
Research Article
Phys Med Rehabil Int. 2018; 5(5): 1157.
Efficacy of Dietary Sugarcane Product on Bowel Function and Blood Sugar Level in Adult Diabetic Patients: A Randomised Controlled Trial
Amatya B1,2,3*, Lee SY1,2,3*, Elmalik A1,2,3*, Lowe M1,2, Khan F1,2,3
¹Department of Rehabilitation Medicine, Royal Melbourne Hospital, Parkville, Victoria, Australia
²Australian Rehabilitation, Research Centre, Royal Melbourne Hospital, Parkville, Victoria, Australia
³Department of Medicine (Royal Melbourne Hospital), University of Melbourne, Parkville, Victoria, Australia
*Corresponding author: Bhasker Amatya, Department of Rehabilitation Medicine, Royal Melbourne Hospital, 34-54 Poplar Road, Parkville, Victoria 3052, Australia
Received: September 24, 2018; Accepted: October 24, 2018; Published: October 31, 2018
Abstract
Objectives: To assess effectiveness of sugarcane-based food product (NutriKane-DTM) in improving blood sugar level control and bowel dysfunction in adult diabetic subjects in rehabilitation settings.
Methods: Consecutive diabetic patients (n=51) admitted to a tertiary inpatient rehabilitation unit randomized to either an intervention group (n=25) who received additional NutriKane-D™ or the control group (n=26) (usual treatment). Assessments were at admission (T0), discharge (T1) and 3-months post-discharge (T2) using validated measures: Functional Independence Measure (FIM), Appraisal of Diabetes Scale (ADS) and Diabetes Health Profile (DHP).
Results: Mean age of participants’ was 68.9±12.7 (range 25.6-91.8) years, 69% were male, most had musculoskeletal dysfunction. At discharge (T1), both groups showed significant improvement in their everyday function (FIM), which maintained at 3-months post-discharge (T2). At T1, compared with controls, the intervention group showed greater adjustment in their management of diabetes (ADS, p <0.001, r = 0.7) and psychosocial adjustment (DHP) - activity subscales scores (p <0.001, r = 0.7). At T2, compared with controls, the intervention group (72%) reported either stable and/or improved bowel function, maintained significant improvement in ADS scores (p <0.001, r = 0.7) and DHP activity subscales (p <0.05, r = 0.6). Although there was a trend in reduction of blood glucose level in the intervention group (compared with controls), it did not reach significance. No between-groups difference was noted for other subscales.
Conclusion: Sugarcane-based food supplements may improve diabetic and bowel management issues in patients in rehabilitation settings. Further research is needed for longer-term outcomes with larger cohorts.
Keywords: Rehabilitation; Diabetes; Dietary supplement; Bowel function
Introduction
Consumption of natural products from whole plant sources including gluten-free grains, soy, vegetables such as sugarcane varieties (sucrose removed), improve intestinal and digestive health [1-4]. Sugarcane fibre reportedly lowers the Glycaemic Index (GI) of most carbohydrate classes, using the Australian standard method of calculation (when taken with a meal) [1]. One locally available food product – NutriKane™, high in micronutrients (including essential trace elements, polyphenolic and flavonoid antioxidants), and high quality fibre (soluble/ insoluble, and resistant starch), is reported to be effective in improving intestinal and digestive health of patients admitted to sub-acute care-settings [5,6]. This product is high in bioavailable chromium, a micronutrient essential in the body’s ability to process blood glucose. It is a component in co-factor Chromodulin which facilitates insulin binding to its receptor [7-11]. Additionally, NutriKane-D™ inhibits the growth of pro-inflammatory bacteria, whilst promoting growth of probiotic bacteria and increasing production of short chain fatty acids [12].
Dietary product processed from sugarcane has been linked to improved blood glucose management due to its natural resistant starches found in red sorghum [2,13]. A number of small case studies (and anecdotal data) suggest that NutriKane-D™ may improve blood glucose level (BSL) [1,14]. It can be an option as a natural alternative for a low carbohydrate/low intestinal diet. Previously, NutriKane-D™ product was found to be an effective component of overall intestinal health strategy [5,6], approved by Food Standards Australia and New Zealand, and available in the local market. The objective of this study was to evaluate beneficial effects of this dietary product NutriKane-D™, on BSL management and digestive health compared with usual care in adult diabetic mellitus (DM) patients admitted to a tertiary rehabilitation unit.
Methods
Setting
This study was conducted in the Rehabilitation Unit at the Royal Melbourne Hospital (RMH), a tertiary referral centre in Victoria, Australia (HREC approval number: 2015.312). This service has medically supervised 40-beds with an active ambulatory program (including a community therapy and domiciliary rehabilitation services, specialist outpatient clinics etc.). It specializes in neurological, cancer, musculoskeletal, amputee and pain rehabilitation. The rehabilitation unit has well-established interdisciplinary rehabilitation programs for complex disabilities (including bowel and bladder programs, DM management) in line with high-quality policies, procedures, and practices.
