
Case Report
A Proteomics. 2015;2(1): 1007.
Proteomics Profiling of Heterozygous and Homozygous Patients with ABCA1 Gene Mutation: A Tangier Disease Molecular
Ucciferri N1, Rocchiccioli S1*, Puntoni M1,2 Bigazzi F2, Cecchettini A1,3, Citti L1 and Sampietro T2
1Institute of Clinical Physiology-CNR Pisa, Italy
2Fondazione Toscana Gabriele Monasterio Pisa, Italy
3Department of Clinical and Experimental Medicine, University of Pisa, Italy
*Corresponding author: Rocchiccioli S, Institute of Clinical Physiology-CNR, Via Moruzzi 1, 56124 Pisa, Italy
Received: November 18, 2014; Accepted: February 04, 2015; Published: February 10, 2015
Abstract
Tangier Disease (TD) is a rare inherited disorder with approximately 100 worldwide identified cases. Alpha-lipoprotein deficiency is the main characteristic of this disease, associated with a virtual absence of High Density Lipoproteins (HDL) in blood. Additional symptoms are mild hypertriglyceridemia, neuropathy and enlarged, orange-colored tonsils. Genetically TD is caused by mutations in the ABCA1 gene, which prevent the release of cholesterol and phospholipids from cells, leading to the accumulation of lipids within cells and body tissues.
In this work a TD patient and his parental heterozygous were examined from a proteomics point of view. Plasma as well as proteome and secretome of circulating monocytes were analyzed.
Plasma proteins underlined in TD the imbalance of lipid trafficking and metabolism, associated with the stimulation of pro-inflammatory pathways. Proteome and secretome of monocytes highlighted an extensive down regulation of mitochondrial enzymes and vesicular trafficking agents along with a substantial cytoskeletal rearrangement, suggesting a reduced activation state of monocytes from TD homozygous patient.
This work is the first proteomics profiling of heterozygous and homozygous TD phenotypes and it suggests a TD case as a model to understand general mechanisms of lipid transport and metabolism and their linkage to inflammatory processes.
Keywords: Tangier disease; Protemics profiling; Monocyte proteomics; Rare disease
Case Presentation
Tangier Disease (TD) is an autosomal recessive genetic disorder, described for the first time in 1961 [1] and characterized by impaired HDL-mediated cholesterol efflux and abnormal intracellular lipid trafficking and turnover. TD patients accumulate cholesterol in body tissues; show a reduced level of HDL, disturbances of nerve functions, premature atherosclerosis and a high incidence of Coronary Artery Disease (CAD). TD is a rare genetic disorder, with less than 100 cases reported in the literature, and patients present both alleles of ABCA1 gene mutated; this gene encodes a member of the ATPBinding Cassette (ABC) transporter family [2, 3]. Carriers of a single ABCA1 mutation (heterozygotes) display an intermediate phenotype with a 50% reduction in the ABCA1-mediated cell cholesterol efflux [4]. Despite the commitment of a unique documented gene, TD is characterized by high variability in phenotypic manifestations, either qualitatively or quantitatively, in terms of clinical severity and organ involvement. In brief there is not a direct correlation between the gene mutation and the numerous and various clinical features described in TD patients.
ABCA1 is the major responsible transporter for clearing cholesterol from macrophages and, since cholesterol accumulation in arterial macrophages is atherogenic, this pathway has a clear involvement in the progression and/or regression of cardiovascular diseases [5]. Due to its central role in the modulation of cholesterol homeostasis, ABCA1 is an attractive target for drug development, but the molecular actors of the ABCA1 lipid pathway are not completely revealed. Studies are needed to understand how lipids are translocated across the plasma membrane and to identify associated proteins that modulate this pathway. Proteomics studies would help to disclose intracellular and secreted factors involved in dysfunction of lipid trafficking and in its clinical manifestation suggesting new targets for therapeutic interventions that might modulate ABCA1 activity assisting the traffic of cholesterol from cells and tissues.
In this paper, proteomics analyses of monocytes and plasma of a TD patient compared with his heterozygous father are reported. The clinical characterization, proband’s kindred and plasma lipoprotein profiles of these two cases have been already published in Sampietro et al. [6] and in Puntoni et al. [7] (a brief description is also presented in the Supplementary Information).
