
Research Article
Austin J Public Health Epidemiol. 2025; 12(1): 1173.
Impact of GeneXpert MTB/RIF Assay for the Detection of Pulmonary and Extrapulmonary Tuberculosis from Clinical Samples
Pradhabane G1, Muthukumar A2, Blasundaram RM3, Prakash V4, Vadakunnel MJ4, Ramachandra V4, Palavesam S4, Subaramani S5 Muthaiah M5*, Soundappan G5 and Fredrick A6
1Department of Biotechnology, Indira Gandhi College of Arts and Science, Indira Nagar, Puducherry, India
2Department of Environmental Science, Central University, Kasaragod, Kerala, India
3Department of Biochemistry, Queen Mary’s College, Madras, Tamil Nadu, India
4Institute of Basic Medical Sciences, University of Madras, Chennai, Tamil Nadu, India
5Department of Microbiology, State TB Training and Demonstration Centre, Intermediate Reference Laboratory, Government Hospital for Chest Diseases, Puducherry, India
6State TB Control Office, Chennai, Tamil Nadu, India
*Corresponding author: Dr. Muthuraj Muthaiah, Department of Microbiology, State TB Training and Demonstration Centre, Intermediate Reference Laboratory, Government Hospital for Chest Diseases, Puducherry, India Email: drmuthurajm@gmail.com
Received: January 06, 2025; Accepted: January 28, 2025; Published: January 31, 2025
Abstract
Background: Tuberculosis (TB) is a remains major infectious disease, particularly in developing countries. Diagnosing TB remains challenging, and the rise of drug-resistant strains poses a serious risk in resource-limited settings. Therefore, improving TB diagnosis is a global priority for effective control. This study aimed to evaluate the diagnostic accuracy of the GeneXpert MTB/RIF assay for identifying both pulmonary TB and extrapulmonary TB. Additionally, it assessed the performance of the GeneXpert system in detecting rifampicin resistance among the patients involved.
Methods: Clinical samples were collected from patients undergoing clinical and radiological evaluations for either Pulmonary Tuberculosis or Extrapulmonary Tuberculosis. These samples were processed and tested for Mycobacterium tuberculosis presence using the GeneXpert assay.
Results: Its sensitivity is 99.8% for pulmonary tuberculosis, and specificity is 99.9% for extrapulmonary tuberculosis. Among the 15.01% of individuals suspected of having tuberculosis, the positivity rates vary by group: 7.54% in people living with HIV (PLHIV), 2.22% in paediatric patients, 20.53% in smearnegative cases, 14.45% in individuals with X-ray results suggestive of TB, and 16.97% in vulnerable groups according to Active Case Finding guidelines. Furthermore, 9.78% of contacts of TB and drug-resistant TB patients tested positive, along with 14.88% in EPTB cases. Contacts of TB and Drug-resistant TB patients have an increased risk, with 23.76% diagnosed with rifampicinresistant tuberculosis. Patients who had contact with TB or DRTB patients have 7.87 times the odds of developing rifampicin-monoresistant TB compared to those without such contacts.
Conclusion: The GeneXpert MTB/RIF assay is a rapid and highly sensitive method for diagnosing PTB and EPTB. Its simplicity and accuracy make it an impressive and valuable tool for detecting M.tuberculosis and rifampicin resistance.
Keywords: Mycobacterium tuberculosis; GeneXpert; Rifampicin resistant; Sensitivity; Specificity; Positive Predictive Value; Negative Predicative Value
Abbreviations
RR-TB: Rifampicin-resistant Tuberculosis; PBS: Phosphate- Buffered Saline; DR-TB: Drug-Resistant Tuberculosis; DS-TB: Drug- Sensitive Tuberculosis; MDR TB: Multidrug-Resistant Tuberculosis; TB: Tuberculosis; MTB: Mycobacterium Tuberculosis; NTM: Non- Tuberculous Mycobacteria; EPTB: Extrapulmonary Tuberculosis; PTB: Pulmonary Tuberculosis; CI: Confidence Interval; HIV: Human Immunodeficiency Virus; WHO: World Health Organization; PMDT: Programmatic Management of Drug-Resistant Tuberculosis; NAAT: Nucleic Acid Amplification Test; CSF: Cerebrospinal Fluid; OR: Odds Ratio
Introduction
Tuberculosis (TB), caused by the bacterium Mycobacterium tuberculosis, is one of the leading causes of mortality worldwide and ranks among the deadliest infectious diseases. Despite extensive global efforts, TB continues to be a significant public health threat, particularly in developing and underdeveloped countries. The World Health Organization (WHO) estimates that in 2023, approximately 10.8 million people were infected with TB, consisting of 55% men, 33% women and 12% children and young adolescents. That year, TB resulted in an estimated 1.25 million deaths, including 161,000 individuals living with HIV. Eight countries accounted for more than two-thirds of the global total of TB cases: India, Indonesia, China, the Philippines, Pakistan, Nigeria, Bangladesh, and the Democratic Republic of the Congo. Notably, the top five countries together accounted for 56% of the global TB burden [1]. Diagnosing tuberculosis (TB) can be difficult due to its nonspecific symptoms and the paucibacillary nature of the disease. Accurately detecting M.tuberculosis is essential for diagnosing TB, which primarily affects the lungs and is transmitted through respiratory means. The GeneXpert MTB/RIF assay (Xpert) is a diagnostic tool that has significantly improved the accuracy of TB detection in clinical settings, offering enhanced sensitivity and specificity [2]. While early detection of TB can be challenging, Xpert has increased the effectiveness of the diagnostic process. However, the accuracy of Xpert can vary based on different diagnostic specimens and the sites of TB infection. Therefore, selecting the appropriate specimens is crucial when using Xpert to identify suspected TB cases. This study assesses the effectiveness of Xpert in diagnosing various types of TB using different specimens.
