Research Article
Austin J Psychiatry Behav Sci. 2014;1(6): 1031.
Visual Hallucinations in Parkinson's Disease: a Hierarchy of Impairments Involving Perception, Source Monitoring and Reasoning
Edelstyn NMJ1*, Drakeford JL2 and Ellis SJ3
1School of Psychology, Keele University, UK
2School of Psychology, Staffordshire University, UK
3University Hospital of North Staffordshire, UK
*Corresponding author: Nicky Edelstyn, School of Psychology, Keele University, Keele, Staffordshire ST5 5BG, UK
Received: July 15, 2014; Accepted: August 12, 2014; Published: August 13, 2014
Abstract
Up to 45% of patients with Parkinson's disease (PD) will develop visual hallucinations (VH) at some point in their illness. Although medication, depression, illness duration and ophthalmic abnormalities are identified as risk factors for VH-PD, specific perceptual and cognitive impairments may also play a role. The aim of this study was therefore to explore a hierarchy of low level perceptual processes, imagery and high level executive functions linked to reasoning in groups of VH and non VH PD.
This study investigated 18 patients with non dementing idiopathic PD. Nine patients had a history of VH. The VH and non VH PD groups were matched for demographic (age, gender), neuropsychological (premorbid and current levels of functioning) and clinical characteristics (disease duration, motor symptom severity, daily levodopa medication) apart from presence of VH in the index group. The VH-PD and non VH PD groups completed tests of bottom-up object processing and recognition, visual imagery, and top-down executive functions such as response inhibition, response suppression, source monitoring and spatial and probabilistic reasoning.
Compared to the non VH-PD group, VH-PD patients showed impairments in object perception and recognition impairments in cases when key identifying details were obscured. They also made more source misattribution errors, where self-generated images were misattributed to an external source. Finally, abnormalities in reasoning were evident. On the other hand, there were no differences between the VH-PD and non VH-PD groups on measures of visual perception using canonical views of objects, spatial perception, visual imagery, and other measures of executive function (initiation and suppression of responses, decision-making and self-monitoring).
The findings are discussed in relation to models of delusion and hallucination formation.
Keywords: Parkinson's disease; Visual hallucinations; Visual Perception; Visual Recognition; Source Memory; Reasoning
Introduction
Hallucinations occur in the waking state and are defined as perceptions in the absence of environmental/external stimulation of the relevant sensory organ (see [1]. Surveys of psychiatric disturbance in Parkinson's disease (PD) report prevalence estimates of visual hallucinations (VH) between 8 to 45% [2-4]. The evolution of VH follows a progressive pattern, starting with vivid dreams, night terrors and nightmares, before gradually appearing during wakefulness and becoming increasingly more frequent. At first patients may retain insight into their VH; however their ominous nature is reflected in the VH becoming accompanied by paranoid and delusional ideation before leading to a permanent confusional state [5-9] Predisposing vulnerability factors include illness duration, medication, age, depression, sleep disturbance and cognitive decline [10]. Peripheral visual impairment also appears to be a contributory factor, as dim lighting i.e. use of scotopic vision appears to increase the frequency and duration of VH [11], in a manner similar to that described in Charles Bonnet Syndrome [12,13], a condition characterized by recurrent vivid VH in the presence of normal cognition and insight [14].
VH in PD develop within a multisystem neurodegenerative condition, which originate with the progressive loss of dopamine producing neurons in the substantia Ingra pars compact and ventral tegmental area [15]. This midbrain neuropathology in turn causes abnormal dopaminergic modulation of the striatum, leading to the hallmark motor signs of bradyphrenia, rigidity and tremor [16].
As a close functional relationship exists between the midbrain and neocortical areas including the prefrontal cortex [17] and the hippocampus (for a review, see [18], it is not surprising to find evidence in PD of deficits in both prefrontal-dependent functions which include planning, problem-solving, reasoning [19,20], response inhibition [21], working memory [22] and hippocampus-dependent primary memory processes that support delayed recall and the recollection of episodic details during recognition [21-23].
Although not traditionally included in the pathophysiological/ neuro pathological description of PD, several lines of evidence show abnormal dopaminergic modulation of the visual system is also present. Supporting evidence for this proposal is briefly outlined below.
First, macaque monkeys chronically treated with 1-methyl- 4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) as a model of PD, show morphological impairments in dopaminergic retinal neurons, particularly amacrine cells, the main neuronal subtype postsynaptic to dopaminergic cells. Additionally, electrical synapses among all classes of retinal cells, as well as chemical synapses between amacrine and rod bipolar cells, are deteriorated in parkinsonian monkeys. These results highlight that the scotopic visual pathway is severely impaired in the parkinsonian condition and provide a morphological basis for a number of abnormalities found in electrophysiological and psychophysical trials in PD patients and animal models [24].
Second, abnormal delays in visual evoked potentials (VEP) are reported in untreated de novo PD [25]. Furthermore, VEP abnormalities in PD were eliminated [25], or reduced in VH-PD [26], by the dopamine precursor levodopa.
Third, functional brain imaging reveals reduced activation of the lateral occipital cortex and extra striate temporal areas several seconds before image recognition in VH-PD compared to groups of non VH PD and healthy volunteers [27].