In brief, molecular factors and activation pathways directly responsible of the pathological phenotype are highlighted and the TD case has been suggested as model to obtain insights in possible mechanisms responsible for lipid dysfunctions and eventually, as a consequence, for atherosclerosis initiation.
Proteomics Profiling
TD homozygous proteomics profile was compared with his parental heterozygous (for the ABCA1 mutation). This choice was suggested by the fact that genetic background is akin and both patients are subjected to the same clinical treatments for cardiovascular disease, even if the heterozygous is mildly affected. In this way it was possible to approximate differences in the proteomics profiles due mostly to the different expression of the pathology so as to extrapolate factors involved in dyslipidemia as a main cause of early onset of atherosclerosis.
Using a shot-gun proteomics strategy (for details see Supplementary Information), we were able to identify 197 proteins in depleted plasma samples of both patients (Table S1).
46 plasma proteins (Table 1) were found differentially expressed between TD patient and his heterozygous father. In particular 22 proteins resulted down-regulated and 24 up-regulated in homozygous with respect to heterozygous. Among the downregulated in the homozygous, 13 different apolipoproteins were particularly interesting. In addition, also Paraoxonase 1 has been found down-regulated in homozygous patient plasma. This enzyme has been suggested to be involved in the protection against oxidative modification of lipoproteins and consequently against pivotal events leading to atheroma formation [8,9].These results, although preliminary, confirm that plasma proteomics can evidence the imbalance of lipid trafficking and metabolism in TD, suggesting a distinctive characteristic fingerprint, provided with a panel of correlated elements. Among the differentially expressed proteins some are linked to inflammation, such as Orosomucoid 1 and 2 and Kininogen 1. Kininogen 1 has been found over expressed in plasma of homozygous patient and it seems to be a key mediator of inflammation [10]. On the other hand, Orosomucoid 1 and 2 resulted down-expressed in homozygous plasma and, besides being involved in pro-inflammatory responses [11,12], they also exert a possible role as lipid carrier in blood circulation [13,14]. Moreover and of note, 4 differentially expressed proteins are related to different growth factor pathways: Vasorin may act as inhibitor of TGFbeta signaling [15], Insulin like Growth Factor Binding Protein 4 (IGFBP4) and Insulin-like Growth Factor-Binding Protein Complex Acid Labile Subunits (IGFALS) are key elements of the IGF pathway and Hepatocyte Growth Factor Activator (HGFA) that seems to play role in inflammatory processes [16]. In conclusion, plasma proteome of TD patient proves a general dysregulation of lipid trafficking and metabolism, associated with the stimulation of growth factor and inflammatory pathways.
Accession
Peak Name
Homo vs Hetero
p-value
Log (Fold Change)
Q04756
HGFA_HUMAN
Hepatocyte growth factor activator
DOWN
4.55E-05
0.847184
P02647
APOA1_HUMAN
Apolipoprotein A-I
DOWN
0.00017
0.771506
P02654
APOC1_HUMAN
Apolipoprotein C-I
DOWN
0.00054
0.726025
P00738
HPT_HUMAN
Haptoglobin-relatedprotein
DOWN
0.00072
0.350024
P00915
CAH1_HUMAN
Carbonicanhydrase 1
DOWN
0.00186
0.847092
P02652
APOA2_HUMAN
Apolipoprotein A-II
DOWN
0.00255
0.695098
P27169
PON1_HUMAN
Serum paraoxonase/arylesterase 1 (PON 1)
DOWN
0.00291
0.483498
P02042
HBD_HUMAN
Hemoglobinsubunit delta (Delta-globin)
DOWN
0.00394
0.362336
P00739
HPTR_HUMAN
Haptoglobin (Zonulin)
DOWN
0.00401
0.513322
O75636
FCN3_HUMAN
Ficolin-3 (Collagen/fibrinogen domain-containing lectin 3 p35)
DOWN
0.00597
0.330745
P00746
CFAD_HUMAN
Complement factor D(Adipsin)
DOWN
0.00684
0.503916
O95445
APOM_HUMAN
Apolipoprotein M
DOWN
0.00827
1.142308
P19652
A1AG2_HUMAN
Alpha-1-acid glycoprotein 2 (Orosomucoid-2)
DOWN
0.01005
0.435858
P22792
CPN2_HUMAN
Carboxypeptidase N subunit 2
DOWN
0.01067
0.552475
P35542
SAA4_HUMAN
Serum amyloid A-4 protein
DOWN
0.01249
0.63467
O14791
APOL1_HUMAN
Apolipoprotein L1
DOWN
0.01259
0.567598
P00488
F13A_HUMAN
Coagulation factor XIII A chain
DOWN
0.01438
0.720657
Q6UXB8
PI16_HUMAN
Peptidase inhibitor 16
DOWN
0.01584
1.509586
P02775
CXCL7_HUMAN
Platelet basic protein (PBP)
DOWN
0.02249
0.664629
P05090
APOD_HUMAN
Apolipoprotein D
DOWN
0.02422
0.332828
P02656
APOC3_HUMAN
Apolipoprotein C-III
DOWN
0.02636
0.844092
P80108
PHLD_HUMAN
Phosphatidylinositol-glycan-specific phospholipase D
DOWN
0.02891
0.720058
O43866
CD5L_HUMAN
CD5 antigen-like (IgM-associated peptide)
UP
4.06E-05
-0.6351
P01871
IGHM_HUMAN
Ig mu chain C region
UP
0.00013
-0.52174
Table 1: Differentially expressed proteins in plasma samples.
