
Special Article – Brain Injury Rehabilitation
Phys Med Rehabil Int. Int. 2018; 5(4): 1155.
Altered Dopamine Bioavailability and Increased Spasticity following Repetitive Blast-induced Traumatic Brain Injuries in Rats
Tsuda S1,2, Golam M1,2, Jiamei H1,2, Rachel NL¹, Bernavil PY¹, Richardson KD¹, Yang Z³, Wang KKW³, Thompson FJ1,2,4 and Bose PK1,2,5*
1Brain Rehabilitation Research Center of Excellence, Malcom Randall VA Medical Center, North Florida/South Georgia Veterans Health System, USA
2Department of Physiological Sciences, University of Florida, USA
3Department of Emergency Medicine, University of Florida, USA
4Department of Neuroscience, University of Florida, USA
5Department of Neurology, University of Florida, USA
*Corresponding author: Bose PK, Brain Rehabilitation Research Center of Excellence, Malcom Randall VA Medical Center, North Florida/South Georgia Veterans Health System, 1601 Archer Rd, Gainesville, FL, 32608-1197, USA
Received: September 17, 2018; Accepted: October 22, 2018; Published: October 29, 2018
Abstract
Purpose: The majority of veterans and military personnel exposed to blastinduced traumatic brain injuries (bTBIs) suffer from hyperreflexia (spasticity/ rigidity). Unfortunately, its pathophysiology has remained largely unknown, leaving limited treatment option for its management. The purpose of this study was to investigate alterations in dopamine bioavailability in the key neuronal substrates that regulate hyperreflexia following repetitive bTBIs in rats.
Methods: A bTBI was induced by an overpressure blast-wave on the day 1, 4, and 7 (total of 3 injuries) in 7 adult male rats, while another cohort of 7 age- and sex-matched animals were prepared as the sham. On the day 8, the velocitydependent ankle torques (VDATs) and the amplitudes of electromyography (EMG) signals of the triceps surae muscles were measured in all animals. On the day 9, the animals were euthanized to collect the motor/sensory cortex (MCx) and the vestibular nuclei (VN) for the determination of the dopamine contents by high performance liquid chromatography with electrochemical detection (HPLC/ ECD).
Results: Following repetitive bTBIs, the VDATs were significantly increased at all tested angular velocities, compared to the sham-treated animals. The amplitudes of EMG signal were also significantly increased at 6 out of 8 different angular velocities in the bTBI group. Furthermore, the HPLC/ECD analysis of the dopamine bioavailability showed a general decrease in the MCx and a significant increase in the VN following bTBIs.
Conclusion: Repetitive bTBIs can induce the motor reflex dysregulation (i.e., hyperreflexia), which might be due, in part, to the bTBI-induced altered dopamine bioavailability in the MCx and/or VN.
Keywords: Blast traumatic brain injury; Spasticity; Dopamine; Motor/ sensory cortex; Vestibular nuclei; Rats
Abbreviations
bTBI: Blast Traumatic Brain Injury; VDAT: Velocity-Dependent Ankle Torque; EMG: Electromyography; MCx: Motor/Sensory Cortex; VN: Vestibular Nuclei; HPLC/ECD: High-Performance Liquid Chromatography with Electrochemical Detection; RMS: Root Mean Square; FPI: Fluid Percussion Injury; FSCV: Fast Scan Cyclic Voltammetry; SN: Substantia Nigra; TH: Tyrosin Hydroxylase; ir: Immunoreactive; CCI: Controlled Cortical Impact Injury.
Introduction
In the war fields, such as Iraq and Afghanistan, active duty soldiers have been exposed to high explosives (e.g., grenades and landmines) [1], which often induces a blast traumatic brain injury (bTBI). As a result, these soldiers suffer from a variety of disorders, including spasticity [2,3]. Spasticity has been defined as an upper motor neuron disorder characterized by a velocity-dependent increase in muscle tone caused by the increased excitability of the stretch reflex [4,5]. This neurological disorder induces deficits in physical mobility [6] as well as gait and balance [7], negatively affecting their participation in active duty as well as the quality of life. However, currently, there is no consensus for effective treatment for TBI-induced spasticity.
Although various pharmacotherapies are available to attenuate spasticity following brain injury, various adverse effects have been reported in a significantly high percentage of spasticity patients [8-12]. Therefore, it is important to improve our understanding of neuropathology of TBI-induced spasticity to guide the development of safe and effective alternative treatments.
