILI influenza like illnesses
Table 2: Virus characteristics.
Clinical Outcomes in Virus versus Non-Virus Groups
Pulmonary outcomes were not different in the virus versus the non-virus group (Table 3). Neither the number of patients that developed ARDS, (virus: 3 (50%) versus non-virus: 16 (37%) (p=0.51), nor the severity of ARDS (virus: moderate/severe ARDS 33% versus non-virus: 16% (p = 0.31) were significantly different (Figure 1).
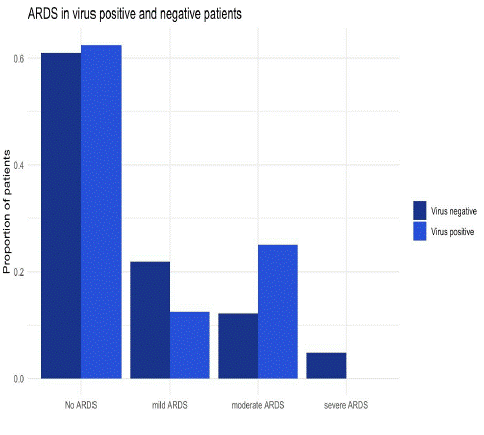
Figure 1: Bar Chart Plot: development of ARDS in virus and non-virus groups (p = 0.510).
Respiratory virus present in miniBAL
YES (n=6)
NO (n=43)
p value
Pulmonary outcomes
Lowest P/F ratio after surgery
Median (IQR)
274 (199 - 349)
188 (139 - 267)
0.15
Lowest A-a gradient after surgery
Median (IQR)
0.8 (0.7 - 0.9)
0.9 (0.6 - 1.5)
0.52
ARDS
No ARDS
n (%)
3 (50)
27 (63)
0.51
mild ARDS
n (%)
1 (16)
9 (21)
moderate ARDS
n (%)
2 (33)
5 (12)
severe ARDS
n (%)
0 (0)
2 (5)
Mechanical ventilation time (min)
Median (IQR)
804 (637 - 990)
767 (663 - 1227)
0.70
Reintubation
n (%)
0 (0.0)
5 (12)
1.00
Severe complications
Re-thoracotomy
n (%)
0 (0.0)
5 (12)
1.00
Cardiopulmonary resuscitation
n (%)
0 (0.0)
1 (2)
1.00
Cardiac tamponade
n (%)
0 (0.0)
3 (7)
1.00
Mortality
n (%)
0 (0.0)
2 (5)
1.00
Other
Infection
n (%)
0 (0.0)
4 (9)
1.00
Delirium
n (%)
1 (16.7)
9 (21)
1.00
ICU stay in hours
Median (IQR)
23 (21 - 39)
24 (22 - 48)
0.50
ICU readmission
n (%)
0 (0)
1 (2)
1.00
IQR interquartile range, P/F ratio PaO2/FiO2 ratio, A-a gradient Alveolar-arterial oxygen gradient, ARDS adult respiratory distress syndrome, ICU intensive care unit.
Table 3: Clinical outcome in virus versus non-virus groups.
Duration of mechanical ventilation (min) (virus: 804 (637–990) versus non-virus: 767 (663-1227), p 0.70) and ICU stay (hours) (virus: 23(21-39) versus non-virus: 24(22-48, p=0.50) were similar in both groups. Furthermore, more severe complications were seen in the non-virus group, though absolute numbers are small (Table 3).
Discussion
In this explorative prospective cohort study of randomly chosen adult asymptomatic cardiac surgery patients, we demonstrated the presence of respiratory viral RNA/DNA in six out of 49 patients (12%). In these six patients, the viral RNA/DNA titre was generally low (high CT-value) and did not differ in or out of the respiratory virus season. Although moderate to severe ARDS occurred more often in the virus group (33%) than in the non-virus group (16%), a significant association of respiratory tract viral RNA/DNA with unfavourable (pulmonary) outcomes, could not be established.
Previous studies reported variably on the clinical impact of the presence of respiratory viruses on surgery outcomes. Three large studies showed that the ILI season was unfavourably linked to clinical outcomes after surgery [9,21,22]. However, in these studies no direct proof was provided that a viral infection was the causal factor in adverse outcomes following cardiac surgery. In fact, in our study, respiratory viruses were detected both in and out of the ILI season, with no significant differences in pulmonary outcomes. This virus independent occurrence of ARDS is consistent with paediatric studies of respiratory viruses in immunocompetent cardiac surgery patients [29,33]. Moreover, in a large (n=1407) multicentre Dutch cohort of acutely admitted adults requiring invasive mechanical ventilation (also including 4% adult cardiothoracic patients) with 28 days of follow up, presence of virus was not associated with the number of ICU free days nor with crude mortality [34].