The central cognitive processes implicated in the development of VH in PD have not been extensively explored. Previous studies of hallucinations and delusions in other illnesses, such as schizophrenia for example, suggest that their presence is marked by a bias towards attributing an image which has been generated by oneself, to an external source, termed reality or source monitoring errors [28,29]. Source monitoring refers to the normal process by which perceived and imagined events are discriminated in memory. Memories originating from experienced events have more contextual, perceptual and meaningful information than memories derived from internally generated events such as dreams and fantasies [30,31]. However, if perceptual qualities of imagined events are unusually vivid, they may be more difficult to discriminate from perceived events. This may occur if reflective processes recruit an overabundance of perceptual processes during imagination. Another possible source of deficit in source monitoring is that healthy volunteers often refer to supporting memories and contextual information to substantiate their perceptions. A reduction in the amount of contextual information usually associated with perceived events or an increase in the contextual information associated with imagined events may produce deficits in reality monitoring [32]. If there is a disruption in the retrieval of contextual memories or the coding of these memories initially, the context of the input of the stimulus is likely to be reduced and the memory may be experienced as more isolated, foreign, and unreal to the individual. A deficit in the ability of an individual to access information during recall of real versus imagined events may similarly disrupt reality monitoring [31]. Another way in which internally derived imagination and externally derived memories are distinguished is through reasoning. Such processes include retrieving additional information from memory and considering if the target memory could have been perceived or self-generated given these other specific memories or general knowledge [33]. Garety and colleagues have found evidence of probabilistic reasoning biases in some hallucinating and deluded patients [34,35]. These biases were a tendency to make decisions on the basis of very little information (jumping to conclusions) and to express high levels of certainty (over-confidence). They also were more responsive to disconfirming information than were non psychotic controls, changing their decisions more rapidly. The material in the studies was neutral (concerning judgments about jars of colored beads), in a deliberate attempt to investigate judgments that were impersonal or emotionally loaded [36]. Were the first to investigate source monitoring in 22 VH-PD, 22 non VH-PD patients and 22 healthy controls. Their study revealed that VH-PD is marked by dissociation between (deficient) source monitoring, visual perception and (intact) visual imagery, spatial perception and imagery. These findings suggest that VH in PD could stem from a combination of faulty perceptual processing of environmental events/stimuli, and reduced reliance on contextual recollection during source monitoring.
Charles Bonnet Syndrome shows that vivid VHs can be precipitated by sensory deprivation alone. And it may be the case that abnormal dopamine in the visual pathway in PD may also contribute to the qualitative nature of the type of VH experienced in PD. However, unlike cases of Charles Bonnet Syndrome, VH-PD is marked by cognitive impairment and diminished insight [37].
The studies reviewed so far suggest that abnormalities in the processing of images, beginning at the level of the retina and continuing up to occipito-temporal areas may be risk factors for VH in PD. So that degraded images present the visual system with anomalous images which can be misinterpreted. However, visual perceptual and visual recognition impairments are only part of the story. Studies of other psychopathologies, such as the Capgras delusion, suggest that impairments in prefrontal-dependent executive functions linked to abnormal reasoning also play a critical role. Capgras delusion is characterized by the abnormal belief that one or a small number of highly significant or familiar others have been replaced by imposters who bear a close physical similarity to the original/s [38]. The delusion can be part of a broader psychotic illness such as paranoid schizophrenia or schizoaffective disorder, but can also develop in isolation within the context of a neurological condition, such as stroke or head injury [38]. Functional and structural imaging of a number of these cases shows Capgras delusion to be associated with the presence of focal right hemisphere lesions involving both prefrontal and parietal/temporal neocortical areas [39-41]. The co-occurrence of anterior and posterior lesions, together with evidence of executive and perceptual impairments in Capgras delusion (for example [40], is consistent with the proposal that abnormal prefrontal-dependent reasoning or reality testing styles or bias' fails to discount well-established perceptual disturbances [42].
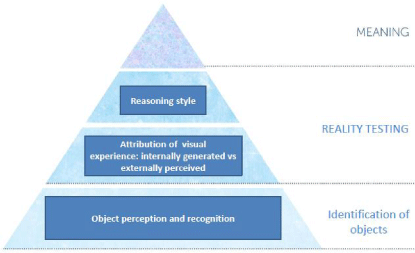
In sum, these separate lines of evidence suggest that VH in PD may arise out of a hierarchy of perceptual and cognitive processing abnormalities which include a bottom-up retinal and posterior cortical impairment in the visual encoding and perception of fine detail which leads to the misidentification of objects in the environment; second, an increased propensity to believe that internally generated mental images are real items occurring in the environment; and finally, a top-down/anterior cortical reasoning deficiency in which VH-PD patients fail to eliminate an irrelevant hypothesis and are more likely to omit one or more appropriate hypotheses from consideration. The model is illustrated in Figure 1.
Figure 1: Model showing the sensory, perceptual and cognitive processes contributing to visual hallucinations in Parkinson's disease. The levels start with sensory encoding, perception and recognition at the bottom, reality testing second and reasoning third. When the 3 levels are combined they generate the fourth level: meaning.
