
Research Article
Austin J Public Health Epidemiol. 2025; 12(2): 1178.
Trend in Prevalence of Tuberculosis among Adult and Adolescent Clients at a Tertiary Military Hospital in Lagos, Nigeria – Retrospective Review
Harrison NE1*, Elekwa CE1, Nnadi TN1, Lawal I2, Adamu Y2, Ifechineke N2, Ugwu AO3, Ani-Ugwu NK4, Ogoh GS4, Meri H2 and Okeji NAE5
1Center for Infectious Diseases, 68 Nigerian Army Reference Hospital Yaba Lagos, Nigeria
2Walter Reed Army Institute of Research (WRAIR) – Africa, Abuja, Nigeria
3Obstetrics and Gynaecology Department, 68 Nigerian Army Reference Hospital Yaba Lagos, Nigeria
4Department of Medicine (Pulmonology unit), Lagos University Teaching Hospital
5Department of Medicine, 68 Nigerian Army Reference Hospital Yaba Lagos, Nigeria
6Ministry of Defence Health Implementation Programme Abuja, Nigeria
*Corresponding author: Dr Harrison NE, Center for Infectious Diseases, 68 Nigerian Army Reference Hospital Yaba Lagos, Nigeria Email: alokechugwu@gmail.com
Received: June 14, 2025 Accepted: July 17, 2025 Published: July 21, 2025
Abstract
Background: Recently Tuberculosis (TB) has returned as the world’s leading cause of death from a single infectious agent, since after the pandemic. It is an infectious disease and a major global health threat. Currently, Nigeria is among the 30 high-burden countries for TB, TB/HIV, and MDR/RR-TB. This study aimed to determine the trends in the prevalence of TB diagnosed in 68 Nigerian Army Reference Hospital Yaba over seven years period.
Methodology: This was a cross-sectional hospital-based retrospective study. A total of 5187 adolescent and adult (15 years and above) clients’ data was extracted from a seven-year record (January 2013 to December 2019) from the presumptive TB patients referred to the laboratory for diagnosis using GeneXpert.
Conclusion: The TB and Rifampicin-resistant TB prevalence over the seven years shows that strengthening of TB services by early diagnosis timely and effective treatment remains essential in limiting transmission and ultimately achieving TB elimination.
Keywords: TB; Trend; Prevalence; TB/HIV co-infection
Introduction
Tuberculosis (TB) is a communicable disease that poses a serious threat to global health. Despite being preventable and treatable, it ranks as the first most common infectious killer after the decline in cases of coronavirus disease during the pandemic (COVID-19) and the 13th greatest cause of mortality globally [1-3]. Mycobacterium tuberculosis is estimated to infect about one-fourth of the world's population. The risk of tuberculosis following infection is influenced by several factors, the most significant is a compromised immune system [4]. Globally, an estimated 10.6 million people, including 5.8 million men, 3.5 million women, and 1.3 million children, were diagnosed with tuberculosis in 2022 [5,6]. There are notable geographical differences in the spatial distribution of TB incidence. Nearly 70% of all TB cases occur in Southeast Asia and Africa, where the majority of high-incidence countries are situated [7,8]. TB disease has a significant death rate (about 50%) if treatment is not received [1]. Through close contact, approximately 10 to 15 persons can contract TB each year from individuals with active tuberculosis [9,10]. Nigeria is among the 30 high-burden TB, DR-TB and TB/HIV countries globally and ranks first in Africa with a prevalence of 4.4%, closely followed by Democratic republic of Congo and south Africa respectively. (WHO 2023 GTB report) [11]. Early diagnosis, timely and effective treatment are cornerstones in the effort for TB control, prevent drug resistance, limit the transmission, and ultimately achieve the elimination of TB as a public health threat [6,12-15]. Interestingly, TB is curable and treatable. Although, despite recent advancements in global efforts, TB remains a public health concern, and the HIV/AIDS epidemic has worsened its eradication.