Participants
All patients consecutively admitted to the rehabilitation ward who met inclusion criteria, were eligible to participate in the study. The inclusion criteria were: adults (over 18 years) with medically documented DM (Type 1 or 2: ICD-10 Codes: E10-E11), ability to communicate and understand English; able to provide informed consent and the clinical judgment of the admitting rehabilitation physician that the program would probably be beneficial for the individual. Patients were excluded if they were already on sugarcanebased food supplements (including NutriKane), unable to consume solid fibrous matter and those with severe cognitive issues, unstable medical, neurological or psychiatric disorders, and/or were pregnant.
Patients who met the inclusion criteria were invited to participate in this study by a researcher, who explained the study in detail. Those providing signed consent (signed by the patient or by the patient’s legally acceptable representative) were recruited (February 2016 to March 2017). They were able to withdraw from the study at any time. The participant diagnostic subgroups included various musculoskeletal and/or neurological conditions.
Procedure
Randomization: As part of routine practice every patient admitted to the rehabilitation ward underwent clinical assessments including diabetes status, bladder/bowel dysfunction, pain, personal care needs and functional ability by the rehabilitation team. Following initial assessments, all patients were screened for eligibility and invited to participate in the study by an independent medical practitioner. Those providing a written consent were recruited and assigned a study-identification number. All participants underwent baseline-structured interview (T0) conducted by an independent clinician (medical) using standardized instruments (see measures). An independent project officer used a computer-generated block randomization to allocate participants to either the control (routine care) or treatment groups (NutriKane-D™) in a 1:1 ratio. Opaque sealed envelopes for concealed allocation ensured approximate balance between the treatment and control groups. Accessors were blinded to the participant group allocation. Those in the control group were managed with routine care (detailed in intervention section below). The treating therapy teams treated all patients on the ward based on clinical need, consistent with standard practice.
Intervention: The interdisciplinary rehabilitation program at the RMH incorporates medical, nursing and allied health (physiotherapy, occupational therapy, speech therapy, social work, dietetics, neuropsychology) input, tailored to promote patient education, selfmanagement skills and functional independence. DM is one of the common co-morbidity in the rehabilitation unit. The team promotes open communication and monitors relevant outcomes such as patient ability to identify symptoms (of hypo- and hyperglycaemia etc.), compliance with food and medication, blood sugar checks, exercise and maximizing function (mobility, activities of daily living); and addressing factors relating to participation. As per standard hospital protocol, those with DM also receive regular input from endocrinologists, dieticians and diabetes educators/nurses. The duration, content and outcome of the rehabilitation program were documented daily.
All participants in the treatment group received an oral nutritional supplement (NutriKane-D™) (Appendix 1), administered twice daily by the registered nurse on the ward, in addition to usual interdisciplinary care. While the participants in the control group received usual interdisciplinary care as per routine ward practice. Compliance with program and adverse effects were recorded daily from the medical charts, product-accountability records maintained and cross-checked by investigators. NutriKane-D™ was supplied by the Medikane Pty Ltd. The investigators and approved representative (pharmacist) ensured secure storage (at room temperature) on the ward. At discharge, participants in the intervention group were provided with required stock of the product to take home (total of 90 days including inpatient days), with detailed product information. A dedicated phone number was made available to all study participants, five days a week (from 9 am to 5 pm) to address any questions or concerns from patients (and caregivers). A research assistant phoned the intervention group participants once every week to remind them of the nutritional supplements, bowel and DM management regime. Overall compliance (including inpatient and at participants’ home for 90 days) was set as consumption at least 80% of the product.
Assessments: A face-to-face structured interview technique was used for assessments using standardized instruments (see Measures section). Assessment time points were at admission (T0), on discharge from the ward (T1) and 3-months post-discharge (by telephone) (T2). All outcome assessments were completed by independent assessors (rehabilitation physicians and research officer) who received 3 halfday training sessions in assessment and data collection. They did not share information about participants or assessments, and received separate case report forms at each interview. These assessments took approximately 30 minutes. The assessors did not prompt patients, but provided assistance for those who had difficulty with completing the questionnaires. The study comprised the following phases:
Baseline assessment (at admission) (T0) - assessments collated within 24 hours of admission to the service. It included demographics (age, gender, marital status, education, employment), diseaserelated characteristics (diagnosis, symptoms, medications and comorbidities) and assessments using standardized instruments. As per standard hospital protocols BSL were monitored, on the ward and glycosylated hemoglobin (HbA1c) was noted (see measurement section below). Any patient concerns/comments were captured in an open-ended questionnaire.
Assessment at discharge (T1): The same tools at T0 were utilized and final BSL were analysed, (see statistics section below). Adverse events during rehabilitation (such as falls, injury during treatment, hypoglycaemia etc.) were noted. A log book was provided to follow the same pattern of BSL monitoring at home.