The aim of the current study was to explore this proposed hierarchy of perceptual, recognition and reasoning deficits in groups of VH and non VH PD patients. The design of the study has, in part, been informed by the functional model for object recognition [43]. This model specifiess 3 levels of object representation: an initial representation which represents 2-D geometry; the second level is a viewer-centered object description which represents the spatial relations of visible surfaces from the viewer's position; the third level is a 3-D object-centered representation in which objects are independent of the viewer's position. Because the object-centered representation specified the 3-D structure of the object in a relatively standard form, it is at this level that perception and recognition converge. Accordingly, impairments in initial object representation are predicted in the VH-PD compared to the non VH-PD group, and for these impairments to be most marked for stimuli where the 2-D features are degraded (where there is overlap with viewing real-world objects under conditions of dim lighting/scotopic vision). If impairments exist in forming an initial representation of objects, then deficits should also be evident in tasks that assess object matching when key identifying features are either obscured or the objects are seen from unusual view-points.
The presence of prefrontal-dependent executive dysfunction is a core feature of PD where broad-ranging deficits span response initiation, working memory, attention, decision-making and reasoning. However, in VH-PD, a disproportionate decline is predicted in a source monitoring task specifically related to a tendency to misattribute internally-generated items as externally-perceived. Furthermore, VH-PD is predicted to mark by a relatively greater impairment in executive functions linked reasoning compared to non VH PD, whereas no differences are expected between the two groups on other executive processes such as response initiation and response suppression.
Methods
Participants
Eighteen patients with idiopathic non-dementing PD were recruited from the outpatient clinic at the Department of Neurology, University Hospital of North Staffordshire. Patients were in the mild to severe stages of the disease with a mean [44] severity stage of 3 (SD=0.62). Patients were assigned to groups according to whether they had experienced VHs in the last 3 months, or had never experienced VHs. Nine VH-PD (6 male, 3 female) patients and 9 non VH-PD (6 male, 3 female) patients were matched for gender, age (U=39.50, p=.95) and the following neuropsychological and clinical characteristics: current levels of mental functioning (Mini- Mental Status Examination [MMSE], [45]: U = 24.50, p = .17, and Cambridge Examination for Mental Disorders of the Elderly - Revised [CAMCOG], [46]: U = 25.00, p = .18); premorbid crystalline IQ (National Adult Reading Test [NART], [47]: U=38.00, p = .85); depression (Hamilton Depression Inventory [HDI], [48]: U=19.50, p =.06); illness duration (U = 35.00, p = .65); disease severity (Modified [44]: U = 27.00, p = .29); and the motor subsection of the Unified Parkinson's Disease Rating Scale (UPDRS; [49]: U = 29.00, p = .33).
VH-PD and non VH-PD groups were also matched on the following medication: daily D2 agonist dosage (U = 24.50, p = .16); daily L-dopa dosage (U = 37.00, p = .78); daily monoamine-oxidase-B-inhibitor (U = 39.00, p = .85); and daily Catechol-o-methyltransferase inhibitor (U = 40.00, p =1.00). The demographics and clinical characteristics for both patient groups are summarized in Table 1.
VH-PD
NonVH-PD
Mean (SD)
Mean (SD)
Age
67.78 (5.91)
67.33 (6.85)
Gender: male/female
6/3
6/3
MMSE
27.89 (1.54)
28.89 (0.93)
CAMCOG: Total Score
88.22 (7.84)
92.56 (4.59)
Premorbid IQ (NART)
104.11 (9.39)
102.89 (11.60)
Depression (HDI)
21.38 (8.24)a
14.17 (9.27)b
Illness Duration (years)
9.22 (4.84)
7.50 (3.00)
Hoehn and Yahr Stage
3.17 (0.66)
2.78 (0.57)
UPDRS (motor subsection)
14.44 (8.16)
12.11 (7.01)
L-dopa (mg/day)
572.22 (265.88)
650.00 (302.08)
Dopamine Receptor Agonist (mg/day)
6.17 (9.29)
6.56 (4.39)
Monamine-oxidase-B-inhibitor (mg/day)
4.67 (5.07)
5.00 (5.00)
Catechol-o-methyltransferase inhibitor (mg/day)
22.22 (66.67)
177.78 (533.33)
Table 1: Demographic and clinical characteristics of VH-PD and non VH-PD.
Exclusion criteria for the study included: a history of substance abuse (including alcohol); learning difficulties (including dyslexia); a co-morbid neurological condition; a history of psychiatric illness (including clinical depression); a first-degree relative with a diagnosed psychiatric illness; previous injury rendering unconsciousness for more than 6 hours; English not their first language; and a MMSE score of less than 26. Any patients taking amantadine and anticholinergic medication were also excluded. All patients were community dwelling with normal or corrected vision and hearing.
The study had local research ethics committee approval and all participants provided written informed consent. Each patient took part in three 90-minute sessions and their participation in the study extended over a 10-14 day period. Screening and matching tests were counterbalanced during the first testing session. Assessment of visual object perception and recognition, spatial processing, visual and spatial imagery, executive function and logical reasoning, were counterbalanced across the remaining 2 sessions. The order of test administration was counterbalanced across all participants.
Neuropsychological tests
Current and premorbid levels of functioning
The MMSE [45] and the CAMCOG [46] were used to measure current levels of mental functioning, and the NART [47] provided an estimate of premorbid crystalline IQ. Mood was measured with the HADS [48].