Methods
Study Settings
Early morning sputum samples (2-4 ml) were collected in presterilized 50 ml Falcon tubes and processed at ten NAAT (Nucleic Acid Amplification Test) sites from January to December 2023 using GeneXpert for M.tuberculosis and Rifampicin-resistance detection. Extra pulmonary samples were processed at the Intermediate Reference Laboratory with GeneXpert instruments. In this study, we successfully enrolled a total of 37695 tuberculosis (TB) suspects from Puducherry, contributing 9,554 participants. Additionally, we collected data from nine neighboring districts in Tamil Nadu, which included Villupuram (2,837 participants), Kallakuruchi (3,243), Cuddalore (2,412), Tiruchirappalli (7,592), Perambalur (2,164), Thiruvarur (3,721), Nagapattinam (1,021), Thanjavur (2,077), and Tiruvannamalai (3,074). This comprehensive enrolment highlights our significant effort to address TB suspicion in the region.
Sputum Sample Processing for GeneXpert MTB/RIF Assay
The GeneXpert sample reagent was added to each sputum specimen at a 2:1 (v/v) ratio using a sterile pipette. The sputum cup was shaken vigorously 10 to 20 times and then incubated at room temperature for 15 minutes, with at least one shake during incubation. Afterward, the sample should be liquefied without clumps. Each GeneXpert MTB/RIF cartridge was labelled with the lab accession number, either by writing on the cartridge or using the provided transfer pipette. The liquefied sample was drawn into the pipette and transferred into the cartridge. It’s crucial to ensure the lab numbers match. After labelling, the pre-printed barcode on the cartridges was scanned, and the cartridge was loaded into the GeneXpert instrument. A green light indicates the test has started, and the results print automatically when complete. Wait for the system to release the door before removing the cartridge, and dispose of used cartridges in a biohazard waste container [3-4].
Tissues Sample Processing for GeneXpert MTB/RIF Assay
Lymph nodes and tissue samples were cut into small pieces with sterile tools and placed in a sterile mortar. Approximately 2 mL of sterile phosphate-buffered saline (PBS) was added, and the mixture was ground into a homogeneous suspension. Next, 0.7 mL of this homogenized sample was transferred to a sterile tube, followed by the addition of 1.4 mL of GeneXpert MTB/RIF Sample Reagent. The mixture was shaken vigorously for at least 10 seconds and incubated at room temperature for 10 minutes, with additional shaking. After incubation, 2 mL of the processed sample was transferred to a GeneXpert MTB/RIF cartridge, ensuring the correct laboratory number was recorded. The barcode was scanned, and the cartridge was loaded into the GeneXpert instrument. Testing initiated when the green light stopped blinking, and results were printed automatically. The cartridge was removed only after the system unlocked the door, and used cartridges were disposed of in a biohazard container [5].
CSF Samples Processing for GeneXpert MTB/RIF Assay
If the cerebrospinal fluid (CSF) sample is less than 2 mL, add an equal volume of GeneXpert MTB/RIF sample reagent and transfer about 2 mL of the mixture into the GeneXpert cartridge. Load it into the instrument as per the manufacturer’s instructions. For samples over 2 mL, transfer the entire volume to a sterile conical tube and centrifuge for 15 minutes at 4000 rpm. Discard the supernatant in a suitable disinfectant. Add 2 mL of GeneXpert reagent to the deposit and transfer 2 mL to the cartridge. Ensure the laboratory number matches the cartridge and sputum cup. Switch on the GeneXpert, scan the pre-labelled barcode, and load the cartridge. Start the test by clicking the appropriate button—when the green light stops blinking, the test has begun. Wait for the green light to turn off at the end of the test, then remove the cartridge and dispose of it in the biohazard container [3].