Thereby leading to mortality and morbidity, particularly in developing countries like Nigeria [16]. Several factors have been fingered to be associated with TB prevalence and transmission. These include Poverty and Inequality, malnutrition, Overcrowding/ poor housing, homelessness, Lack of Public Health Infrastructure, HIV infection, Smoking, substance misuse and Diabetes [17-27]. Additionally, the effect of TB is not only limited to the respiratory system where it has been shown to cause lung damage, bronchiectasis, pneumothorax, lung collapse, pleurisy, effusions, empyema and ultimately respiratory failure if untreated, but it is also implicated in so many extra pulmonary manifestations, such as genital TB causing infertility, miliary Tb, and TB meningitis. It has also been shown to affect other organs such as the skin, kidney, liver and even the joints [17-27].
This study, therefore, aimed to determine the trends in the prevalence of TB diagnosed in 68 Nigerian Army Reference Hospital Yaba over seven years period.
Methodology
Study Design, Sampling Method, and Data Collection
This was a cross-sectional hospital-based retrospective study. Seven-year data from January 2013 to December 2019 was extracted from the facility TB laboratory and treatment registers. Adults and adolescents =15 years old were included.
Study Setting
This study was conducted at 68 Nigerian Army Reference Hospital Yaba (68NARHY), Lagos. This tertiary military hospital offers specialized treatment to both officers and men of the Nigerian Army and civilians [28]. It also has a unit dedicated to care for infectious disease patients including TB, HIV, COVID-19, syphilis, Hepatitis and other sexually transmitted infections [28]. Care can be assessed every day of the week. This unit is headed by a team of specialists including physicians, laboratory scientists, nurses and other staff.
Laboratory Diagnosis
All the collected samples were processed in the laboratory as per standard procedures for the GeneXpert technique and testing was carried out on the MTB/RIF test platform (GeneXpert, Cepheid) [28,29]. The results were interpreted by the GeneXpert diagnosis system from the measured fluorescent signals and displayed automatically as: Negative (MTB not detected), Positive (MTB detected. RIF Resistant not detected, MTB detected, RIF Resistant detected, MTB detected, RIF Indeterminate), Invalid and Error
Inclusion Criteria
This includes all patients who sought care in our facility with presumptive diagnosis of TB during the period of study.
Exclusion criteria- All patients that presented for TB testing but were confirmed to be negative were excluded from the study and alternative diagnosis considered.
Data Analysis and Statistics
Variables of interest were extracted from the TB laboratory records and recorded in Microsoft Excel. This was later imported and analysed using Statistical Package for Social Sciences 29 Armonk, NY: IBM Corp (SPSS® package version 29) was used to analyze the data. The findings were presented using descriptive statistics such as frequency and percentage. Continuous variables such as age were summarized as means and standard deviations. Bivariate and multivariate logistic regression were also used to evaluate the association between independent and dependent variables. The statistical significance level was set as < 0.05.
Ethical Consideration
IRB approval was obtained from 68 Nigerian Army Reference Hospital Ethical Review Committee with ethical approval number 68NARHY/EC/004.
The ethical principle of Helsinki was followed throughout the study.
Results
A total of 5202 cases were evaluated during the period of review, however, 5187 met the inclusion criteria and their data was collected for the study. This makes it a retrieval rate of 99.7%.
The demographic characteristics showed that 52.1% of the clients were males while 47.9 % were females. Concerning their age distribution, 13.7% were 15-24years, 24.7% were 25-34 years, 27.4 were 35-44years, 18.1% were 45-54years, 9.1% were 55-64years and 7.0% were 65years and above, with a mean age of 40.05±14.3 years. The average annual TB prevalence over the seven years was 16.4% and the average annual rifampicin-resistant TB was 1.2% (Table 1). The annual TB prevalence ranged from 28.7% in 2013 to 8.7% in 2019 and the annual rifampicin-resistant TB ranged from 3.3% in 2013 to 0.5% in 2019 (Figure 1).
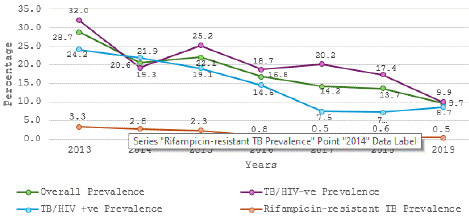
Figure 1: Trends in TB and Rifampicin-resistant TB Prevalence.