Mechanisms of TBI-induced spasticity are attributed to the dysregulation of the neurotransmitter and neuromodulatory control of supraspinal descending tracts, such the corticospinal and vestibulospinal tracts [5,13,14]. Although TBI has been reported to induce alterations in dopamine, an important neuromodulator, in its major source regions (i.e., the nigrostriatal system) [15-24], TBIinduced changes in dopamine levels in the motor/sensory cortex (MCx) and vestibular system have not been reported.
Therefore, the purpose of this study was to investigate potential alterations in the dopamine bioavailability in the executive-motor (motor/sensory cortex) and posture-motor (vestibular) brain regions following repetitive mild bTBIs in a clinically relevant rat model, mimicking the exposure of soldiers to the war fields.
Materials and Methods
Animals
A total of 14 adult male rats were randomly distributed to bTBI and sham treatment groups (n = 7 per group). The protocols of the experiments were approved by the Institutional Animal Care and Use Committee of the University of Florida and North Florida/South Georgia Veterans Health System. Efforts were made to minimize the number of animals used and post-trauma complications. Animals were paired in the cages in a 12h light/dark cycle with controlled room temperature and humidity at an American Association for Laboratory Animal Science-Accredited facility. Food and water were given ad libitum.
bTBI
An overpressure blast-wave brain injury was induced to each animal in the bTBI group on the day 1, 4, and 7, as previously described [25]. Briefly, following surgical plane of deep anesthesia with 3% isoflurane, the animal’s body was wrapped around the animal holder in a prone position exposing only his head from the horizontal shock tube with an open end. The head was placed on a flexible mesh surface to reduce the surface reflection of blast waves as well as the formation of secondary waves which could potentially exacerbate the injury. Then, each animal in the bTBI group was subjected to a blast wave for 2.0–2.5 milliseconds with the peak pressure of 30 poundforce per square inch, while each animal in the sham treatment group received only anesthesia. This system produced a blast waveform with a positive pressure followed by a negative pressure. After surgery, the animals were kept in an temperature regulated incubator under constant surveillance until they awaken as indicated by head lifting and other volitional movements. At that point, they were transferred to warm recovery units (i.e. cages with one end on a heating pad or temperature-controlled incubators) until they are able to eat and drink on their own.
VDAT and EMG
On the 8th day, the VDATs and time-locked EMG were simultaneously recorded during the ankle dorsiflexion in all animals to assess the spasticity/rigidity, using methods that we previously described [4,7,26-28]. Briefly, animals were immobilized in a customdesigned trunk restraint device and the hindlimbs were secured to permit a normal range of ankle rotation. Using an electromechanical shaker (model 405; Ling Dynamic Systems, Royston Herts, UK), a series of controlled 12-degree dorsiflexion was produced at various velocities (49, 136, 204, 272, 350, 408, 490, and 612 degrees per second) with 3-second intervals. During the dorsiflexion, the lengthening resistance of the triceps surae muscles was measured by quantifying the VDATs and EMG. An EMG electrode was inserted in a skin fold over the distal convergence of the triceps surae muscles, while a reference electrode was placed in a skin fold over the greater trochanter. Raw EMG and root mean square (RMS) of EMG bursts were recorded simultaneously with the ankle torques. The data were acquired and analyzed using a digital acquisition system with LabVIEW graphic programming (version 8.2; National Instruments, Austin, TX).
HPLC/ECD
On the 9th day, after the animals were euthanized with Euthasol, the MCx and vestibular nuclei (VN) were collected to be snap-frozen in liquid nitrogen. Then, all samples were stored at -80°C until the HPLC/ECD analysis. Tissues were sonicated in the 0.1M perchloric acid (50μL/mg tissue) and centrifuged at 40,000g for 20min. Supernatants were filtered through the 0.2μm pore and the protein concentration of each sample was determined by the bicinchoninic acid assay. The standard solution was prepared in the 0.1 M perchloric acid. The contents of the mobile phase were 0.1mM EDTA, 100mM phosphoric acid, 100mM citric acid, 0.06% 1-octanesulfonic acid, and 8% acetonitrile based on the specification manual of Antec Scientific, the Netherlands. The dopamine contents of the samples were determined using a HPLC ALEXYS 100 2D system equipped with electrochemical detection (DECADE II) from ANTEC Leyden (Zoeterwoude, Netherlands).
Statistical analysis
Between-group differences were analyzed using unpaired t-tests. The data were expressed as mean ± SEM. P values less than 0.05 were considered to be statistically significant. Data analysis was performed using the GraphPad Prism 4 software (GraphPad Software).