Visual object and spatial perception, object recognition and imagery
The Visual Object and Space Perception battery (VOSP; [50] comprises of four spatial subtests (dot-counting, position discrimination, number location and cube analysis) and four object perception sub-tests (incomplete letters, silhouettes, object decisions and progressive silhouettes). Visual object recognition was measured using the foreshortened view subtest and the minimal view subtest of the Birmingham Object Recognition Test (BORB; [51]. In the foreshortened view subtest the main identifying features of the object is maintained when viewed from an unusual viewpoint, whereas in the minimal feature view subtest the main identifying feature of the object is obscured when viewed from an unusual viewpoint. In both the foreshortened and minimal view subtest participants are required to identify which of two pictures show the target object. The spatial imagery subtest of the Multidimensional Aptitude Battery [52] was used to provide a measure of spatial imagery. In this task, participants are required to use mental rotation to notice differences in a series of figures. Participants are required to work as quickly and as accurately as they can by selecting the correct answer from a choice of 5 options.
Visual imagery ability was measured using 10 structured imagery questions similar to those detailed by [53]. The questions were designed to elicit a mental image that could be used to provide a one word answer to questions on the following topics: shape (e.g. "A ruler is longer than it is wide - true or false?"), colour (e.g. "Is a holly leaf darker green than grass - yes or no?"), and letters (e.g. "Is the capital letter B formed by straight lines, curved lines, or both?"). The 16-item vividness of visual imagery questionnaire (VVIQ; [54] was also used to provide a measure of subjective imagery. The image summoned for each item was rated along a 5-point scale of vividness (1-perfectly clear, to 5-no image at all), once with eyes open, and then with eyes closed. In addition to these questionnaires, 4 sets of 10 semantic decision questions similar to those detailed in [36], were asked concerning both visual and non-visual living and non-living items, e.g. Living-visual: Does an eagle have small talons? Living-non-visual: Are monkey's primates? Non-living-visual: Does a tractor have large tyres? Non-living-non-visual: Is a thimble used for knitting?
Executive function
Estimates of response initiation and response suppression, and spatial reasoning were derived from The Hayling Tests 1 and 2, and the Brixton Test, respectively [55]. The Wisconsin Card Sorting Test (WCST; Heaton, Chelune, Talley et al., 1989) and verbal fluency (phonetic, semantic, and alternating) were used to provide measures of decision-making, monitoring, and reasoning. Finally, deductive reasoning was assessed using a version of the XT-task [56].
The XT-task required the selection of one of two stimuli that were presented together, one to the left and one to the right of the screen. Upon selection the response was fed back immediately and appeared as a circle around the selected stimulus with the message correct or incorrect presented on screen for 600ms below the stimulus pair. The stimuli comprised of XT letters that differed in color (blue or red), size (large or small), and location (left or right); thus creating eight features during each trial i.e. X or T, blue or red, left or right, and large or small. One of these features was relevant to the task at any moment and was determined by the computer program. During the experiment the relevant feature changed without notice after 10, 12 or 14 trials (mean = 12 trials) with a maximum of 30 changes. A failure to make a response within 3000ms following stimulus presentation resulted in the recording of an incorrect response. XT reasoning performance was measured by the number of stimuli that was used before the correct rule was found and this was averaged across all trials.
Source monitoring task
The source monitoring task was similar to that used by [36]. The stimuli included 48 targets (24 words for the imagery trials, 24 pictures for the perception trials) presented at study and 24 distractors (12 words, 12 pictures) randomly intermixed with the targets and presented at test. All stimuli were presented individually on 120mm X 80mm cards. The pictures were taken from a set of standardized Snodgrass pictures [57]. At test, 24 targets (12 words, 12 pictures) were reinstated in the same format and the remaining 24 targets (12 words, 12 pictures) were presented in the opposite format. This produced four conditions: 1) picture at encoding, picture at retrieval (picture-picture); 2) picture at encoding, word at retrieval (picture-word); 3) word at encoding, picture at retrieval (word-picture); and 4) word at encoding, word at retrieval (word-word). The allocation of stimuli to be viewed as a word or as a picture was counterbalanced.
Prior to the experiment participants were given a short practice test to help familiarize them with the procedure. At study, participants were presented with each target (picture or word) for 5000ms and asked to imagine the picture or word as a black and white drawing and to provide an estimate in seconds of how long it would take to draw the item. For words, participants were told to base their estimated drawing time on the image and not the complexity or size of the object. Once the study phase was complete, there was a 15 minute filled delay before testing in which participants were engaged in tests of fluency and executive function. At test, participants were presented with the 48 previously presented targets randomly intermixed with the 24 distracters. Participants were asked to state 'yes' if they had previously seen the item before or 'no' if the item was new. For all recognized items, regardless of whether these were correct or incorrect, participants were asked to state whether it had been previously presented at study as a word (imaged) or as a picture (percept). Performance measures reported are the mean number of correct and incorrect source judgments for each of the four conditions: picture-picture, picture-word, word-picture, and word- word.
Results
The data was not normally distributed so non-parametric analysis was conducted using Mann Whitney U tests. Data is reported for 8 VH-PD patients on the following tests: WCST, Brixton, VIAQ, XT reasoning task and source monitoring task, and for 8 non VH-PD patients on the XT reasoning task due to one patient in each group who was lost to follow up.