For all that, within the ILI season, the number of circulating respiratory viruses, and thus the risk of viral infection, remains undoubtedly higher. The preoperative assessment by the thoracic surgeon and anaesthesiologist, however, clearly succeeded in allowing only patients for surgery that showed no clinical signs of viral infections. Nevertheless, as our results indicate, patients with very low viral loads, hence subclinical viral infections, do undergo surgery. However, and notwithstanding their low number, we found this not to be associated with an unfavourable clinical outcome compared to elective patients without presence of respiratory viruses. This result should of course be interpreted with caution. While more non pulmonary complications occurred in the non-virus group, we cannot rule out the possibility that, in the virus group, this might have masked a possible effect of the virus itself, and multivariate correction was only possible to a limited extent due to the small sample size. While earlier studies suggested including viral screening in the preoperative assessment (and possibly even vaccination) to prevent pulmonary complications after cardiac surgery, our study suggests that surgery on patients without symptoms of a viral infection is safe. Our results hence do not support a policy to include PCR-based preoperative screening for respiratory viruses in asymptomatic patients and/or postpone surgery in all asymptomatic patients with preoperatively detectable virus RNA/DNA in respiratory samples, though the heterogeneity of the data and small sample size make that results are difficult to interpret.
Screening for high CT values (low viral loads) of selected respiratory viruses - irrespective of the season- might still contribute to predict unfavourable outcomes. Some of these viruses (IVA, IVB [9,21,22], RSV [29,30,33], PIV [35], and SARS-CoV-2 [36,37], HSV [38,10], and CMV [41] are more pathogenic and can in themselves lead to the development of viral pneumonitis. Since our panel of viruses also included low pathogenic viruses, such as HCoV 229E, NL63, HKU1, OC43, PIV1-4, hMPV, and RV, we found a variety of different viruses in our patients of which there were only four that were associated with an unfavourable outcome in other studies. Hence, future research into higher pathogenic viruses and ARDS after cardiac surgery patients remains useful. Furthermore, there was only one patient with a CT value lower than 35. This could be indicative of a very early stage of the infectious process or, just the opposite, be indicative of viral clearance. Moreover, since viral detection assays were only performed in miniBAL samples and not in nasopharyngeal swabs, we formally cannot exclude that some patients, classified as virus negative in our study, actually did carry virus in the upper airways that later (after collection of the last miniBAL sample) could have resulted in a lower airway infection. Therefore, results should be interpreted with caution. Finally, on top of the fore mentioned restrictions, another significant limitation of our study is its insufficient power to properly correct for baseline differences, or to perform (propensity score) matched analyses. Larger studies are warranted and results from study could be used for power calculations.
Notwithstanding the findings in our study, viral (re)activity is still likely to be one of the many factors contributing to the development of ARDS in patients following major surgery [9,11]. It is known that major surgery, transfusions and certain comorbidities (diabetes mellitus, cardiovascular disease, obesity and old age) are associated with dysregulation of innate and adaptive immunity, including impaired antigen presenting capacity, impaired production of pro-inflammatory mediators, inhibition of T-cell activity, dysregulation of cytokine production, disruption of Th1 and Th17 pathways, and increased apoptosis of immune cells [15]. This makes the body vulnerable to reactivation and a more severe clinical course of respiratory viruses [15]. Subsequent disruption of the alveolar side of the endothelial/alveolar interface eventually leads to impaired gas exchange, disturbed P/F ratio, ARDS and in the end a protracted postoperative clinical course.
Conclusion
In this study we have demonstrated the presence of respiratory viruses in the perioperative phase in 12% of cardiac surgery patients that had no symptoms of a respiratory infection. Only low amounts of often low pathogenic viruses - as indicated by high CT-values –were detected and similarly present in and out of the ILI season. Although in our study the presence of respiratory viral genetic material was not associated with worse pulmonary outcomes after cardiac surgery, larger investigations are needed to determine the importance of the different types of respiratory viruses and the importance of viral load on the perioperative course after heart surgery.