Assessment at 3-months following discharge (T2): An independent blinded research officer conducted a telephone follow-up of all participants who had completed both T0 and T1 interviews. The information obtained was similar to the T1 and final BSL reported by participants, (including any HbA1c values obtained in the community) following discharge. The assessor did not have access to previous assessments, treatment schedules or treating rehabilitation therapy team documentation. Review of the blood monitoring log book for the BSL (including daily blood glucose profile) for the previous three days was obtained.
All assessments were secured and filed, and opened only at the time of data entry into a special study database by an independent data entry officer.
Measurement
Participant demographic and clinical characteristics: This included: demographic information (age, gender, marital status, education level, employment); premorbid history (BMI, alcohol use, conditions and limitations, psychiatric history, and other major co-morbidities); disease-related information (diagnosis, date of diagnosis, type, complication,) and DM-related information (DM diagnosis, DM type/duration, treatment status including medications, adverse effects and complications).
Bowel assessment: Clinical assessment of bowel dysfunction was initially completed by admitting ward medical officer or nurse, consistent with existing practice. A research medical officer (independent of the ward or treating team) then completed further assessment using standardized instruments at admission (within 48 hours of admission and discharge). Any issues/ participant comments were captured in an open- ended questionnaire in data collection forms. Further, the following validated measures were used. The Bristol stool chart [15] determined stool consistency, patterns or changes in bowel habit and the effectiveness of treatment. The stool was categorized in 7 different types (types 1 and 2 indicate constipation, 3 and 4 being the “ideal stools”, and 5–7 tend towards diarrhea).
The Wexner Faecal Incontinence Score (WFIS) [16] measured faecal incontinence and symptom severity. The score was derived from a rating of frequency of the type of incontinence and whether the participant’s lifestyle was altered by incontinence (0=no incontinence or impact, 20 = worse possible incontinence and impact).
Activity limitation: The Functional Independence Measure (FIM) [17] assessed function (activity) and need for assistance. The patient dependency was measured in the 13 items: Self-care, Transfers, Locomotion, Sphincter control and Cognition subscales.
DM related measure: HbA1c and daily BSL (as per routine ward practice) were collected in the ward. Relevant DM-related information (medications, current symptoms, adverse events etc.) were noted.
The Appraisal of Diabetes Scale (ADS) [18]: a self-report 7-item questionnaire assessed individual’s thoughts about coping with DM. The items included: distress, control, uncertainty, anticipated future deterioration, coping, and effect on life goals on a 5-point adjectival scale scored (1= none at all to 5 total amount). The scores were summed to produce a score from 0-35 (0 representing the least and 35 the greatest impact of DM).
The Diabetes Health Profile (DHP-18) [19], comprised 18 items for 3 dimensions: psychological distress (6 items: dysphoric mood, feelings of hopelessness, irritability), barriers to activity (7 items: perceived limitation to activity, operant anxiety), and disinhibited eating (5 items: lack of eating control, response to food cues and emotional arousal). Each item has a four-point adjectival scale; items are summed within the three dimensions and transformed to produce a score from 0-100 (where 0 represents no dysfunction).
Statistical analysis
The SPSS Version 21 (IBM SPSS Products, USA) was used for all statistical analyses. Data on participant demographics, diseasecharacteristics was presented with descriptive numbers and percentages. There are no minimal clinically important differences (MCIDs) available to determine study power to demonstrate the superiority of Nutrikane-D™ compared with usual care regarding mean change in HbA1c from baseline to 3 months between the intervention and control groups. It was envisaged that this pilot study can be used to power a future larger trial. Comparisons of demographic and clinical characteristics between groups were conducted using univariate analyses of variance for continuous variables and likelihood ratio based ?2 test for symmetry and marginal homogeneity for categorical measures.
Primary endpoint was the mean change in HbA1c from baseline (T0) to 3 months (T2) and/or change in average 3-days blood sugar level from T0 to discharge T1 and T2. Change in BSLs (for each blood sugar profile) was based on the mean value of first 3 days of admission, last 3 days before discharge and last 3 days before the 3-months follow-up. Given the nature of the data, nonparametric statistical techniques compared change scores (T0 – T1, T0 – T2) for the control and treatment groups. Clinically important changes were estimated as effect sizes (ES, r), using Cohen’s criteria (0.2 = small, 0.5 = medium, 0.8 = large effect) [20,21]. Analysis of differences (within and between treatments) was performed using Wilcoxon’s matched-pair signed-rank test regarding BMI, and the blood sample measurements. Descriptive statistics was generated for each scale in the study. The level of significance was set at ‘p value’ of <0.05 (2-sided).