P02768
ALBU_HUMAN
Serumalbumin
UP
0.0014
-0.50844
P06331
HV209_HUMAN
Ig heavy chain V-II region ARH-77
UP
0.00152
-0.60634
P04208
LV106_HUMAN
Ig lambda chain V-I region WAH
UP
0.00274
-0.87145
P01834
IGKC_HUMAN
Ig kappa chain C region
UP
0.00321
-0.35637
P35858
ALS_HUMAN
Insulin-like growth factor-binding protein complex acid labile subunit (ALS)
UP
0.00506
-0.37728
P01042
KNG1_HUMAN
Kininogen-1 (Alpha-2-thiol proteinase inhibitor)
UP
0.00524
-0.32611
P04430
KV122_HUMAN
Ig kappa chain V-I region BAN
UP
0.00608
-0.61736
P01591
IGJ_HUMAN
Immunoglobulin J chain
UP
0.00997
-1.05921
P01743
HV102_HUMAN
Ig heavy chain V-I region HG3
UP
0.01589
-0.81718
P06316
LV107_HUMAN
Ig lambda chain V-I region BL2
UP
0.01694
-2.00561
P04220
MUCB_HUMAN
Ig mu heavy chain disease protein (BOT)
UP
0.01833
-0.36182
P01876
IGHA1_HUMAN
Ig alpha-1 chain C region
UP
0.01903
-0.30019
P04433
KV309_HUMAN
Ig kappa chain V-III region VG (Fragment)
UP
0.02037
-0.62005
P01880
IGHD_HUMAN
Ig delta chain C region
UP
0.02129
-0.64278
P01859
IGHG2_HUMAN
Ig gamma-2 chain C region
UP
0.02168
-0.64733
P01861
IGHG4_HUMAN
Ig gamma-4 chain C region
UP
0.02441
-0.66096
P18428
LBP_HUMAN
Lipopolysaccharide-bindingprotein
UP
0.02811
-0.69375
P13645
K1C10_HUMAN
Keratin, type I cytoskeletal 10
UP
0.02953
-0.64284
P01857
IGHG1_HUMAN
Ig gamma-1 chain C region
UP
0.03532
-0.37459
P01717
LV403_HUMAN
Ig lambda chain V-IV region Hil
UP
0.03595
-1.08395
P03952
KLKB1_HUMAN
Plasma kallikrein
UP
0.03806
-0.59386
O00187
MASP2_HUMAN
Mannan-binding lectin serine protease 2
UP
0.04597
-1.06298
Table 1 : (1 of 2 )
Although ABCA1 is expressed in many tissues, the accumulation of cholesterol in TD interests mostly macrophages [17]. An early event in atherogenesis is the recruitment of monocytes from the peripheral blood vessel intima as a consequence of high amounts of lipids and high levels of protein oxidation. Monocytes transmigrate into the vessel wall and differentiate into macrophages. Macrophages are well known to exert an important role in atheroprotection/ atheroformation, removing excess of cholesterol from tissues. Reverse Cholesterol Transport (RCT) is a pathway responsible of the transport of accumulated cholesterol from the vessel wall to the liver for excretion, this process preventing inflammation and atherosclerosis [18]. Many studies indicate that the inflammatory process impairs RCT [19]. ABCA1 is the major cholesterol efflux system in macrophages, it plays a crucial role in the modulation of inflammatory response [20] and in mice its knockout increases inflammatory cell infiltration [21]. Monocytes are the circulatory precursors of macrophages and play a central role under several pathophysiological conditions, particularly when inflammatory reactions are involved. For these reasons we were interested in analyzing the proteome and the secretome of monocytes from a TD patient and to compare these profiles with those of the heterozygous father.