References
- Kogan A, Preisman S, Levin S, Raanani E, Sternik L. Adult respiratory distress syndrome following cardiac surgery. J Card Surg. 2014; 29: 41-6.
- Sanfilippo F, Palumbo GJ, Bignami E, Pavesi M, Ranucci M, et al. Acute Respiratory Distress Syndrome in the Perioperative Period of Cardiac Surgery: Predictors, Diagnosis, Prognosis, Management Options, and Future Directions. J Cardiothorac Vasc Anesth. 2022; 36: 1169-79.
- Stephens RS, Shah AS, Whitman GJ. Lung injury and acute respiratory distress syndrome after cardiac surgery. Ann Thorac Surg. 2013; 95: 1122-9.
- Su IL, Wu VC, Chou AH, Yang CH, Chu PH, et al. Risk factor analysis of postoperative acute respiratory distress syndrome after type A aortic dissection repair surgery. Medicine (Baltimore). 2019; 98: e16303.
- Bellani G, Laffey JG, Pham T, Fan E, Brochard L, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. Jama. 2016; 315: 788-800.
- Asimakopoulos G, Taylor KM, Smith PL, Ratnatunga CP. Prevalence of acute respiratory distress syndrome after cardiac surgery. J Thorac Cardiovasc Surg. 1999; 117: 620-1.
- Kogan A, Segel MJ, Ram E, Raanani E, Peled-Potashnik Y, et al. Acute Respiratory Distress Syndrome following Cardiac Surgery: Comparison of the American-European Consensus Conference Definition versus the Berlin Definition. Respiration. 2019; 97: 518-24.
- Milot J, Perron J, Lacasse Y, Létourneau L, Cartier PC, et al. Incidence and predictors of ARDS after cardiac surgery. Chest. 2001; 119: 884-8.
- Groeneveld GH, van Paassen J, van Dissel JT, Arbous MS. Influenza Season and ARDS after Cardiac Surgery. N Engl J Med. 2018; 378: 772-3.
- Li Y, Wei H. Lipopolysaccharide “two-hit” induced refractory hypoxemia acute respiratory distress model in rats. J Huazhong Univ Sci Technolog Med Sci. 2009; 29: 470-5.
- Nieman G, Searles B, Carney D, McCann U, Schiller H, et al. Systemic inflammation induced by cardiopulmonary bypass: a review of pathogenesis and treatment. J Extra Corpor Technol. 1999; 31: 202-10.
- Mathis MR, Duggal NM, Likosky DS, Haft JW, Douville NJ, et al. Intraoperative Mechanical Ventilation and Postoperative Pulmonary Complications after Cardiac Surgery. Anesthesiology. 2019; 131: 1046-62.
- Lellouche F, Delorme M, Bussières J, Ouattara A. Perioperative ventilatory strategies in cardiac surgery. Best Pract Res Clin Anaesthesiol. 2015; 29: 381-95.
- Reis Miranda D, Gommers D, Struijs A, Dekker R, Mekel J, et al. Ventilation according to the open lung concept attenuates pulmonary inflammatory response in cardiac surgery. Eur J Cardiothorac Surg. 2005; 28: 889-95.
- Fragkou PC, Moschopoulos CD, Karofylakis E, Kelesidis T, Tsiodras S. Update in Viral Infections in the Intensive Care Unit. Front Med (Lausanne). 2021; 8: 575580.
- Bludau M, Hölscher AH, Bollschweiler E, Leers JM, Gutschow CA, et al. Preoperative airway colonization prior to transthoracic esophagectomy predicts postoperative pulmonary complications. Langenbecks Arch Surg. 2015; 400: 707-14.
- D’Journo XB, Bittar F, Trousse D, Gaillat F, Doddoli C, et al. Molecular detection of microorganisms in distal airways of patients undergoing lung cancer surgery. Ann Thorac Surg. 2012; 93: 413-22.
- D’Journo XB, Michelet P, Papazian L, Reynaud-Gaubert M, Doddoli C, et al. Airway colonisation and postoperative pulmonary complications after neoadjuvant therapy for oesophageal cancer. Eur J Cardiothorac Surg. 2008; 33: 444-50.
- Lei S, Jiang F, Su W, Chen C, Chen J, et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. E Clinical Medicine. 2020; 21: 100331.
- Li YK, Peng S, Li LQ, Wang Q, Ping W, Zhang N, et al. Clinical and Transmission Characteristics of Covid-19 - A Retrospective Study of 25 Cases from a Single Thoracic Surgery Department. Curr Med Sci. 2020; 40: 295-300.