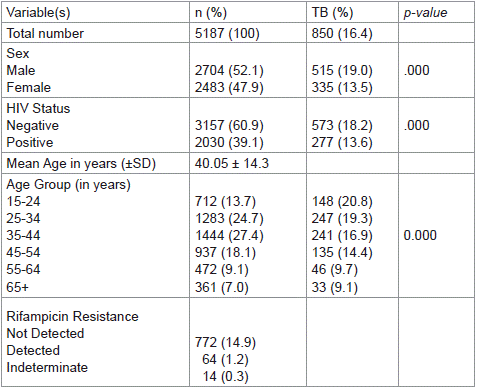
Table 1: Socio-demographic and clinical characteristics of the clients.
The binary logistic regression analysis model showed that the effect of sex and HIV status on the prevalence of TB was statistically significant with adjusted OR (1.519 P value 0.000) and (1.394 P value 0.000) respectively. Males had 1.5 times the odds of being diagnosed with TB than females using GeneXpert, and HIV-negative had 1.3 times the odds of being diagnosed with TB using GeneXpert than HIV-positive. In addition, older age was associated with a reduction in the likelihood of being diagnosed with TB using GeneXpert (Table 2).
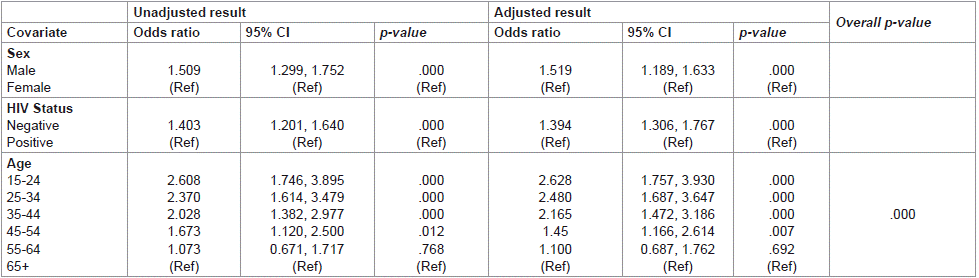
Table 2: Logistic regression analysis for factors associated with TB Prevalence.
Discussion
The study aimed to assess the trends in the prevalence of tuberculosis over a seven-year period. We show that the trend of TB over the years is on the decline, and this is similar to the observation from other studies that noted a decline in the incidence of TB from 2015 to 2021 [3,9]. Bai et al in their study observed that the decline in the incidence of TB in middle- and low-income countries and regions was significant than the decline observed in the high-income regions [9]. This decline in the TB prevalence/incidence observed in these studies may imply that the TB control strategies are effective.
The trend in TB prevalence over the seven-year period among HIV co-infected clients was noted to be lower when compared with that of HIV non-infected clients. Several studies have shown varying effects of HIV coinfection on the TB yield by GeneXpert. Some studies observed that the use of Xpert significantly improved the diagnosis of HIV-associated TB compared to microscopy, due to its increased sensitivity [30–32]. On the other hand, some studies observed similar yield on GeneXpert and microscopy in clients with HIV coinfection [33-35]. The observation from our study may be attributed to immunosuppression from HIV confection leading to paucibacillary TB. In addition, clients with immunosuppressive conditions like HIV are at high risk for Non-Tuberculous Mycobacterium (NTM) which is not missed by the GeneXpert technique [36,37].
Males had 1.5 times the odds of being diagnosed with TB than females using GeneXpert in our study and this observation is similar to other studies in the literature which shows that globally more men than women have TB [38,39]. Several studies have attempted to explain the differential TB infection rates between men and women in terms of biology such as vulnerability; males being more vulnerable, difficulty diagnosing TB in females, and differences in immune response [39,40-45]. Another possible reason could be economic leading to women having less access to TB treatment and prevention services than men and are unlikely to undergo sputum smear examination [39].
The average annual Rifampicin resistance TB in our study is 1.2% with a range of 3.3% to 0.5%. This observation from our study agrees with the Global TB reports by WHO in 2016, which show low levels of MDR/RRTB (< 3%) in new TB patients in various regions globally [46]. In addition, this finding is similar to an earlier study in Lagos that reported a Rifampicin-resistant TB prevalence of 2% and another study in Kaduna that reported 1.25% [47,48]. Nevertheless, a higher prevalence of Rifampicin-resistant TB has been reported in other studies within Lagos (17.6%, 23.4%) and outside, 49.1% in India, 16.7% in Ethiopia, and …in Burundi [49-53].