Neuropsychological tests
Executive function
Measures of executive function are presented in Table 2.
VH-PD
nonVH-PD
Mean (SD)
Mean (SD)
WCST
Trials to complete first category
42.88 (43.24)
18.56 (17.06)
Number of categories completed
2.63 (2.07)**
4.33 (1.50)
Percent of perseverative errors
25.88 (9.42)**
17.67 (8.44)
Percent of conceptual level responses
45.00 (19.65)
55.56 (18.41)
Failure to maintain a set
2.38 (2.20)**
0.78 (0.97)
Haylinga
Response initiation & suppression
4.11 (2.42)
4.56 (1.89)
Brixtona
Reasoning
2.38 (2.13)**
4.89 (1.62)
XT
Reasoning Task
9.68 (1.42)*
8.32 (0.90)
Fluency
Phonemic: FAS (total)
10.76 (7.41)
12.48 (4.40)
Semantic (total)
19.17 (6.52)
21.52 (5.76)
Alternating (total)
10.54 (6.10)**
15.33 (2.34)
Table 2: Executive function, Reasoning and Fluency for VH-PD and non VH-PD.
Relative to non VH-PD patients, VH-PD patients completed fewer categories (U= 17.50, p<.05, one-tailed), made more preservative errors (U= 17.50, p<.05, one-tailed) and failed to maintain a set (U = 18.00, U< .05, one-tailed) on the WCST. However, there were no significant difference between VH-PD and non VH-PD in the number of trials to complete a category (U = 26.00, p = .18, one-tailed) or in the number of conceptual level responses (U = 24.00, p = .14, one-tailed) on the WCST.
VH-PD patients also exhibited deficits on the Brixton Test of Spatial Anticipation (U = 12.00, p<.05, one-tailed) and total Alternating fluency (U = 12.50, p< .05, one-tailed) compared to non- VH-PD patients.
There were no significant differences between VH-PD patients and non-VH-PD patients on the Hayling Tests of Sentence Initiation and Suppression (U = 37.50, U = .40, one-tailed), total phonemic FAS fluency (U = 28.50, U = .15, one-tailed) or total Semantic fluency (U = 29.00, U = .16, one-tailed).
Performance data (means and standard deviations) for the XT reasoning task are presented in Table 2 and graphed in Figure 2. VH-PD patients required more trials to find the rule compared to non VH-PD patients (U = 16.00, U = .05, one-tailed).
Figure 2: The mean number of trials needed to find the rule on the XT reasoning tasks for PD-VH and non VH-PD patient groups.
Visual perception and object recognition and visual imagery
Performance on visual perception, object recognition and visual imagery is presented in Table 3. VH-PD patients were impaired on the silhouettes (U = 20.00, p< .05, one-tailed) object recognition subtest of the VOSP compared to non VH-PD patients. However, performance on the remaining 3 object recognition and 4 spatial performance subtests of the VOSP did not differ between VH-PD and non VH-PD patient groups (all p-values > .15). VH-PD patients also exhibited deficits on the foreshortened (U = 22.50, p< .05, one-tailed) and the minimal feature (U = 22.00, p< .05, one-tailed) view subtests of the BORB compared to non VH-PD patients. VH-PD and non VH-PD patients did not differ significantly on tests of spatial and visual imagery (all p-values > .15).
VH-PD
nonVH-PD
Mean (SD)
Mean (SD)
VOSP
Incomplete letters (20)
19.11 (0.78)
19.00 (0.87)
Silhouettes (30)
19.56 (4.33)*
23.33 (3.74)
Object decision (20)
17.22 (2.39)
17.89 (1.83)
Progressive silhouettes (20)
9.00 (3.00)
9.33 (2.00)
Dot counting (10)
10.00 (0.00)
9.89 (0.33)
Position discrimination (20)
19.78 (0.44)
19.89 (0.33)
Number location (10)
8.44 (1.24)
8.78 (1.92)
Cube analysis (10)
8.67 (1.66)
9.56 (0.73)
BORB
Foreshortened subtest
24.33 (0.87)*
25.00 (0.00)
Minimal feature subtest
24.11 (0.33)*
24.56 (0.73)
Spatiala
Scaled score
45.22 (10.16)
49.11 (13.38)
VIAQ
Shapes (10)
8.38 (1.51)
8.67 (1.00)
Letters (10)
9.50 (0.54)
9.11 (1.05)
Colours (10)
8.00 (1.51)
8.78 (1.09)
Living visual (10)
8.88 (0.99)
9.33 (0.87)
Living non visual (10)
8.88 (0.64)
9.00 (0.71)
Non-living visual (10)
9.50 (0.76)
9.44 (0.73)
Non-living non-visual (10)
10.00 (0.00)
9.78 (0.44)
VVIQ (80)
29.94 (7.84)
30.94 (9.36)
Table 3: Object Recognition, perception and visual imagery for VH-PD and nonVH-PD.