Results
Of the 82 diabetics patients admitted to the rehabilitation unit during the study period, 51 patients agreed to participate and provided written consent. Of these, 25 were allocated to the treatment group to receive additional NutriKane-D™ supplement and 26 to the control group, who received usual care. Three participants in the control group were lost to follow-up (1 deceased and 2 uncontactable) at the 3-month follow-up assessment (T2) (Figure 1). The median length of inpatient stay was 17 days (inter quartile range (IQR) 12.5 – 27 days). There was 94% compliance with treatment program in the intervention, as per the a priori compliance definition. No adverse events were reported in either group.
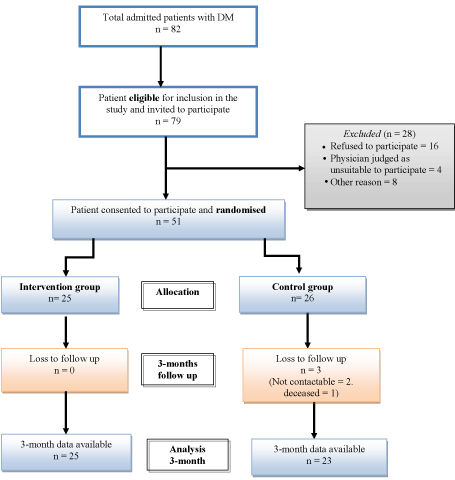
Figure 1: Flow chart of recruitment process.
Baseline characteristics
The baseline participant characteristics s are summarised in Table 1. Both groups were well-matched for demographic and clinical characteristics. Participants’ mean age was 68.9±12.7 (range 25.6 - 91.8) years, majority were caucasian (86%) and male (n= 35, 69%). The main diagnosis in both groups was musculoskeletal conditions, with hypertension as a predominant comorbidity and 84% ‘at risk’ of falls. The control group had slightly longer DM disease duration (mean (M) = 16.7±16.0 years) compared with the treatment group (M = 10.8±9.3 years), this however, was not statistically significant. The intervention group had greater body mass index (BMI) than controls, but was not statistically significant. The blood glucose profiles and HbAc1 were comparable between both groups. There was no significant difference between participants lost to followup and those who provided 3 months follow-up results in terms of gender, age, disease duration and median scores for measures used.
Intervention group
(n = 25)
Control group
(n = 26)
Characterisitics
n, (%)(unless stated different)
P value
Demographic factors
Age (years) [Mean±SD (range)]
66.8±12.0 (25.6-83.8)
70.9±13.2(41.5-91.7)
0.153
Male
17 (68.0)
18 (69.2)
1.000
Ethnicity – Caucasian
20 (80.0)
24 (92.3)
0.366
Living with
Alone
8 (32.0)
9 (34.6)
0.586
Partner/Family
16 (64.0)
17 (65.4)
Education
Secondary
16 (64.0)
15 (57.7)
0.890
Tertiary
6 (24.0)
7 (26.9)
Retired
17 (68.0)
20 (76.9)
0.744
Clinical characterisitics
Main Diagnosis
Orthpaedic
16 (64.0)
19 (73.1)
0.524
Neurological conditions
6 (24.0)
4 (15.4)
Others
3 (12.0)
3 (11.5)
LOS - inpatient (days) [Mean±SD (range)]
21.48±12.8 (4-54)
21.6±16.2 (6-75)
0.974
DM Type I
2 (8.0)
7 (26.9)
0.076
DM Type II
23 (92.0)
19 (73.1)
10.8±9.3 (1-34)
16.7±16.0 (1-49)
0.122
On DM medication
21 (84.0)
23 (88.5)
0.703
Body weight (Kg)[Mean±SD]
92.3 (27.3)
88.0 (23.7)
0.540
BMI [Mean±SD]
32.2 (9.0)
30.1 (6.6)
0.361
Co-morbidities
Hypertension
18 (72.0)
18 (69.2)
1.000
IHD
7 (28.0)
6 (23.1)
0.755
Depression
4 (16.0)
3 (11.5)
0.703
On opioids
12 (48.0)
18 (69.2)
0.124
Impairments/symptoms
Visual
15 (60.0)
15 (57.7)
0.663
Hearing
4 (16.0)
9 (34.6)
0.145
Cognition
1 (4.0)
2 (7.6)
0.612
Sensory
5 (20.0)
6 (23.1)
0.855
Speech
2 (8.0)
1 (3.8)
0.610
Falls risk
23 (92.0)
22 (84.6)
0.160
DVT risk
18 (72.0)
19 (73.1)
0.103
BMI: Body Mass Index; DM: Diabetes Mellitus; DVT: Deep Vein Trhombosis; IHD: Ishamic Heart Disease; LOS: Length of Stay; n: Total number; SD: Standard Deviation.
Table 1: Socio-demographic characteristics of participants (n = 51).