Ethical Consideration
The study was approved by the Ethics and Scientific Review Committee at the General Hospital Institute, Puducherry, and conducted according to WHO guidelines and the National Tuberculosis Elimination Program.
Statistical Analysis
Logistic regression analysis was used to evaluate the odds ratio for multidrug-resistant/rifampicin-resistant tuberculosis, utilizing MedCalc software (version 22.026) [6].
Results
Of 37695 samples, 28141 were collected from nine adjoining districts of Tamil Nadu, while 9,554 samples came from Puducherry state. Among the 37695 samples, 74.45% (29114) were classified as pulmonary cases, and 21.94% (8,581) were categorized as extrapulmonary cases. Additionally, 87.08% of the samples were considered presumptive TB, whereas 12.92% were deemed presumptive DR-TB. Furthermore, of the 37695 samples, 92.36% (34814) were received from the public sector, and 7.64% (2,881) were obtained from the private sector, as illustrated in Figure 1. Of 29114 pulmonary samples, 21.42% (6,236) were positive for tuberculosis, and 1.65% (479) were rifampicin-resistant. Among 8,581 extrapulmonary samples, 10.72% (920) were positive for tuberculosis, and 0.35% were rifampicin-resistant, as shown in Figure 2. Out of 32825 presumptive patient samples, 15.01% (4927) tested positive for tuberculosis, with 0.63% (208) being rifampicin-resistant. Among 4870 presumptive DR-TB patient samples, 45.77% (2229) tested positive for tuberculosis, and 6.18% were rifampicin-resistant, as shown in Figure 3.
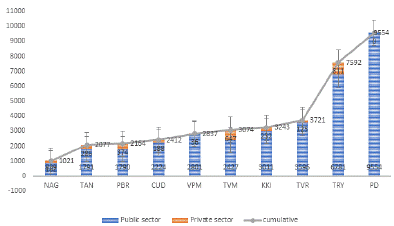
Figure 1: Total number of samples enrolled for this study from public and
private sector.
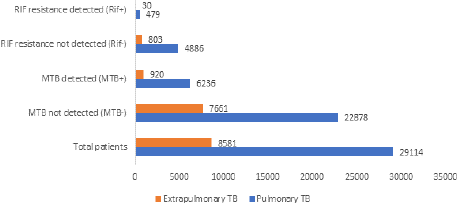
Figure 2: Prevalence of tuberculosis and rifampicin-resistant tuberculosis
among pulmonary and extrapulmonary samples.
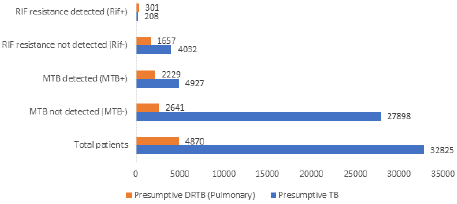
Figure 3: Prevalence of tuberculosis and rifampicin-resistant tuberculosis
among presumptive DRTB and Presumptive TB
Of the 15.01% of presumptive tuberculosis cases, the positivity rates were as follows: 7.54% in people living with HIV (PLHIV), 2.22% in pediatric cases, 20.53% in smear-negative cases, 14.45% in cases with X-ray suggestive of TB, 16.97% in vulnerable groups (according to Active Case Finding guidelines), 9.78% among contacts of TB and drug-resistant TB patients, 14.88% in extrapulmonary TB, and among those offered upfront molecular tests as presented in Table 1. Contacts of TB and DRTB patients have a heightened risk, with 23.76% diagnosed with rifampicin-resistant tuberculosis. Among the 15.01% presumptive drug-resistant tuberculosis cases, positivity rates were 40.90% for newly notified TB patients (who underwent universal drug susceptibility testing), 40.48% for previously treated TB patients (also tested), and 83.45% for non-responders (including patients with drug-sensitive TB and high-risk TB). Both newly notified and previously treated TB patients demonstrate a higher likelihood of having rifampicin-resistant tuberculosis. From 2,881 samples collected from the private sector, 26.25% tested positive for pulmonary TB, while 18.37% tested positive for EPTB. The overall positivity rate for presumptive TB was 18.98%, and the prevalence of rifampicin-resistant cases among those with presumptive drugresistant TB was 7.11%. Table 2 presents the sensitivity, specificity, positive predictive value, and negative predictive value accuracy of the GeneXpert assay for all presumptive tuberculosis and presumptive drug-resistant tuberculosis samples. The sensitivity and specificity of GeneXpert for pulmonary tuberculosis are 99.8% and 99.9%, respectively. For extrapulmonary tuberculosis, the sensitivity and specificity of GeneXpert are 98.7% and 99.8%, respectively.