The difference in the various prevalence rates could be due to the extent and burden of MDRTB/RRTB in the geographical location, study methods, sample size, completeness record, and data-keeping.
This study has some limitations: The data is from one hospital; hence the findings may not be generalized. Other information that would have been useful in our analysis, such as the client’s status as new or re-treatment, was unavailable. Furthermore, for the HIV coinfected client data on their viral load, CD4 count, and ARV use were also not indicated in the TB laboratory registers.
Conclusion
This study provides insights into the prevalence of tuberculosis among adult and adolescent clients at a military hospital in Lagos, Nigeria, over a seven-year period. The findings reveal a decline in TB prevalence, highlighting the effectiveness of the current prevention and control measures. However, sustained efforts and continued research, strengthening of TB services by early diagnosis and timely effective treatment remain essential in limiting transmission of TB and ultimately achieving TB elimination.
Informed Consent
This was a descriptive retrospective study and did not contain any data with human participants performed by the authors and thus did not require informed consent.
Data Availability
The authors are available and ready to supply the data upon any request through the corresponding author.
AI Declaration
No form of regenerative AI such as ChatGPT, COPILOT, DEEPSTEEK was used during the preparation of this manuscript.
References
- World Health Organization (WHO). Global Tuberculosis Report 2022 [Internet]. 2022.
- World Health Organization (WHO). Global Tuberculosis Report 2021[Internet]. 2021.
- Villar-Hernández R, Ghodousi A, Konstantynovska O, et al. Tuberculosis: current challenges and beyond. Breathe. 2023; 19: 220166.
- World Health Organization (WHO). Operational handbook on tuberculosis. Module 1: prevention - tuberculosis preventive treatment [Internet]. 2020.
- World Health Organization (WHO). Tuberculosis [Internet]. 2023.
- World Health Organization (WHO). Global Tuberculosis Report 2019 [Internet]. 2019.
- Chakaya J, Khan M, Ntoumi F, Aklillu E, Fatima R, Mwaba P, et al. Global Tuber¬culosis Report 2020 – reflections on the global TB burden, treatment and prevention efforts. Int J Infect Dis. 2021; 113: 7–12.
- ANI-UGWU NK, Ugwu AO, Ohagwu IC, Omisakin SI, Harrison NE, Anas RF, et al. Comparative Analysis of the Diagnostic Role of Induced Sputum Versus Expectorated Sputum in Patients with Presumptive Diagnosis of Tuberculosis. Academic Medicine & Surgery. 2025; 1-7.
- Bai W, Ameyaw EK. Global, regional and national trends in tuberculosis incidence and main risk: a study using data from 2000 to 2021. BMC Public Health. 2024; 24: 12.
- Khademi G, Bahreini A, Saeidi M. Tuberculo¬sis. Past, Present and Future. 2016.
- Nigerian Federal Ministry of Health. National Tuberculosis, Leprosy and Buruli Ulcer Control Program; 2023 Annual Report.
- Pablos-Mendez A, Knirsch CA, Barr R G, Lerner BH & Frieden TR. Nonadherence in tuberculosis treatment: predictors and consequences in New York City. Am J Med. 1997; 102: 164–170.
- Bradford WZ. et al. The changing epidemiology of acquired drug-resistant tuberculosis in San Francisco, USA. Lancet. 1996; 348: 928–931.
- Cegielski JP, et al. Extensive Drug Resistance Acquired During Treatment of Multidrug-Resistant Tuberculosis. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2014.
- Olarewaju S, Olanrewaju O, Folorunso E, Babatunde A, Temitayo-Oboh A, Abdulsalam S, et al. Treatment Outcome of Tuberculosis Patients Registered at DOTS Centre in Ogbomoso, Southwestern Nigeria: A 4-Year Retrospective Study Tuberculosis Research and Treatment. 2014.
- Yusuff AA, Idoko A, Tommy DS, et al. Burden of Tuberculosis and Challenges Facing Its Eradication in West Africa Int J Infect. 2019; 6: e92250.
- Ugwu, AO, Harrison NE, Haruna M, Ayeni SA. Genital Tuberculosis in a Nigerian Woman with Primary Infertility. Nigerian Journal of Medicine. 2023; 32: p 445-448.