Monocyte proteome analysis identified 198 proteins while 128 proteins were found in secretome. Proteins are reported in (Supplementary Data Table S2 and Table S3 respectively). They were classified based on their localization (Figure 1) using Uniprot database (www.uniprot.com), while the secretome proteins were also subdivided, according to the secretion pathway (Figure 2), in classically or non classically secreted, as predicted by the SecretomeP 2.0 Server (https://www.cbs.dtu.dk/services/SecretomeP/).
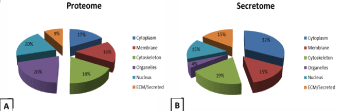
Figure 1: Monocyte proteome (A) and secretome (B). Identified proteins were classified according to their localization.
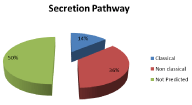
Figure 2: Monocyte secreted proteins classified according to the intracellular
pathways (classically or non classically secreted) as predicted by SecretomeP
Server.
Monocyte whole proteome analysis brought to the identification of 198 proteins, of which, so much as 47 resulted differentially expressed (Table 2). Four principal functional groups may be underlined: a)- cytoskeleton organization (Coronin 1, Profilin 1, Tropomyosin 3, S100A6, Gelsolin, Thymosin beta4, Actin-related protein 3 and Macrophage-capping protein), b)- mitochondrial functions(Superoxide dismutase, Cytochrome C oxidase, ATP synthase beta, Thioredoxin-dependent peroxide reductase, Heat shock cognate 71 protein, Heat shock 60 kDa protein, Heat shock 10 kDa protein, Dihydroprolyl dehydrogenase, Hydroxyacylcoenzyme A dehydrogenase, Trifunctional enzyme subunit beta, Complement component 1 Q subcomponent-binding protein), c)- vesicular trafficking regulation (RAS-related protein Rab-8B, RAS-related protein Rab-8A, RAS-related protein Rab-10, ADPribosylation factor 1) and d)-defense activities (Myeloperoxidase, Cathepsin G, Lysozyme C, Plasma serine protease inhibitor, Alpha-2-macroglobulin). It is interesting to note that all groups are somehow related to monocyte-macrophage transition since differentiation is triggered by mitochondrial functions, induces a dramatic rearrangement of cytoskeleton due to increasing motility and enhances vesicular trafficking for more intense intra- and intercellular interactions, these including also defense strategies.
Accession
Peak Name
Protein name
Homo
vs
Hetero
p-value
Log (Fold Change)
P08311
CATG_HUMAN
Cathepsin G
DOWN
0.00509
-0.81137
O00299
CLIC1_HUMAN
Chlorideintracellularchannelprotein 1
DOWN
0.00429
-0.98615
P06703
S10A6_HUMAN
Protein S100-A6
DOWN
0.03129
-0.71595
Q92930
RAB8B_HUMAN
Ras-related protein Rab-8B
DOWN
0.00329
-1.00065
P61006
RAB8A_HUMAN
Ras-related protein Rab-8A
DOWN
0.02228
-1.4238
P61026
RAB10_HUMAN
Ras-relatedprotein Rab-10
DOWN
0.00993
-0.81693
P11142
HSP7C_HUMAN
Heat shock cognate 71 kDa protein
DOWN
0.0011
-0.61458
P06396
GELS_HUMAN
Gelsolin
DOWN
0.00136
-0.64524
P06753
TPM3_HUMAN
Tropomyosin alpha-3 chain
DOWN
0.03406
-0.55569
P31146
COR1A_HUMAN
Coronin-1A
DOWN
0.01454
-0.54048
P14625
ENPL_HUMAN
Endoplasmin
DOWN
0.00317
-0.63045
P84077
ARF1_HUMAN