Stratification of patients
Total
MTB not detected (MTB-)
MTB detected (MTB+)
RIF resistance not detected (Rif-)
RIF resistance detected (Rif+)
Percentage of
TB positivityPercentage
of Rr TBPresumptive TB
PLHIV out of presumptive TB
2374
2195
179
155
10
7.54
5.59
Paediatric out of presumptive TB
2257
2207
50
49
0
2.22
0
Smear Negative, X-ray suggestive of TB
11233
8927
2306
2088
113
20.53
4.9
Other Vulnerable group (as per ACF guidelines)
1820
1557
263
197
10
14.45
3.8
Contacts of TB & DRTB patients
595
494
101
77
24
16.97
23.76
EP TB
7639
6892
747
669
26
9.78
3.48
Upfront Molecular test offered
4026
3427
599
191
6
14.88
1
Presumptive DRTB (Pulmonary)
Notified TB patients (New)- UDST
3846
2273
1573
1210
268
40.9
17.04
Notified TB patients (Previously treated) -UDST
462
275
187
165
19
40.48
10.16
Non-responders (DS TB & Hr TB)
562
93
469
282
14
83.45
2.99
Private sector
Pulmonary TB
1939
1430
509
472
15
26.25
2.95
EPTB
942
769
173
134
4
18.37
2.31
37695
30539
7156
5689
509
18.98
7.11
Table 1: Prevalence of TB and rifampicin resistant tuberculosis among various categories of presumptive TB and presumptive DRTB cases.
Number
Percentage
(%)Xpert
Sensitivity (%)Xpert
Specificity (%)Xpert Positive
Predictive ValueXpert Negative
Predictive ValueDisease Prevalence (%)
Accuracy (%)
with 95% CI
with 95% CI
(%) with 95% CI
(%) with 95% CI
with 95% CI
with 95% CI
PTB
29114
74.45
99.87(99.75-99.94)
99.92(99.88-99.95)
99.71(99.54-99.82)
99.97(99.93-99.98)
21.38(20.92-21.86)
99.91(99.87-99.94)
EPTB
8581
21.94
99.45(98.73- 99.82)
99.84(99.73-99.92)
98.70(97.73-99.25)
99.93(99.84-99.97)
10.64(9.99-11.31)
99.80(99.68-99.88)
Presumptive TB
32825
87.08
99.82(99.65-99.92)
99.91(99.87-99.94)
99.51(99.28-99.67)
99.97(99.94-99.98)
14.96(14.58-15.35)
99.93(99.89-99.95)
Presumptive DRTB (Pulmonary)
4870
12.92
99.82(99.54-99.95)
99.77(99.51-99.92)
99.73(99.40-99.88)
99.85(99.60-99.94)
45.73(44.32-47.14)
99.92(99.82-99.97)
Presumptive TB:
PLHIV out of presumptive TB
2374
6.07
99.44(96.91-99.99)
99.91(99.67-99.99)
98.88(95.68-99.72)
99.95(99.68-99.99)
7.50(6.47-8.63)
99.87(99.63-99.97)
Paediatric out of presumptive TB
2257
5.77
97.96(89.15-99.95)
99.91(99.67-99.99)
96.00(85.72-98.97)
99.95-(99.69-99.99)
2.17(1.61-2.86)
99.87(99.61-99.97)
Smear Negative, X-ray suggestive of TB
11233
28.72
100.00(99.84-100.00)
99.99(99.94-100.00)
99.96(99.69-99.99)
100.00(99.96-100.00)
20.52(19.78-21.28)
99.99(99.95-100.00)
Other Vulnerable group (as per ACF guidelines)
1820
4.65
99.62(97.89-99.99)
99.87(99.54-99.98)
99.24(97.03-99.81)
99.94(99.55-99.99)
14.40(12.81-16.09)
99.84(99.52-99.97)
Contacts of TB & DRTB patients
595
1.52
100.00(96.31-100.00)
99.40(98.25-99.88)
97.03(91.36-99.02)
100.00(99.26-100.00)
16.47(13.58-19.70)
99.50(98.53-99.90)
EP TB
7639
19.53
99.60(98.82-99.92)
99.90(99.79-99.96)
99.06(98.06-99.55)
99.96(99.87-99.99)
9.73(9.07-10.41)
99.87(99.76-99.94)
Upfront Molecular test offered
4026
10.29
99.83(99.07-100.00)
99.97(99.84-100.00)
99.83(98.83-99.98)
99.97(99.79-100.00)
14.88(13.79-16.02)
99.95(99.82-99.99)
Presumptive DRTB (Pulmonary):
Notified TB patients (New)- UDST
3846
9.83
99.94(99.65-100.00)
99.87(99.62-99.97)
99.81(99.41-99.94)
99.96(99.69-99.99)
40.85(39.29-42.42)
99.90(99.73-99.97)
Notified TB patients (Previously treated) -UDST