Source monitoring task
The recognition and source judgments for each condition in the source monitoring task are presented in Table 4. There were no significant differences in recognition performance for picture-picture (U = 27.50, p = .21, one-tailed), word-word (U = 30.00, p = .29, one-tailed), picture-word (U = 34.00, p = .44, one-tailed), word-picture (U = 31.50, p = .34, one-tailed). There were also no significant differences in correct source for picture-picture (U = 31.00, p = .33, one-tailed), word-word (U = 35.00, p = .47, one-tailed), picture-word (U = 34.00, p = .43, one-tailed), or word-picture (U = 19.00, p = .06, one-tailed), however, correct source for word-picture was approaching significance, which suggested that VH-PD patients had a tendency towards poor source memory for word-picture than non VH-PD patients. There were also no significant differences between VH-PD and non VH-PD patients for incorrect source for picture-picture (U = 32.00, p = .53, one-tailed), word-word (U = 22.00, p = .09, one-tailed), and picture-word (U = 35.00, p = .67, one-tailed). However, VH-PD patients did make significantly more source errors for word-picture (U = 15.00, p< .05, one-tailed) than non VH-PD patients by misattributing an internally generated image as a real percept.
Condition
Group
Recognition scores of old items
Correct source scores
Incorrect source scores
(Encoding-Retrieval)
Mean (SD)
Mean (SD)
Mean (SD)
Picture-Picture
VH-PD
8.50 (2.00)
8.50 (2.00)
0.00 (0.00)
NonVH-PD
8.89 (3.26)
8.67 (3.24)
0.22 (0.67)
Word-Word
VH-PD
7.75 (2.61)
5.50 (4.24)
2.25 (1.91)
NonVH-PD
6.78 (2.54)
5.56 (2.55)
1.22 (1.64)
Picture-Word
VH-PD
4.88 (1.96)
4.63 (2.26)
0.25 (0.26)
NonVH-PD
4.78 (2.39)
4.56 (2.35)
0.22 (0.44)
Word-Picture
VH-PD
5.88 (2.36)
2.00 (2.83)*
3.88 (2.23)**
NonVH-PD
6.11 (3.79)
4.44 (3.64)
1.67 (1.94)
Table 4: Source monitoring performance for VH-PD and non VH-PD patients.
Discussion
Previous behavioral and functional brain imaging research indicates that impaired visual perception and visual recognition (for example,[27,36] and errors identifying the source of mental images such that internally generated images are misattributed to the external environment [36], characterize the behavioral profile of visually hallucinating (VH) patients with Parkinson's Disease (PD) compared to non VH-PD. So, for example, in the study reported by Barnes et al., VH-PD patients showed dissociation between (deficits in) object perception, recognition memory, source attribution and (spared) spatial processing and visual imagery. The presence of low level perceptual errors in this study is consistent with the later functional magnetic resonance imaging evidence of reduced activation of the lateral occipital cortex, and extra striate visual temporal areas just before image recognition in VH-PD compared to non VH-PD [27].
According to Coltheart, [58] two factor theory of monothematic delusion, and by extension here, to visual hallucinations, at least two cognitive abnormalities must be present: an anomalous perceptual experience [59] and a reasoning impairment [42]. Faulty perception is cited as giving the anomalous experience its content while the reasoning impairment prevents its rejection. A variety of different studies converge to support a reasoning abnormality as a risk factor for delusions. For example, [60] reported that people with delusions required less information to arrive at a definite decision than persons without a delusion or people with a depressive disorder, a phenomenon called "jumping to conclusions" and was interpreted as an argument for disturbed cognitive processes in the case of (persecutory) delusion. So, the purpose of the reported study was to replicate previous work showing the presence of faulty "bottom-up" perception and extend this by exploring "top-down" executive functions that had a differential weighting on reasoning.
The key behavioral findings from this case report can be summarized as follows: first, impairments in object perception and recognition impairments, when key identifying details are obscured, and source attribution errors, where self-generated images were misattributed to an external source, mark the behavioral profile of VH-PD compared to non VH-PD. Second, the perceptual and source attribution errors in VH-PD dissociate from visual perception using canonical views of objects, spatial perception and visual imagery. Indicating therefore that, VH-PD is not simply a subgroup with generalized cognitive impairment. Third, these replicated findings (see [36] were present in relatively small groups of PD patients, which speaks to the robustness of the perceptual and cognitive profiles described. Finally, our study is consistent with Coltheart et al.'s two factor theory, we show for the first time (as far as the authors are aware), that VH-PD show abnormal reasoning compared to non VH-PD. Furthermore reasoning abnormalities dissociate from other executive functions showing that VH-PD is not a marker of generalized executive dysfunction.
The following key findings from our study, summarized below, should be considered against the background of our relatively small sample size, and the limitation this poses for interpretation of results (for example, false positive results leading to an over-estimation of the magnitude of associations). Accordingly, we suggest that VH in PD is associated with a hierarchy of bottom-up (visual perceptual and recognition processes) and top-down (reasoning) risk factors. In the present case, these impairments represent stable or mediating vulnerability factors rather than transient indicators of dysfunction- since none of the VH-PD group were actively hallucinating during the neuropsychological testing sessions. These "inherent" weaknesses within the perceptual and cognitive structures remain hidden when lighting is good, visual objects are well defined etc but may be artificially exposed in the laboratory by challenging the processing systems with the particular stimuli and tasks found most difficult. Such deficits, when combined with impaired cognitive reasoning operate in a complex interaction to produce VHs in PD.
Acknowledgement
The authors would like to thank the research participants for agreeing to take part and Moyra Mortby, MSc, for help with data collection. This study was funded by a Fast Track grant K-0611 from the Parkinson's disease Society.