ADP-ribosylationfactor 1
DOWN
0.00311
-0.87542
P05164
PERM_HUMAN
Myeloperoxidase
DOWN
0.00178
-0.81517
P08575
PTPRC_HUMAN
Receptor-type tyrosine-protein phosphatase C
DOWN
0.03603
-0.96159
P04179
SODM_HUMAN
Superoxidedismutase [Mn], mitochondrial
DOWN
0.02356
-1.86513
P09622
DLDH_HUMAN
Dihydrolipoyldehydrogenase, mitochondrial
DOWN
0.00493
-1.1493
P10606
COX5B_HUMAN
Cytochrome c oxidasesubunit 5B, mitochondrial
DOWN
0.00089
-1.5417
P10809
CH60_HUMAN
60 kDa heat shock protein, mitochondrial
DOWN
0.00071
-1.05006
P61604
CH10_HUMAN
10 kDa heat shock protein, mitochondrial
DOWN
0.00384
-1.08989
Q16836
HCDH_HUMAN
Hydroxyacyl-coenzyme A dehydrogenase, mitochondrial
DOWN
0.02882
-1.51385
P06576
ATPB_HUMAN
ATP synthase subunit beta, mitochondrial
DOWN
0.02171
-0.65835
P30048
PRDX3_HUMAN
Thioredoxin-dependent peroxide reductase, mitochondrial
DOWN
0.02955
-1.02142
Table 2: Differentially expressed proteins in monocyte proteome samples.
Q13011
ECH1_HUMAN
Delta(3,5)-Delta(2,4)-dienoyl-CoAisomerase, mitochondrial
DOWN
0.0026
-1.13024
P55084
ECHB_HUMAN
Trifunctional enzyme subunit beta, mitochondrial
DOWN
0.00162
-1.0893
Q07021
C1QBP_HUMAN
Complement component 1 Q subcomponent-binding protein, mitochondrial
DOWN
0.01677
-1.11609
Q14240
IF4A2_HUMAN
Eukaryotic initiation factor 4A-II
DOWN
0.02237
-1.11038
P63241
IF5A1_HUMAN
Eukaryotic translation initiation factor 5A-1
DOWN
0.03193
-0.85348
P05154
IPSP_HUMAN
Plasma serine proteaseinhibitor
DOWN
0.02319
-1.1857
P01023
A2MG_HUMAN
Alpha-2-macroglobulin
DOWN
0.01839
-0.85005
P02765
FETUA_HUMAN
Alpha-2-HS-glycoprotein
DOWN
0.00435
-1.34102
P61626
LYSC_HUMAN
Lysozyme C
DOWN
0.01812
-1.54991
P04264
K2C1_HUMAN
Keratin, type II cytoskeletal 1
UP
0.00435
0.773886
P60174
TPIS_HUMAN
Triosephosphateisomerase
UP
0.04817
0.537814
P00558
PGK1_HUMAN
Phosphoglyceratekinase 1
UP
0.00521
0.614203
P40121
CAPG_HUMAN
Macrophage-cappingprotein
UP
0.03962
0.815598
P00488
F13A_HUMAN
Coagulation factor XIII A chain
UP
0.0026
0.830138
P13647
K2C5_HUMAN
Keratin, type II cytoskeletal 5
UP
0.031
0.954622
Q9NZT1
CALL5_HUMAN
Calmodulin-likeprotein 5
UP
0.01543
0.843285
P07737
PROF1_HUMAN
Profilin-1
UP
0.0115
0.177598
P35527
K1C9_HUMAN
Keratin, type I cytoskeletal 9
UP
0.00304
1.021316
P62328
TYB4_HUMAN
Thymosin beta-4
UP
0.00614
1.665154
P61158
ARP3_HUMAN
Actin-relatedprotein 3
UP
0.011
0.967938
P08514
ITA2B_HUMAN
Integrinalpha-IIb
UP
0.03896
1.792178
P26447
S10A4_HUMAN
Protein S100-A4
UP
0.0369
2.142443
Q9HBT7
ZN287_HUMAN
Zinc finger protein 287
UP
0.02335
1.781582
Table 2 : (1 of 2 )
Indeed, mitochondrial functions produce Reactive Oxygen Species (ROS), which are recognized triggers of monocyte activation [22]. Normally, the diffusion of ROS and damaged mitochondrial contents into the cytosol is prevented by autophagy. During autophagy cytosolic constituents are enclosed within a double-layered lipid vesicle addressed to fuse with lysosomes for degradation and recycling of the internal contents. Impaired autophagy may interfere with mitochondrial turnover [23].