462
1.18
99.47(97.06-99.99)
99.64(97.99-99.99)
99.47(96.34-99.92)
99.64(97.49-99.95)
40.48(35.97-45.11)
99.57(98.45-99.95)
Non-responders (DS TB & Hr TB)
562
1.44
99.57(98.47-99.95)
97.85(92.45-99.74)
99.57(98.34-99.89)
97.85(91.94-99.45)
83.45(80.12-86.43)
99.29(98.19-99.81)
Private sector:
Pulmonary TB
1939
4.96
100.00(99.28-100.00)
99.93(99.61-100.00)
99.80(98.62-99.97)
100.00(99.74-100.00)
26.20(24.25-28.22)
99.95(99.71-100.00)
EPTB
942
2.41
98.82(95.81-99.86)
99.35(98.50-99.79)
97.11(93.34-98.77)
99.74(98.98-99.93)
18.05(16.64-2065)
99.26(98.47-99.70)
Table 2: Diagnostic Performance of GeneXpert for the detection of Tuberculosis abd Rifampicin-resistance among pulmonary and extrapulmonary samples
The findings of this study indicate that patients with smearnegative tuberculosis and X-ray results suggestive of TB have significantly higher odds of developing additional diseases, with an odds ratio of 1.87 (95% CI: 1.76-1.99). Additionally, contacts of both TB and drug-resistant TB patients also face increased odds for the development of diseases, reflected by an odds ratio of 1.16 (95% CI: 0.94-1.44), as illustrated in Table 3. In contrast, non-responders to treatment (including those with drug-sensitive TB and those with isoniazid monoresistant TB) exhibit an even greater odds ratio for disease development, with an odds ratio of 7.3 (95% CI: 5.80-9.19), as shown in Table 4. Table 5 presents the results of a multivariable logistic regression analysis examining various factors associated with the development of rifampicin-monoresistant tuberculosis. Notably, people living with HIV (PLHIV) who were presumptive TB patients had 1.47 times the odds of becoming rifampicin-monoresistant. Among patients with smear-negative results, those with X-ray findings suggestive of TB had 2.19 times the odds of becoming rifampicinmonoresistant compared to those who were smear-negative without X-ray suggestions. Patients classified as part of vulnerable groups also faced a higher risk of developing rifampicin monoresistance, with an odds ratio of 1.15 compared to those not in vulnerable groups. Additionally, patients who had contacts with TB or drug-resistant TB (DRTB) patients had 7.87 times higher odds of becoming rifampicinmonoresistant than those who did not have such contacts. Table 6 further indicates that notified TB patients had a higher odds ratio of 3.01 for developing rifampicin monoresistance.
Study variables
MTB detected
MTB not detected
Total n(32825)%
Odds ratio
95%Cl
p-value
Presumptive TB
PLHIV out of presumptive TB
No
4748
25703
30451
Yes
179
2195
2374
0.44
0.38-0.52
0.0001
Paediatric out of presumptive TB
No
4877
25691
30568
Yes
50
2207
2257
0.12
0.09-0.16
0.0001
Smear Negative, X-ray suggestive of TB
No
2621
18971
21592
Yes
2306
8927
11233
1.87
1.76-1.99
0.0001
Other Vulnerable group (as per ACF guidelines)
No
4664
26341
31005
Yes
263
1557
1820
0.95
0.83-1.09
0.4918
Contacts of TB & DRTB patients
No
4826
27404
32230
Yes
101
494
595
1.16
0.94-1.44
0.176
Extra pulmonary
No
4180
21006
25186
Yes
747
6892
7639
0.54
0.50-0.59
0.0001
Upfront Molecular test offered
No
4328
24471
28799
Yes
599
3427
4026
0.99
0.90-1.08
0.8029
Table 3: Multivariable logistic regression analysis of presumptive TB diagnosis n (32825).