References
- Collerton D, Perry E, McKeith I. Why people see things that are not there: A novel Perception and Attention Deficit model for recurrent complex visual hallucinations. Behavioral and Brain Sciences. 2005; 28: 737-757.
- Aarsland D, Larsen JP, Cummins JL, Laake K. Prevalence and clinical correlates of psychotic symptoms in Parkinson disease: a community-based study. Arch Neurol. 1999; 56: 595-601.
- Celesia GG, Barr AN. Psychosis and other psychiatric manifestations of levodopa therapy. Arch Neurol. 1970; 23: 193-200.
- Fénelon G, Mahieux F, Huon R, Ziégler M. Hallucinations in Parkinson's disease: prevalence, phenomenology and risk factors. Brain. 2000; 123: 733-745.
- Korczyn AD. Hallucinations in Parkinson's disease. Lancet. 2001; 358: 1031-1032.
- Graham JM, Grünewald RA, Sagar HJ. Hallucinosis in idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1997; 63: 434-440.
- Haeske-Dewick HC. Hallucinations in Parkinson's disease: characteristics and associated clinical features. International Journal of Geriatric Psychiatry. 1995; 10: 487-495.
- Sanchez-Ramos JR, Ortoll R, Paulson GW. Visual hallucinations associated with Parkinson disease. Arch Neurol. 1996; 53: 1265-1268.
- Sweet RD, McDowell FH, Feigenson JS, Loranger AW, Goodell H. Mental symptoms in Parkinson's disease during chronic treatment with levodopa. Neurology. 1976; 26: 305-310.
- Ozer F, Meral H, Hanoglu L, Ozturk O, Aydemir T, Cetin S, et al. Cognitive impairment patterns in Parkinson's disease with visual hallucinations. J Clin Neurosci. 2007; 14: 742-746.
- Korczyn AD, Brooks DJ, Brunt ER, Poewe WH, Rascol O, Stocchi F. Ropinirole versus bromocriptine in the treatment of early Parkinson's disease: a 6-month interim report of a 3-year study. 053 Study Group. Mov Disord. 1998; 13: 46-51.
- Howard R, Levy R. Charles Bonnet syndrome plus: Complex visual hallucinations of Charles Bonnet syndrome type in late paraphrenia.0 International Journal of Geriatric Psychiatry. 2004; 9: 399-404.
- Teunisse RJ, Cruysberg JR, Hoefnagels WH, Verbeek AL, Zitman FG. Visual hallucinations in psychologically normal people: Charles Bonnet's syndrome. Lancet. 1996; 347: 794-797.
- Siatkowski RM, Zimmer B, Rosenberg PR. The Charles Bonnet syndrome. Visual perceptive dysfunction in sensory deprivation. J Clin Neuroophthalmol. 1990; 10: 215-218.
- Hornykiewicz O. Dopamine (3-hydroxytyramine) and brain function. Pharmacol Rev. 1966; 18: 925-964.
- Parkinson J. An essay on the shaking palsy. 1817. J Neuropsychiatry Clin Neurosci. 2002; 14: 223-236.
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986; 9: 357-381.
- Lisman J, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005; 46: 703-713.
- Beatty WW, Monson N. Problem solving in Parkinson's disease: comparison of performance on the Wisconsin and California Card Sorting Tests. J Geriatr Psychiatry Neurol. 1990; 3: 163-171.
- Morris RG, Downes JJ, Sahakian BJ, Evenden JL, Heald A, Robbins TW. Planning and spatial working memory in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988; 51: 757-766.
- Edelstyn NM, Mayes AR, Condon L, Tunnicliffe M, Ellis SJ. Recognition, recollection, familiarity and executive function in medicated patients with moderate Parkinson's disease. J Neuropsychol. 2007; 1: 131-147.
- Shepherd TA, Edelstyn NM, Mayes AR, Ellis SJ. Second-generation dopamine agonists and recollection impairments in Parkinson's disease. J Neuropsychol. 2013; 7: 284-305.
- Edelstyn NM, Shepherd TA, Mayes AR, Sherman SM, Ellis SJ. Effect of disease severity and dopaminergic medication on recollection and familiarity in patients with idiopathic nondementing Parkinson's. Neuropsychologia. 2010; 48: 1367-1375.
- Cuenca N, Herrero MT, Angulo A, de Juan E, Martínez-Navarrete GC, López S, et al. Morphological impairments in retinal neurons of the scotopic visual pathway in a monkey model of Parkinson's disease. Journal of Comparative Neurology. 2005; 493: 261-273.
- Bodis-Wollner I, Yahr MD, Mylin L, Thornton J. Dopaminergic deficiency and delayed visual evoked potentials in humans. Ann Neurol. 1982; 11: 478-483.
- Onofrj M, Bonanni L, Albani G, Mauro A, Bulla D, Thomas A. Visual hallucinations in Parkinson's disease: clues to separate origins. J Neurol Sci. 2006; 248: 143-150.
- Meppelink AM, de Jong BM, Renken R, Leenders KL, Cornelissen FW, van Laar T. Impaired visual processing preceding image recognition in Parkinson's disease patients with visual hallucinations. Brain. 2009; 132: 2980-2993.