In brief, the extensive down regulation of mitochondrial enzymes and vesicular trafficking agents along with cytoskeletal and defense proteins in monocyte proteome (Figure 3) suggest a reduced activation ability of monocytes from TD homozygous patient.
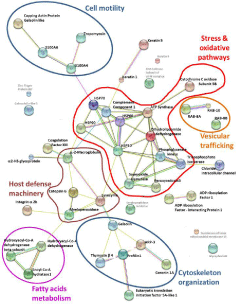
Figure 3: Interaction network of differentially expressed proteins in monocyte
proteome. Top scoring and significant proteins were submitted to String
database and resulted image was adapted.
As expected, a lower number of proteins were identified in the secretome (128) and of these only 16 resulted differentially released within 24 h (Table 3). The majority (8 out of 16) are actin binding or related factors, underlining a reassessment of monocyte cytoskeleton.
Accession
Peak Name
Protein Name
Homo
vs
Hetero
p-value
Log (Fold Change)
P61160
ARP2_HUMAN
Actin-relatedprotein 2
DOWN
0.00059
-0.73265
P31146
COR1A_HUMAN
Coronin-1A
DOWN
0.02114
-0.39996
Q9Y490
TLN1_HUMAN
Talin-1
DOWN
0.02877
-0.32292
P00558
PGK1_HUMAN
Phosphoglyceratekinase 1
DOWN
0.02932
-0.41883
P07996
TSP1_HUMAN
Thrombospondin-1
DOWN
0.04812
-0.65607
P07437
TBB5_HUMAN
Tubulin beta chain
DOWN
0.04965
-0.35121
P62937
PPIA_HUMAN
Peptidyl-prolylcis-trans isomerase A
UP
0.00154
0.465786
O15145
ARPC3_HUMAN
Actin-relatedprotein 3
UP
0.0023
0.687822
P60842
IF4A1_HUMAN
Eukaryotic initiation factor 4A-I
UP
0.00544
0.403022
P60660
MYL6_HUMAN
Myosin light polypeptide 6
UP
0.00622
0.860975
Q01518
CAP1_HUMAN
Adenylylcyclase-associatedprotein 1
UP
0.01958
0.294693
Q9NY33
DPP3_HUMAN
Dipeptidylpeptidase 3
UP
0.02472
0.360369
P32119
UP
0.02762
0.540569
P50395
UP
0.02791
0.965144
P61978
HNRPK_HUMAN
Heterogeneousnuclearribonucleoprotein K
UP
0.03703
0.474348
P08670
VIME_HUMAN
Vimentin
UP
0.04041
0.285031
Table 3: Differentially expressed proteins in monocyte secretome samples.
Conclusion
In conclusion, it was possible to obtain a preliminary plasma fingerprint and a monocyte molecular map of Tangier disease. This study could be preparatory for deeper and more specific analyses that could help to understand general mechanisms of lipid transport and metabolism and their linkage to inflammatory processes.
Limitation of this work is the difficulty to establish if the results obtained with a TD patient can be extrapolated to other TD patients, due to the infrequence of this genetic disorder. Nevertheless, this case can be exploited as model for the study of general, common mechanisms in cell biology.
References
- Fredrickson DS, Altrocehi PH, Avioli LV, Goodman DWS, Goodman HC. Tangier disease. Combined clinical staff conference at the National Institutes of Health. Ann Intern Med. 1961; 55: 1016–1031.
- Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, et al. Mutations in ABC1 in Tangier disease and familial highdensity lipoprotein deficiency. Nat Genet. 1999; 22: 336–345.
- Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, et al. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet. 1999; 22: 352–355.
- Brousseau ME, Schaefer EJ, Dupuis J, Eustace B, Van Eerdewegh P, Goldkamp AL, et al. Novel mutations in the gene encoding ATP binding cassette 1 in four tangier disease kindreds. J. Lipid Res. 2000; 41: 433–441.
- Oram JF. Molecular basis of cholesterol homeostasis: lessons from Tangier disease and ABCA1. Trends Mol Med. 2002; 8: 168-173.