Study variables
MTB detected
MTB not detected
Total n(4870)%
Odds ratio
95%Cl
p-value
Presumptive DRTB (Pulmonary)
Notified TB patients (New)- UDST
No
656
368
1024
Yes
1573
2273
3846
0.39
0.34-0.45
0.0001
Notified TB patients (Previously treated)
No
2042
2366
4408
Yes
187
275
462
0.79
0.65-0.96
0.0166
Non-responders (DS TB & Hr TB)
No
1760
2548
4308
Yes
469
93
562
7.3
5.80-9.19
0.0001
Table 4: Multivariable logistic regression analysis of presumptive DRTB diagnosis n (4870).
Study variables
RR detected n(189)%
RR not detected n(4245)%
Total n(4434)%
Odds ratio
95%Cl
p-value
Presumptive TB
PLHIV out of presumptive TB
No
179
4090
4269
Yes
10
155
165
1.47
0.76-2.84
0.2468
Paediatric out of presumptive TB
No
189
4196
4385
Yes
0
49
49
0.22
0.01-3.64
0.2927
Smear Negative, X-ray suggestive of TB
No
76
1939
2015
Yes
113
2306
2419
1.25
0.93-1.68
0.1405
Other Vulnerable group (as per ACF guidelines)
No
179
4048
4227
Yes
10
197
207
1.15
0.60-2.21
0.6787
Contacts of TB & DRTB patients
No
165
4168
4333
Yes
24
77
101
7.87
4.85-12.77
0.0001
Extra pulmonary
No
163
3576
3739
Yes
26
669
695
0.85
0.56-1.30
0.4591
Upfront Molecular test offered
No
183
4054
4237
Yes
6
191
197
0.7
0.30-1.59
0.3897
Table 5: Multivariable logistic regression analysis for the detection of Rifampicin-resistant among presumptive TB cases n (4434).
Study variables
RR detected n(301)%
RR not detected n(1657)%
Total n(1958)%
Odds ratio
95%Cl
p-value
Presumptive DRTB (Pulmonary)
Notified TB patients (New)- UDST
No
33
447
480
Yes
268
1210
1478
3.01
2.06-4.38
0.0001
Notified TB patients (Previously treated)
No
282
1492
1774
Yes
19
165
184
0.61
0.37-01.00
0.0482
Non-responders (DS TB & Hr TB)
No
287
1375
1662
Yes
14
282
296
0.24
0.14-0.41
0.0001
Table 6: Multivariable logistic regression analysis for the detection of Rifampicin-resistant among presumptive DRTB cases n (1958).
Discussion
Tuberculosis remains a significant public health threat, with an increasing death rate, particularly in low-resource settings. Early detection and initiation of proper treatment are crucial to reducing mortality rates. Acid-fast bacillus (AFB) smear microscopy and culture are fundamental for diagnosing tuberculosis. While culture is considered the gold standard for TB diagnosis, it is time-consuming and requires appropriate infrastructure and technical expertise. In contrast, AFB smear microscopy is a rapid and inexpensive option, but its sensitivity can be variable, ranging from 20% to 80%. Additionally, due to its limited specificity, it cannot distinguish between M. tuberculosis and non-tuberculous mycobacteria (NTM). Given these limitations, the fully automated Xpert MTB/RIF assay has been endorsed by the World Health Organization (WHO) as the most rapid test for diagnosing pulmonary tuberculosis. The Xpert MTB/ RIF method is prioritized for diagnosing M. tuberculosis because it is quick, reliable, easy to use, and cost-effective. The GeneXpert system employs DNA PCR technology to detect M. tuberculosis and mutations related to rifampicin resistance simultaneously [7].