Bowel characteristics of participants
The bowel and bladder characteristics of study participants are shown in Table 2. There were no significant differences in participantreported bowel dysfunction at baseline (T0), with majority in both groups considered their problem ‘mild to moderate’. Constipation was the most commonly reported bowel dysfunction followed by diarrhoea. Over half in both groups took oral laxative medication for bowel dysfunction. One-third in both groups were on high fibre diet and half drank >2 litres of fluid per day. At admission, higher number of participants in the intervention group (28%) reported constipation (Bristol stool chart showed Type 1 and 2) compared with 15% of controls, this however, was not statistically significant (Table 2).
Characterisitics
Intervention group
(n = 25)
Control group
(n = 26)
n, (%) (unless stated different)
P value
Bowel dysfunction
0.375
Mild
4 (16.0)
4 (15.4)
Moderate
6 (24.0)
5 (19.2)
Severe
0
3 (11.5)
Bowel dysfunction type
0.144
Constipation
7 (28.0)
11 (42.3)
Diarrohea
3 (12.0)
1 (3.8)
On bowel medication
13 (52.0)
17 (65.4)
0.400
Senna
13 (52.0)
15 (57.7)
0.781
Coloxyl
12 (48.0)
17 (65.4)
0.264
Fibre
0
1 (3.8)
0.490
Special diet (high fibre/high fat)
8 (32.0)
8 (34.6)
0.510
Fluid intake (>2 litre)
7 (28.0)
13 (50.0)
0.110
Bristol stool chart
0.120
Constipatiobn (Type 1-2)
7 (28.0)
4 (15.4)
Diarrohea (Type 5 - 8)
2 (8.0)
4 (15.4)
Bladder Dysfunction
5 (20.0)
8 (30.8)
0.663
Incontinence
3 (12.0)
4 (15.4)
0.520
Urinary tract infection
0
1 (3.8)
Table 2: Bowel and bladder charecteristics of participants (n = 51).
Outcome measurements change scores
Summary data for all outcome measures at different time periods are provided in Table 3.
Scales
Intervention group(Md, IQR)
Control group(Md, IQR)
Mann-Whitney U
T0
(baseline)
T1
(discharge)
T2
(3-month)
T0
(baseline)
T1
(discharge)
T2
(3-month)
Z values^
Effect size^
T1-T0
T2-T0
T1-T0
T2-T0
WFIS (0 - 20)#
4 (1, 7.5)
2 (0, 4)
NA
2 (0.8,4)
0 (0, 3)
NA
-0.584
NA
0.08
NA
Body weight (kg)
90.0 (70.5, 114.5)
91.0 (70, 114)
92.0 (74.0, 105.0)
88.3 (66.5, 106.3)
85.4 (67.5, 107.0)
92.0 (67.0, 104.0)
-0.42
-1.84
0.06
0.26
BMI (kg/m2)
30.7 (25.7, 39.7)
31.0 (25.0, 38.5)
30.0 (24.2, 34.8)
29.7 (24.3, 35.9)
29.7 (24.4, 36.0)
29.7 (25.3, 35.4)
-0.22
-1.25
0.03
0.18
HbA1c (%)^^
6.8 (6.2, 8.7)
NA
7.5 (6.0, 7.6)
7.1 (6.0, 8.2)
NA
7.5 (6.0, 7.6)
NA
-1.15
NA
0.16
BSL (3 days average)
6.9 (6.4, 10.0)
6.9 (5.8, 7.9)
6.5 (5.7, 7.8)
7.5 (6.7, 9.3)
7.1 (6.2, 8.3)
7.8 (6.4, 8.0)
-0.21
-0.80
0.03
0.11
Single item QoL (0 - 6)
2 (1,4)
1 (1,1)
1 (1,2)
1 (1,3.3)
1 (1, 2)
1 (0, 2)
-1.80
-0.65
0.25
0.09
FIM Motor
Total (13-91)
55 (41.5, 65)
79 (75.5, 82)
77 (67.5, 83)
55 (41.5, 65)
76 (67, 81)
75 (65, 79)
-0.29
-0.52
0.04
0.07
Self-care (6-42)
19 (27, 33)
39 (36.5, 40)
38 (33, 39.5)
25 (18, 31)
37.5 (30, 39.3)
36 (31, 38)
-0.491
-0.51
0.07
0.07
Sphincter control(2.14)
12 (11,13)
13 (13, 13)
12 (12, 14)
11 (6,13)
13 (12, 13)
13 (12, 14)
-0.923
-1.03
0.13
0.15
Locomotion (2-14)
3 (2,6.5)
11 (7, 12)
10 (6.5, 12)
6 (3,6)
7.5 (7, 12)
9 (7, 10)
-0.73
-0.91
0.10
0.13
Mobility (3-21)
12 (9,15.5)
18 (18, 19)
18 (15, 18)
13 (9,15)
18 (18, 19)
18 (15, 18)
-0.15
-0.09
0.02
0.01
FIM Cognition
Total (5-35)
35 (32. 35)
35 (33.5, 35)
34 (32, 35)
33.5 (30, 35)
35 (31.8, 35)
35 (31, 35)
-1.64
-1.74
0.23
0.25
Communication (2-14)
14 (14,14)
14 (14, 14)
14 (13, 14)
13 (10,14)
14 (14, 14)
14 (13, 14)
-1.47
-0.943
0.21
0.13
Psychosocial (1-7)
7 (6, 7)
7 (6, 7)
6 (5.5, 7)
5 (5,6)
7 (6.8, 7)
7 (6, 7)
-1.49
-1.42
0.21
0.20
Cognition (2-14)
14 (13, 14)
14 (13, 14)
14 (12.5, 14)
13 (11.5, 13)
14 (12, 14)
14 (12, 14)
-0.95
-0.95
0.13
0.14
ADS (7-35)
21 (19,22)
14 (10.5, 19)
13 (10, 18.5)
13 (11,17)
13 (11,17)
12 (11, 16)
-5.19**
-4.61**
0.73
0.66
DHP-18 Psycho (0-18)
8 (6, 10)
18 (13, 28)
15 (11.5, 23. 5)
5 (4, 7)
17 (12, 23)
15 (11, 25)
-0.99
-1.50
0.14