- Sampietro T, Puntoni M, Bigazzi F, Pennato B, Sbrana F, Dal Pino B, et al. Tangier Disease in Severely Progressive Coronary and Peripheral Artery Disease. Circulation. 2009; 119: 2741-2742.
- Puntoni M, Sbrana F, BigazziF, Sampietro T. TangierDisease. Epidemiology, Pathophysiology, and Management. Am J Cardiovasc Drugs. 2012; 12: 303-311.
- Aviram M, Rosenblat M. Paraoxonases 1, 2 and 3 oxidative stress, and macrophage foam cell formation during atherosclerosis development. Free Radical Biology & Medicine. 2004; 37: 1304–1316.
- Mackness B, Quarck R, Verreth W, Mackness M, Holvoet P. Human Paraoxonase-1 Over expression Inhibits Atherosclerosis in a Mouse Model of Metabolic Syndrome.ArteriosclerThrombVascBiol. 2006; 26: 1545-1550.
- Sainz IM, Isordia-Salas I, Castaneda JL, Agelan A, Liu B, DeLaCadena RA, et al. Balfour Sartor RB, Colman RW. Modulation of Inflammation by Kininogen Deficiency in a Rat Model of Inflammatory Arthritis. Arthritis & Rheumatism. 2005; 52: 2549–2552.
- Hochepied T, Berger FG, Baumann H, Liber C. a1-Acid glycoprotein: an acute phase protein with inflammatory and immunomodulating properties. Cytokine & Growth Factor Reviews. 2003; 14: 25–34.
- Kim Y, Huh JY, Koh YJ, Koh GY, Son HJ, Masuzaki H, et al. AdipocytokineOrosomucoid Integrates Inflammatory and Metabolic Signals to Preserve Energy Homeostasis by Resolving Immoderate Inflammation. J BiolChem. 2010; 285: 22174–22185.
- Ojala PJ, Hermansson M, Tolvanen M, Polvinen K, Hirvonen T, Impola U, et al. Identification of alpha-1 acid glycoprotein as a lysophospholipid binding protein: a complementary role to albumin in the scavenging of lysophosphatidylcholine.Biochemistry. 2006; 45: 14021–14031.
- Zsila F, Mady G. Biliverdin is the endogenous ligand of human serum alpha1-acid glycoprotein. Biochem Biophys Res Commun. 2008; 372: 503–507.
- Ikeda Y, Imai Y, Kumagai H, Nosaka T, Morikawa Y, Hisaoka T, et al. Vasorin, a transforming growth factor beta-binding protein expressed in vascular smooth muscle cells, modulates the arterial response to injury in vivo. PNAS. 2004; 101: 10732–10737.
- Kataoka H, Kawaguchi M. Hepatocyte Growth Factor Activator (HGFA): pathophysiological functions in vivo. FEBS Journal. 2010; 277: 2230-2377.
- Assmann G, von Eckardstein A, Brewer HB. Familial high density lipoprotein deficiency: Tangier disease. Scriver CA, Beaudet AL, Sly WS, Valle D, editors. In: The metabolic and molecular bases of inherited disease. 7th edn, New York, USA: McGraw-Hill. 1995; 2053–2072.
- Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res. 2009; 50: 189-194.
- McGillicuddy FC, de la LleraMoya M, Hinkle CC, Joshi MR, Chiquoine EH, Billheimer JT, et al. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009; 119: 1135-1145.
- Tang C, Liu Y, Kessler PS, Vaughan AM, Oram JF. The macrophage cholesterol exporter ABCA1 functions as an anti-inflammatory receptor. J BiolChem. 2009; 284: 32336-32343.
- Aiello RJ, Brees D, Francone OL. ABCA1-deficient mice: insights into the role of monocyte lipid efflux in HDL formation and inflammation. ArteriosclerThrombVascBiol. 2003; 23: 972-980.
- Zhang Y, Choksi S, Chen K, Pobezinskaya Y, Linnoila I, Liu Z-G.ROS play a critical role in the differentiation of alternativelyactivated macrophages and the occurrence of tumor-associated macrophages. Cell Research. 2013; 23: 898-914.
- Zhang Y, Morgan MJ, Chen K, Choksi S, Liu Z-g. Induction of autophagy is essential for monocyte-macrophage differentiation. Blood. 2012; 119: 2895-2905.