- Vinogradov S, Willis-Shore J, Poole JH, Marten E, Ober BA, Shenaut GK. Clinical and neurocognitive aspects of source monitoring errors in schizophrenia. Am J Psychiatry. 1997; 154: 1530-1537.
- Keefe RSE, Arnold MC, Bayen UJ, McEvoy JP, Wilson WH. Source-monitoring deficits for self-generated stimuli in schizophrenia: multinomial modeling of data from three sources. Schizophrenia Research. 2002; 57: 51-67.
- Johnson MK, Raye CL. Reality monitoring. Psychological Review. 1981; 88: 67-85.
- Johnson MK, Foley MA, Suengas AG, Raye CL. Phenomenal characteristics of memories for perceived and imagined autobiographical events. Journal of Experimental Psychology: General. 1988; 117: 371-376.
- Cohen JD, Servan-Schreiber D. Context, cortex, and dopamine: a connectionist approach to behavior and biology in schizophrenia. Psychol Rev. 1992; 99: 45-77.
- Keefe RSE. The neurobiology of disturbance of the self: autonoetic agnosia in schizophrenia. In: XF Amador & AS David Editors. Insight and Psychosis Oxford: OUP. 1998; 142-173.
- Garety PA, Hemsley DR, Wessely S. Reasoning in deluded schizophrenic and paranoid patients. Biases in performance on a probabilistic inference task. J Nerv Ment Dis. 1991; 179: 194-201.
- Huq SF, Garety PA, Hemsley DR. Probabilistic judgements in deluded and non-deluded subjects. Q J Exp Psychol A. 1988; 40: 801-812.
- Barnes J, Boubert L, Harris J, Lee A, David AS. Reality monitoring and visual hallucinations in Parkinson's disease. Neuropsychologia. 2003; 41: 565-574.
- Barnes J, David AS. Visual hallucinations in Parkinson's disease: a review and phenomenological survey. J Neurol Neurosurg Psychiatry. 2001; 70: 727-733.
- Edelstyn NM, Oyebode F. A review of the phenomenology and cognitive neuropsychological origins of the Capgras syndrome. Int J Geriatr Psychiatry. 1999; 14: 48-59.
- Lewis SW. Brain imaging in a case of Capgras' syndrome. Br J Psychiatry. 1987; 150: 117-121.
- Edelstyn NM, Oyebode F, Barrett K. The delusions of Capgras and intermetamorphosis in a patient with right-hemisphere white-matter pathology. Psychopathology. 2001; 34: 299-304.
- Seltzer B, Sherwin I. A comparison of clinical features in early- and late-onset primary degenerative dementia. One entity or two? Arch Neurol. 1983; 40: 143-146.
- Hemsley DR. Cognitive disturbance as the link between schizophrenic symptoms and their biological bases. Neurology, Psychiatry and Brain Research. 1994; 2: 163-170.
- Ellis AW, Young AW. Human Cognitive Neuropsychology, chapter 3. Psychology Press. 2004.
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology. 1967; 17: 427-442.
- Folstein M, Folstein S, McHugh P. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975; 12: 189-198.
- Roth M, Huppert FA, Mountjoy CQ, Tym E. CAMDEX-R: The Cambridge Examination for Mental Disorders of the Elderly - Revised. Cambridge: Cambridge University Press. 1998.
- Nelson HE. National adult reading test (NART). Windsor (Berks): NFER-Nelson.1982.
- Reynolds WM, Kobak KA. Hamilton Depression Inventory: A Self-report Version of the Hamilton Depression Rating Scale. Psychological Assessment Resources, Odessa, FL. 1995.
- Fahn S, Elton RL. The Unified Parkinson's Disease Rating Scale. In S. Fahn CD, Marsden DB, Calne M Goldstein, Editors. Recent developments in Parkinson's disease. Florham Park, NJ: Macmillan. 1987; 293-304.
- Warrington EK, James M. The Visual Object and Space Perception Battery. Bury St Edmunds: Thames Valley Test Company. 1991.
- Riddoch MJ, Humphreys GW. Birmingham Object Recognition Battery. Hove: LEA. 1993.
- Jackson DN. Multidimensional Aptitude Battery-II Manual. MI: Sigma Assessment Systems, Inc. Burgess. 1998.
- Farah MJ, Hammond KM, Levine DN, Calvanio R. Visual and spatial mental imagery: dissociable systems of representation. Cogn Psychol. 1988; 20: 439-462.
- Marks DF. Visual imagery differences in the recall of pictures. Br J Psychol. 1973; 64: 17-24.
- Burgess PW, Shallice T. The Hayling and Brixton Tests. Thames Valley Test Co. Ltd: Bury St. Edmunds. 1997.
- Cicerone KD, Lazar RM, Shapiro WR. Effects of frontal lobe lesions on hypothesis sampling during concept formation. Neuropsychologia. 1983; 21: 513-524.
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol Hum Learn. 1980; 6: 174-215.
- Coltheart M, Langdon R, McKay R. Schizophrenia and monothematic delusions. Schizophr Bull. 2007; 33: 642-647.
- Maher B. Delusional thinking and perceptual disorder. Journal of Individual Psychology. 1974; 30: 98-113.
- Garety PA, Freeman D. Cognitive approaches to delusions: a critical review of theories and evidence. British Journal of Clinical Psychology. 1999; 38: 113-154.