0.21
Activity (0-21)
7 (6.5, 8.5)
4 (3,5.5)
4 (3, 5)
4 (3, 6)
4 (3, 5)
3 (3, 6)
-4.69**
-3.95**
0.66
0.56
Eating (0-15)
1 (0. 3.5)
1 (0. 3.5)
1 (0. 3)
1 (0. 3)
1 (0. 3)
1 (0. 3)
-0.59
-0.06
0.08
0.01
*Correlation significant at the 0.05 level (2-tailed); ** Correlation significant at the 0.01 level (2-tailed),
^All scores with significant correlation and moderate to large effect size are bolded.
Efffect size are assessed based on Cohen’s criteria: 0.1- 0.2 = small, 0.3 -0.4 = medium, = 0.5 = large) (Pallant J 2013; Cohen J 1998),
# not assessed at 3-month follow-up (T2), as requires face-to-face assessments.
^^ HbA1c test not tested at discharge (T1) as it is recommneded only every 3-months (T2, n=33),
ADS: Appraisal of Diabetes Scale; BMI: body mass index; DHP-18: Diabetes Health Profile; ES: Effect Size; FIM: Functional Independent Measure; HbA1c: Glycated Haemoglobin; IQR: Interquartile Range; Md: Median; n: Total number; WFIS: Wexner Faecal Incontinence Score.
Table 3: Summary of per protocol analysis of outcomes.
Short-Term Outcomes (at discharge - T1): As expected, both group showed significant improvement in activities from baseline at discharge (T1), with no statistically significant difference (FIM subscales). At discharge (T1), compare with controls, the intervention group showed significant improvement in bowel function (WFIS scores) and impact of faecal dysfunction on quality of life (QoL) (single item scale), however, both did not reach significance level (p = 0.572 and 0.071, respectively). A significant difference between groups in favour of the intervention group was seen in ADS total score (p <0.001) with moderate effect size (r = 0.7) indicating greater adjustment to DM or risk for noncompliance with a care regimen, and in DHP activity subscales scores (p <0.001, r = 0.7), signifying better psychosocial adjustment.
At discharge (T1) the intervention group showed greater reduction in mean BSL compared with the control group, however this was not statistically significant (p = 0.932). Similarly, body weight and BMI tends to decrease slightly more in interventions group, however this didn’t reach the significance value (p > 0.05 for both). There was no significant difference in other measures between the groups at discharge.
Long-term Outcomes (3-months follow-up - T2): Most participants were discharged home (n = 48), and both groups maintained their functional improvement (FIM scores) at 3 months follow-up (T2). Majority in the intervention group (72%) indicated that their bowel function improved or had been stable. Despite a reduction in the effect sizes, compared with the control group, significant improvement in the treatment group was maintained at T2 in ADS scores (p <0.001, r = 0.7) and DHP activity subscale (p <0.05, r = 0.6), signifying better adjustment to their condition. No difference between-groups were noted for other subscales. At 3-months follow-up (T2) the intervention group showed tendency towards reduction in 3-days mean BSL and HbAc1 compared with controls, this however, did not reach significance level (p = 0.427 and 0.250 respectively). There was no significant difference in other measures between-groups. Further, no significant between-group differences were found for anthropometrics (body weight, BMI) at T2.
Participants’ satisfaction with the program: At discharge (T1), 92% participants in the intervention group reported satisfaction with the NutriKane-DTM supplement. Interestingly, at 3-month followup (T2), only 62% of the intervention group indicated they would recommend the supplement to others, while only (41%) believed that it improved their bowel and/or DM condition. One-thirds (31.2%) continued intermittent use of NutriKane-DTM due to less than optimal consistency and taste of the product The fibre was well tolerated and no adverse effects were reported.