- Ugwu AO, Makwe CC, Oluwole AA, Okunade KS, Odo CC, Ezeoke CD. et al., Seroprevalence of Hepatitis B, and C Viruses and HIV Infections among Antenatal Women in a Secondary Health Facility in Lagos, Nigeria. West Afr J Med. 2022; 39: 1084-1088.
- Tobing KL, Nainggolan O, Rachmawati F, Manalu HSP, Sagala RD, Kusrini I. The Relationship Between Malnutrition and Tuberculosis (TB) at the Age Group More Than 18 Years Old In Indonesia (Analysis Of The Basic Health Research 2018). In: International Journal of Innovation, Creativity and Change. 2021; p. 332–348.
- Jethani S, Semwal J, Kakkar R, Rawat J. Study of Epidemiological Cor¬relates of Tuberculosis. Indian J Community Health. 2012; 24: 304–309.
- Narasimhan P, Wood J, MacIntyre CR, Mathai D. Risk Factors for Tuberculosis. Pulm Med. 2013; 2013: 828939.
- Zhang C, Zhao F, Xia Y, Yu Y, Shen X, Lu W, et al. Prevalence and risk factors of active pulmonary Tuberculosis among elderly people in China: a population based cross-sectional study. Infect Dis Poverty. 2019; 08: 26–35.
- Hertz D, Schneider B. Sex differences in Tuberculosis. Semin Immunopathol. 2019; 41: 225–237.
- Horton KC, MacPherson P, Houben RMGJ, White RG, Corbett EL. Sex differences in Tuberculosis Burden and notifications in low- and Middle- Income countries: a systematic review and Meta-analysis. PLoS Med. 2016; 13: e1002119.
- Neyrolles O, Quintana-Murci L. Sexual inequality in Tuberculosis. PLoS Med. 2009; 6: e1000199.
- Khan MK, Islam MN, Ferdous J, Alam MM. An overview on Epidemiology of Tuberculosis. Mymensingh Med J. 2019; 28: 259–266.
- Kustanto A. The role of socioeconomic and environmental factors on the number of Tuberculosis cases in Indonesia. Jurnal Ekonomi Pembangunan. 2020; 18: 129-146.
- Harrison NE, Ugwu AO, K.S. Okunade, Ismail L, Olufemi Ayanbode, Elekwa Chinenye, et al. Comparative assessment of veneral disease research laboratory (VDRL) and point of care (POC) tests for diagnosing syphilis among pregnant women at a tertiary health facility in Lagos: A retrospective cohort study. Hellenic Journal of Obstetrics and Gynecology. 2023; 22: 176.
- World Health Organization (WHO. Rapid implementation of the Xpert MTB/RIF diagnostic test. Technical and operational ‘how-to’: practical considerations [Internet]. 2011.
- Lawn SD, Brooks SV, Kranzer K, Nicol MP, Whitelaw A, Vogt M, Bekker LG, Wood R. Screening for HIV-associated tuberculosis and rifampicin resistance before antiretroviral therapy using the Xpert MTB/RIF assay: a prospective study. PLoS Med. 2011; 8: e1001067.
- Sharma SK, Kohli M, Yadav RN, Chaubey J, Bhasin D, Sreenivas V, Sharma R, Singh BK. Evaluating the diagnostic accuracy of Xpert MTB/RIF assay in pulmonary tuberculosis. PLoS One. 2015; 10: e0141011.
- Kaur R, Kachroo K, Sharma JK, Vatturi SM, Dang A. Diagnostic accuracy of xpert test in tuberculosis detection: A systematic review and meta-analysis. J Global Infect Dis. 2016; 8: 32–40.
- Mavenyengwa RT, Shaduka E, Maposa I. Evaluation of the Xpert(R) MTB/ RIF assay and microscopy for the diagnosis of Mycobacterium tuberculosis in Namibia. Infect Dis Poverty. 2017; 6: 13.
- Hanrahan CF, Haguma P, Ochom E, Kinera I, Cobelens F, Cattamanchi A, et al. Implementation of xpert MTB/RIF in Uganda: missed opportunities to improve diagnosis of tuberculosis. Open forum Infect Dis. 2016; 3: ofw068.
- Akanbi MO, Achenbach C, Taiwo B, Idoko J et al. Evaluation of Gene Xpert for routine diagnosis of HIV-associated tuberculosis in Nigeria: A prospective cohort study. BMC Pulmonary Medicine. 2017; 17: 87.