- Martin TJ, Eltorai AEM, Kennedy K, Sellke F, Ehsan A. Seasonality of postoperative pneumonia after coronary artery bypass grafting: A national inpatient sample study. J Card Surg. 2020; 35: 1258-66.
- Mori M, Wang Y, Mahajan S, Geirsson A, Krumholz HM. Associations Between the Severity of Influenza Seasons and Mortality and Readmission Risks After Elective Surgical Aortic Valve Replacement and Coronary Artery Bypass Graft Surgery in Older Adults. JAMA Netw Open. 2020; 3: e2031078.
- Assembly W. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Jama. 2013; 310: 2191-4.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987; 40: 373-83.
- Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994; 47: 1245-51.
- Yurkovich M, Avina-Zubieta JA, Thomas J, Gorenchtein M, Lacaille D. A systematic review identifies valid comorbidity indices derived from administrative health data. J Clin Epidemiol. 2015; 68: 3-14.
- Nederlands Instituut voor Onderzoek van de Gezondheidszorg. 2022.
- Loens K, van Loon AM, Coenjaerts F, van Aarle Y, Goossens H, et al. Performance of different mono- and multiplex nucleic acid amplification tests on a multipathogen external quality assessment panel. J Clin Microbiol. 2012; 50: 977-87.
- Roeleveld PP, Van Rijn AL, de Wilde RBP, van Zwet EW, Wink J, et al. Rhinovirus Detection in the Nasopharynx of Children Undergoing Cardiac Surgery Is Not Associated With Longer PICU Length of Stay: Results of the Impact of Rhinovirus Infection After Cardiac Surgery in Kids (RISK) Study. Pediatr Crit Care Med. 2021; 22: e79-e90.
- van Rijn AL, Roeleveld PP, de Wilde RBP, van Zwet EW, Wink J, Rozendaal L, et al. RISK-Study protocol. J Clin Trials. 2015; 5: 62015.
- Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. Jama. 2012; 307: 2526-33.
- Nashef SA, Sharples LD, Roques F, Lockowandt U. EuroSCORE II and the art and science of risk modelling. Eur J Cardiothorac Surg. 2013; 43: 695-6.
- Moynihan K, Barlow A, Alphonso N, Anderson B, Johnson J, et al. Impact of Viral Respiratory Pathogens on Outcomes After Pediatric Cardiac Surgery. Pediatr Crit Care Med. 2017; 18: 219-27.
- van Someren Gréve F, Juffermans NP, Bos LDJ, Binnekade JM, Braber A, et al. Respiratory Viruses in Invasively Ventilated Critically Ill Patients-A Prospective Multicenter Observational Study. Crit Care Med. 2018; 46: 29-36.
- McFarlane HJ, MacDonald J, Collins TC, Molyneaux PJ, Carman WF. Severe pneumonia after cardiac surgery as a result of infection with parainfluenza virus type 4. J Cardiothorac Vasc Anesth. 2009; 23: 84-6.
- Niknam J, Rong LQ. Asymptomatic patients with coronavirus disease and cardiac surgery: When should you operate? J Card Surg. 2020; 35: 2486-8.
- Yates MT, Balmforth D, Lopez-Marco A, Uppal R, Oo AY. Outcomes of patients diagnosed with COVID-19 in the early postoperative period following cardiac surgery. Interact Cardiovasc Thorac Surg. 2020; 31: 483-5.
- Bruynseels P, Jorens PG, Demey HE, Goossens H, Pattyn SR, et al. Herpes simplex virus in the respiratory tract of critical care patients: a prospective study. Lancet. 2003; 362: 1536-41.
- Linssen CF, Jacobs JA, Stelma FF, van Mook WN, Terporten P, et al. Herpes simplex virus load in bronchoalveolar lavage fluid is related to poor outcome in critically ill patients. Intensive Care Med. 2008; 34: 2202-9.
- Yamaguchi A, Matsumoto S, Hagiwara S, Shingu C, Iwasaka H, et al. Herpes simplex virus pneumonia following mitral valve replacement. Gen Thorac Cardiovasc Surg. 2010; 58: 580-3.
- Zhang Z, Liu X, Sang L, Chen S, Wu Z, et al. Cytomegalovirus reactivation in immunocompetent mechanical ventilation patients: a prospective observational study. BMC Infect Dis. 2021; 21: 1026.