Discussion
To our knowledge this is the first RCT to examine the efficacy of a sugarcane-based nutritional product - within an interdisciplinary rehabilitation service for an adult DM inpatient cohort in the public-hospital system. The findings suggest that multimodal bowel management intervention, with additional dietary sugarcane supplement, improved participants’ bowel symptoms and function, diabetes-related activities and coping with the condition. The fibre was generally well tolerated, with no reported adverse events.
Consistent with previous reports this study found high prevalence of bowel dysfunction in hospitalized individuals [5,22- 24]. As expected, there was significant improvement in overall functional ability (FIM motor/cognition scales) in both groups with multidisciplinary rehabilitation, and gains maintained till 3-months follow-up period. Although treatment with NutriKane-DTM (added to regular rehabilitation regime), did not provided clinically relevant reduction in BSL, the intervention group showed improvement compared with controls without the supplement. Further, there was reduction in the body weight in both groups only at discharge, which was slight more in intervention group participants compared to the control group participants. This maybe attributable to improved education, exercise and improved bowel function as routine part of the rehabilitation program. The effect of added fibre therefore needs further study with bigger sample size with longer-term follow-up beyond the 3 months.
The findings of this study are difficult to compare due to lack of studies in this population and in similar settings elsewhere. The positive effects on various aspects of bowel-related outcomes (QoL, ADS, DHP scores) of participants in the intervention group, (both at discharge and 3-months post-intervention) were independent of the type of DM and achieved irrespective of variability in patient profile and characteristics. There is therefore need for providing structured bowel management programs to patients during their hospital stay and over a longer-term in the community.
There were many challenges in conducting this RCT in a rehabilitation setting [25,26], which included methodological issues (included blinding, compliance and ethical considerations), patient characteristics (requiring individualized approach) and a ‘reallife’ hospital setting. The likelihood of individualized rehabilitation programs, varying participant cognitive and educational ability, compliance with diet and medications, and type and severity of the DM may have influenced overall findings. The impact of individual factors such as therapy, exercise, diet alone on diabetes and blood sugar control were beyond the scope of this pilot study. However, routine multidisciplinary rehabilitation with DM management was consistent with the recommended practice and generalized to all participants irrespective of the group allocation.
This study has number of limitations. It was conducted in a small number of adult patients and selection bias cannot be ruled out as participants were recruited from a single rehabilitation service. Larger sample sizes in multiple centres and different settings are needed to confirm the generalizability of findings. The randomization was not stratified based on DM type, severity or intake of medications (insulin or oral glycaemic agents). However, all participants with DM admitted to the ward during the study period were assessed, irrespective of their demographic or disease status. There was no statistically significant difference in any study variables between participants who completed post-treatment assessment and those lost to follow-up in the control group. This was open label study and blinding of the participants and treating therapists was not possible, however, the assessors were independent of the treating team and blinded to reduce potential bias. Many participants fail to complete BSL diary after discharge as suggested, and average BSL information was collected by the researcher by contacting the participants consequently by phone. Similarly, despite multiple reminders and mailing BSL profile pathology investigation forms (including HbAc1); we fail to get information from over one fourth of the participants. We acknowledge the implication of these in the results. The study was of relatively short duration (only up to 3-months), further glycaemic improvement may have been achieved over a longer period. Further, the overall sample of this study, with older people is representative of the population presenting to rehabilitation services.
We acknowledge that other factors may have impacted participants’ overall bowel function (bladder dysfunction and diseasespecific symptoms such as pain) and anthropometrics parameters (such as weight loss) were not included in used outcome measures. These factors can negatively influence the disease-specific outcomes. A comprehensive report of symptoms and physical and cognitive dysfunction, however, was beyond the scope of this study. The impact of other rehabilitation modalities and interventions, within the DM management is unknown, and needs further research.
In conclusion, the present study provides some evidence that adding simple sugarcane extract in regular dietary regime may improve bowel function for patients with DM and may help in diabetes management. However, more research for longer duration is needed to ascertain the efficacy of the dietary supplements in larger studies.
Acknowledgement
We are grateful to all participants in this study. We particularly wish to thank Dr SF Wong and Dr I Windle for patient assessments; and Ms L Oscari for patient assessments and data entry.
Ethical Approval
The study was approved by the Royal Melbourne Hospital Ethical Committee and informed consent was obtained from all the subjects.
Declaration of Interest
The authors declare that they have no financial or conflict of interest. All NutriKane-D™ was supplied by the Medikane Pty Ltd, Sydney, Australia, free of cost. No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the authors or upon any organisation with which the authors are associated.
References
- Medikane Pty Ltd. NutriKane D™: a natural health treatment. Fact Sheet. Sydney: Medikane Pty Ltd.; 2015.