Almost one in seven individuals seeking evaluation at a public health facility for presumed Tuberculosis had a history of treatment for active TB. Additionally, nearly half of those evaluated had previously undergone treatment for active TB, a rate that is higher than what was reported by Mateyo et al [8]. in Zambia. Our study found that the co-infection rate of HIV and Tuberculosis was 7.54%, while the rate of rifampicin mono-resistance was 5.59%. These figures are higher than those reported by Qi et al [9]. In terms of paediatric tuberculosis prevalence, our study showed a rate of 2.22%. According to Surve et al [10] nearly five percent of new TB cases in India occur among children, which is higher than our findings. Additionally, Mane et al [11] recently reported that the prevalence of paediatric tuberculosis in India is between 6% and 7%. Our study found a prevalence of 20.53% among 11233 smear-negative X-rays suggestive of tuberculosis cases enrolled in this research. This prevalence is lower than the 23.61% reported by Khadka et al [12] in India. Additionally, Moyo et al [13] reported a prevalence of 21.34% of tuberculosis among smear-negative X-rays suggestive of TB cases in South Africa. Reta et al [14] reported a prevalence of pulmonary tuberculosis of 11.70% among key and vulnerable populations living in hotspot settings in Ethiopia, which is lower than the 14.45% reported in our study. In addition, Balakrishnan et al [15] found a TB prevalence of 14.16% among vulnerable populations, a finding that aligns closely with our results. Our study found that the prevalence of tuberculosis and rifampicin-resistant tuberculosis among contacts of TB and DRTB patients was 16.97% and 23.76%, respectively. These rates are lower than the 21.04% reported by Paryani et al [16] in India. In our study, the prevalence of tuberculosis among extrapulmonary patients was 9.78%, while rifampicin-resistant tuberculosis was found in 3.48% of cases. These figures are lower than the 29.7% reported by Singhal et al [17] in India. Extrapulmonary tuberculosis accounts for approximately 20–30% of all active TB cases and primarily affects children and adults with compromised immune systems.
Our study indicates that individuals who are smear-negative and have X-ray results suggestive of tuberculosis are over 1.87 times more likely to develop TB compared to those who are smear-negative but have non-X-ray suggestive results. This finding aligns with research conducted by Kebede et al [18] and a survey from southwest Ethiopia, which shows that smear-negative, non-X-ray suggestive TB patients are 2.7 times more likely to have diseases. Our study indicates that contacts of TB and DRTB patients are at an increased risk of developing tuberculosis, consistent with the findings of Warria et al [19] from Kenya. Our study suggests that people living with HIV who are presumptively diagnosed with tuberculosis, have smear-negative results, show X-ray signs suggestive of TB, belong to vulnerable groups, or are contacts of TB and drug-resistant TB patients are at an increased risk of developing rifampicin-resistant tuberculosis. This finding aligns with the results reported by Qi et al [9], Carter et al [20], and Velayutham et al [21]. This study had two significant strengths. First, it collaborated closely with the National Tuberculosis Elimination Programme, resulting in very few individuals declining participation. The extensive study population allowed for precise estimates of the prevalence and incidence of pulmonary tuberculosis. Second, involving existing health systems and community structures boosted community engagement, contributing to the project's sustainability. This study emphasizes the need for ongoing operational research on the effects of GeneXpert MTB/RIF and other factors related to the diagnosis of tuberculosis and rifampicin-resistant tuberculosis (RR-TB), which could significantly impact TB control programs overall.
Ethical Approval and Consent to Participate
This retrospective study received approval from the Ethics and Scientific Review Committee of the General Hospital Institute in Pondicherry (Approval No. GHIEC/2023/244, dated 08-09-2023), which waived informed consent. The research followed the ethical principles of the Helsinki Declaration, and all data were kept confidential.
Availability of Data and Material
All primary and secondary data are available with the corresponding author and in the Nikshay portal, Government of India, but can be requested from the corresponding author.
Contact no: +91 9944737597
Email ID: drmuthurajm@gmail.com
Competing Interests
The authors declare that they have no conflicts of interest.
Contributors
All authors contributed to the conception and design of the study. SS, MJV, VP, UB, VR, SP, AM, RMB, SSR, GP, and GS all participated in data analysis and interpretation. MM drafted the manuscript, and all authors contributed to revisions and approved the final version.
Acknowledgments
We would like to express our gratitude to the District TB Control officers and staff from the eight neighbouring districts of Tamil Nadu, the team at the Intermediate Reference Laboratory in Puducherry, and the medical officers at the TB treatment and primary health centres for their invaluable support during the data collection process.
References
- World Health Organization. Global tuberculosis report 2023.
- Gong X, He Y, Zhou K, Hua Y and Li Y. Efficacy of Xpert in tuberculosis diagnosis based on various specimens: a systematic review and metaanalysis. Front. Cell. Infect. Microbiol. 2023; 13: 1149741.
- Ramachandra V, Brammacharry U, Muralidhar A, Muthukumar A, Mani R, Muthaiah M, Soundappan G, Frederick A. Assess the Diagnostic Accuracy of GeneXpert to Detect Mycobacterium tuberculosis and Rifampicin-Resistant Tuberculosis among Presumptive Tuberculosis and Presumptive Drug Resistant Tuberculosis Patients. Microbiol Res. 2024; 15: 91–108.