- Lan R, Yang C, Lan L, Ou J, Qiao K, Liu F, Gao Q. Mycobacterium tuberculosis and non-tuberculous mycobacteria isolates from HIV-infected patients in Guangxi, China. Int J Tuberc Lung Dis. 2011; 15: 1669–1675.
- Aliyu G, El-Kamary SS, Abimiku A, Brown C, Tracy K, Hungerford L, et al. Prevalence of non-tuberculous mycobacterial infections among tuberculosis suspects in Nigeria. PLoS One. 2013; 8: e63170.
- World Health Organization (WHO). Tuberculosis in women fact sheet [Internet]. 2019.
- UNDP Discussion Paper: Gender and TB, December 2015.
- Neyrolles O, Quintana-Murci L. Sexual Inequality in Tuberculosis, Plos Medicine. 2009.
- Begum V, de Colombani P, Das Gupta S, et al. Tuberculosis and patient gender in Bangladesh: sex differences in diagnosis and treatment outcome. Int J Tuberc Lung Dis. 2001; 5: 604-610.
- Boeree MJ, Harries AD, et al. Gender Differences in Relation to Sputum Submission and Smear Positive Pulmonary Tuberculosis in Malawi, International Journal of Tuberculosis and Lung Disease. 2000; 4: 882–884.
- Austin JF, Dick JM, et al. Gender Disparity Amongst TB Suspects and New TB Patients According to Data Recorded at the South African Institute of Medical Research Laboratory for the Western Cape Region of South Africa. International Journal of Tuberculosis and Lung Disease. 2004; 8: 435–439.
- Diwan VK, Thorson A. Sex, Gender, and Tuberculosis. The Lancet. 1999; 353: 939–1026; Long, N., E. Johansson et al., Fear and Social Isolation as Consequences of Tuberculosis in Vietnam: A Gender Analysis, Health Policy. 2001; 58: 69–81.
- World Health Organization (WHO). Gender and Tuberculosis [Internet]. 2002.
- World Health Organization. Global tuberculosis report 2016 [Internet]. 2016.
- Idigbe O, Sofola T, Akinosho R, Onwujekwe D, Odiah F, Okoye R. Initial drug resistance tuberculosis amongst HIV seropositive and seronegative prison inmates in Lagos, Nigeria. In: Int Conf AIDS. 1998; 137.
- Olatunji OA, Egbe NE, Vanstawa PA, Alhaji B, Idama D, Famiyesin T, et al. Prevalence of Tuberculosis,Rifampicin resistant Tuberculosis and associated risk factors in Presumptive Tuberculosis patients attending some hospitals in Kaduna, Nigeria. The Nigerian Health Journal. 2023; 23: 641-653.
- Kuyinu YA, Odugbemi BA, Salisu-Olatunji SO, Adepoju FO, Odusanya OO. Characteristics of Mycobacterium Tuberculosis Positive Patients Screened for Drug-Resistant Tuberculosis at a Tertiary Health Facility in Lagos. J Natl Med Assoc. 2018; 10: 89-91.
- Adejumo OA, Olusola-Faleye B, Adepoju V, Bowale A, Adesola S, Falana A, et al. Prevalence of rifampicin resistant tuberculosis and associated factors among presumptive tuberculosis patients in a secondary referral hospital in Lagos Nigeria. Afri Health Sci. 2018; 18: 472-478.
- Menon S, Dharmshale S, Chande C, Gohil A, Lilani S, Mohammad S, et al. Drug resistance profiles of Mycobacterium tuberculosis isolates to first line antituberculous drugs: A five years study. Lung India. 2012; 29: 227–231.
- Adane K, Ameni G, Bekele S, Abebe M, Aseffa A. Prevalence and drug resistance profile of mycobacterium tuberculosis isolated from pulmonary tuberculosis patients attending two public hospitals in East Gojjam zone, northwest Ethiopia. BMC Public Health. 2015; 15: 572.
- Sanders M, Van Deun A, Ntakirutimana D, Masabo JP, Rukundo J, Rigouts L, et al. Rifampicin monoresistant Mycobacterium tuberculosis in Bujumbura, Burundi: results of a drug resistance survey. Int J Tuberc Lung Dis. 2006; 10: 178-183.