- Holt S, Jong V De, Faramus E, Lang T, Brand Miller J. A bioflavonoid in sugar cane can reduce the postprandial glycaemic response to a high-GI starchy food. Asia Pacific J Clin Nutrition. 2003; 12: S66.
- Capuano E. The behavior of dietary fiber in the gastrointestinal tract determines its physiological effect. Crit Rev Food Sci Nutr. 2017; 57: 3543-3564.
- El-Salhy M, Ystad SO, Mazzawi T, Gundersen D. Dietary fiber in irritable bowel syndrome (Review). Int J Mol Med. 2017; 40: 607-613.
- Amatya B, Elmalik A, Lowe M, Khan F. Evaluation of the structured bowel management program in inpatient rehabilitation: a prospective study. Disabil Rehabil. 2016; 38: 544-551.
- Khan F, Amatya B, Ng L, Galea M. Rehabilitation outcomes in persons with spina bifida: A randomised controlled trial. J Rehabil Med. 2015; 47: 734-740.
- Hua Y, Clark S, Ren J, Sreejayan N. Molecular mechanisms of chromium in alleviating insulin resistance. J Nutr Biochem. 2012; 23: 313-319.
- Mertz W. Chromium occurrence and function in biological systems. Physiol Rev. 1969; 49: 163-239.
- Mertz W. Chromium in human nutrition: a review. J Nutr. 1993; 123: 626-633.
- Mertz W. Interaction of chromium with insulin: a progress report. Nutr Rev. 1998; 56: 174-177.
- Rech M, To L, Tovbin A, Smoot T, Mlynarek M. Heavy metal in the intensive care unit: a review of current literature on trace element supplementation in critically ill patients. Nutr Clin Pract. 2014; 29: 78-89.
- Chong RWW, Hewawasam Gamage HH, Kautto L, Paulsen I, McRae C, Ball M, et al. Determination of the effect of dietary fibre on short-chain fatty acid production by the gut microbiome. Poster Abstracts MP15 Islet Society and Australian Islet Study Group VIII Annual Scientific Meeting; July 19 - 21 2015; Charles Perkin Centre, University of Sydney, Sydney, Australia. p 43.
- Prasad MP, Rao BD, Kalpana K, Rao MV, Patil JV. Glycaemic index and glycaemic load of sorghum products. J Sci Food Agric. 2015; 95: 1626-1630.
- Medikane Pty Ltd. NutriKane D™: Shareholder Update. Sydney: Medikane Pty Ltd.; Nov 2014.
- Heaton KW, Lewis SJ. Stool form scale as a useful guide to intestinal transit time. Scandinavian J Gastroenterol. 1997; 32: 920-924.
- Jorge J, Wexner S. Etiology and management of faecal incontinence. Dis Colon Rectum. 1993; 36: 77-97.
- Granger CV, Cotter AC, Hamilton BB, Fiedler RC, Hens MM. Functional assessment scales: a study of persons with multiple sclerosis. Arch Phys Med Rehabil. 1990; 71: 870-875.
- Carey MP, Jorgensen RS, Weinstock RS, Sprafkin RP, Lantinga LJ, Carnrike CL, Jr., et al. Reliability and validity of the Appraisal of Diabetes Scale. J Behav Med. 1991; 14: 43-51.
- Meadows KA, Abrams C, Sandbaek A. Adaptation of the Diabetes Health Profile (DHP-1) for use with patients with Type 2 diabetes mellitus: psychometric evaluation and cross-cultural comparison. Diabet Med. 2000; 17: 572-580.
- Cohen J. Statistical power analysis for the behavioural sciences. 2nd: Lawrence Erlbaum Associates; 1988.
- Pallant j. SPSS survival manual: a step by step guide to data nalysis using IBM SPSS. Berkshire, England: McGraw-Hill Education; 2013.
- Morris AR, Ho MT, Lapsley H, Walsh J, Gonski P, Moore KH. Costs of managing urinary and faecal incontinence in a sub-acute care facility: a "bottom-up" approach. Neurourol Urodyn. 2005; 24: 56-62.
- Chassagne P, Landrin I, Neveu C, Czernichow P, Bouaniche M, Doucet J, et al. Fecal incontinence in the institutionalized elderly: incidence, risk factors, and prognosis. Am J Med. 1999; 106: 185-190.
- Romero Y, Evans JM, Fleming KC, Phillips SF. Constipation and fecal incontinence in the elderly population. Mayo Clin Proc. 1996; 71: 81-92.
- Khan F, Amatya B, Pallant JF, Rajapaksa I, Brand C. Multidisciplinary rehabilitation in women following breast cancer treatment: a randomized controlled trial. J Rehabil Med. 2012; 44: 788-794.
- Khan F, Pallant JF, Brand C, Kilpatrick TJ. Effectiveness of rehabilitation intervention in persons with multiple sclerosis: a randomised controlled trial. J Neurol Neurosurg Psychiatry. 2008; 79: 1230-1235.