- Muthukumar A, Blasundaram RM, Pradhabane G, Kapalamurthy VC, Brammacharry U, et al. Access the Diagnostic Accuracy of Genotypic Assays for The Rapid Detection of Drug-Resistant Mycobacterium Tuberculosis. J Bacteriol Mycol. 2024; 11: 1220.
- Subaramani S, Joes M, Prakash V, Brammacharry U, Ramachandra V, et al. Comparative Analysis of Xpert MTB/RIF and Xpert Ultra Cartridges for Rapid Detection of Tuberculosis: A Retrospective Point-of Care Testing. J Bacteriol Mycol. 2024; 11: 1223.
- MedCalc: MedCalc’s Relative risk calculator. MedCalc Software Ltd, 2024.
- Elbrolosy AM, El Helbawy RH, Mansour OM, Latif RA. Diagnostic utility of GeneXpert MTB/RIF assay versus conventional methods for diagnosis of pulmonary and extra-pulmonary tuberculosis. BMC Microbiol. 2021; 21: 144.
- Mateyo K, Kerkhoff AD, Dunn I, Nteeni MS, Muyoyeta M. Clinical and radiographic characteristics of presumptive tuberculosis patients previously treated for tuberculosis in Zambia. PLoS ONE. 2022; 17: e0263116.
- Qi CC, Xu LR, Zhao CJ, et al. Prevalence and risk factors of tuberculosis among people living with HIV/AIDS in China: a systematic review and metaanalysis. BMC Infect Dis. 2023; 23: 584.
- Surve S, Naukariya K, Shah I. Latent TB in Indian paediatric population: An update on evidence gaps and research needs. Indian Journal of Tuberculosis. 2023; 70: S8-S13.
- Mane SS, Shrotriya P. Current Epidemiology of Pediatric Tuberculosis. Indian J Pediatr. 2024; 91: 711-716.
- Khadka P, Thapaliya J, Basnet RB, Ghimire GR, Amatya J, Rijal BP. Diagnosis of tuberculosis from smear-negative presumptive TB cases using Xpert MTB/Rif assay: a cross-sectional study from Nepal. BMC Infect Dis. 2019; 19: 1090.
- Moyo, Sizulu, et al. Prevalence of bacteriologically confirmed pulmonary tuberculosis in South Africa, 2017–19: a multistage, cluster-based, crosssectional survey, The Lancet Infectious Diseases. 2022; 22: 1172 - 1180.
- Reta MA, Asmare Z, Sisay A, Gashaw Y, Getachew E, Gashaw M, et al. Prevalence of pulmonary tuberculosis among key and vulnerable populations in hotspot settings of Ethiopia. A systematic review and meta-analysis. PLoS ONE. 2024; 19: e0309445.
- Balakrishnan SK, Suseela RP, Mrithyunjayan S, Mathew ME, Varghese S, Chenayil S, et al. Individuals’ Vulnerability Based Active Surveillance for TB: Experiences from India. Tropical Medicine and Infectious Disease. 2022; 7: 441.
- Paryani RH, Gupta V, Singh P, Verma M, Sheikh S, Yadav R, et al. Yield of Systematic Longitudinal Screening of Household Contacts of Pre-Extensively Drug Resistant (PreXDR) and Extensively Drug Resistant (XDR) Tuberculosis Patients in Mumbai, India. Trop Med Infect Dis. 2020; 5: 83.
- Singhal J, Verma RK. Epidemiology and effects of sociodemographic factors on extrapulmonary tuberculosis in Ambala, India. Indian Journal of Tuberculosis. 2024; 71: 242-249.
- Kebede W, Abebe G, Gudina EK, et al. The role of chest radiography in the diagnosis of bacteriologically confirmed pulmonary tuberculosis in hospitalised Xpert MTB/RIF negative patients. ERJ Open Res. 2021; 7: 00708-2020.
- Warria K, Nyamthimba P, Chweya A, Agaya J, Achola M, Reichler M, et al. Tuberculosis disease and infection among household contacts of bacteriologically confirmed and non-confirmed tuberculosis patients. Trop Med Int Health. 2020; 25: 695-701.
- Carter N, Webb EL, Lebina L, et al. Prevalence of subclinical pulmonary tuberculosis and its association with HIV in household contacts of index tuberculosis patients in two South African provinces: a secondary, crosssectional analysis of a cluster-randomised trial. BMC Global Public Health. 2023; 1: 21.
- Velayutham B, Jayabal L, Watson B, Jagadeesan S, Angamuthu D, Rebecca P, et al. Tuberculosis screening in household contacts of pulmonary tuberculosis patients in an urban setting. PLoS ONE 2020; 15: e0240594.