Results
VDAT
Significantly increased VDATs were observed in animals following bTBI (Figure 1B). Although the largest increases were observed at the higher test velocities, significantly increased VDATs during 12-degree dorsiflexion at all tested ankle rotation velocities (49, 136, 204, 272, 350, 408, 490, and 612 degrees per second) were observed in recordings from the bTBI group compared to those from the sham treatment group (p < 0.05, 0.01, and 0.001).
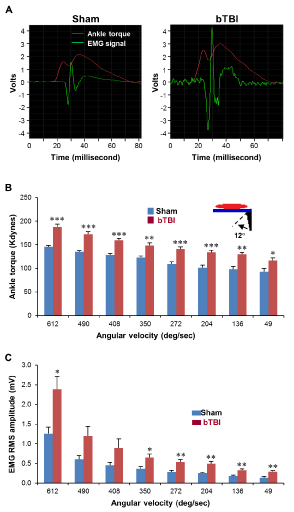
Figure 1: EMG during the dorsi?exion at various rotation velocities. (A)
Representative pictures of the EMG signals of the animals in the sham and
bTBI groups. (B) VDATs during the dorsi?exion at various rotation velocities.
VDATs were measured during the 12-degree dorsi?exion at 49, 136, 204,
272, 350, 408, 490, and 612 degrees per second. Data were obtained from
seven independent animals per group. Values are mean ± SEM. *p < 0.05,
**p < 0.01, ***p < 0.001. (C) EMG-RMS amplitudes were recorded during the
dorsi?exion at 49, 136, 204, 272, 350, 408, 490, and 612 degrees per second.
Data were obtained from seven independent animals per group. Values are
mean ± SEM. *p < 0.05, **p < 0.01.
EMG
EMG burst discharge amplitudes time-locked to the onset of the VDATs (Figure 1A) were significantly increased at relatively lower velocities (49, 136, 204, 272, and 350 degrees per second) as well as the highest velocity (612 degrees per second) of the ankle rotation (p < 0.05 and 0.01, Figure 1C). Increases were the most significant at 49, 136, 204, and 272 degrees per second (p < 0.01).
Dopamine contents in the MCx
The HPLC/ECD-derived data analysis of the experimental samples showed that the relative dopamine contents in the MCx appeared to be decreased following repetitive bTBIs, compared to the animals in the sham treatment group (p = 0.1171, Figure 2B).
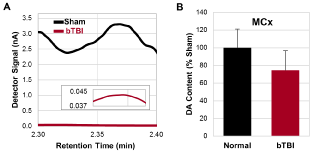
Figure 2: Dopamine contents in the MCx analyzed by HPLC/ECD. (A)
Representative detector signals of dopamine in the MCx. Inlet shows the
signal of bTBI animal with the smaller scale of the vertical axis. (B) Relative
dopamine contents in the MCx converted from the detector signals (p =
0.1171). Data were obtained from seven independent animals per group.
Values are mean ± SD.
Dopamine contents in the VN
The HPLC/ECD-derived data analysis of the experimental samples showed that the relative dopamine contents in the VN (Figure 3A) were significantly increased following repetitive bTBIs, compared to the animals in the sham treatment group (p < 0.01; Figure 3B).
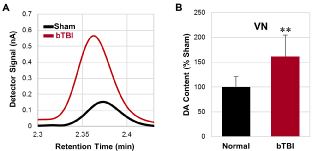
Figure 3: Dopamine contents in the VN analyzed by HPLC/ECD. (A)
Representative detector signals of dopamine in the VN. (B) Relative
dopamine contents in the VN converted from the detector signals. Data were
obtained from seven independent animals per group. Values are mean ± SD.
**p < 0.01.
Discussion
Spasticity following bTBIs
According to a previous study, 70% of veterans and active duty military personnel with impaired consciousness following bTBI (median Glasgow Coma Scale score of 3, n=29) suffered from spasticity requiring medical intervention (approximately 67 days postinjury) [2]. In agreement with this report, the present study showed significantly increased expression of spasticity tested in the hindlimbs in an experimental rodent model of repetitive bTBIs in rats (Figure 1A-C). These changes were revealed as significant increases in the VDATs at all tested velocities recorded in the bTBI animals compared with those recorded in the controls. Significant EMG/ RMS amplitudes time-locked to the VDATs were also observed in recordings from bTBI animals. Collectively, these data indicated significant increases in the lower limb stretch reflexes to displacement. However, the high level of increased EMG/RMS amplitudes observed at the lower velocities are particularly suggestive of increased resting tone. These observations indicate the increased ankle torques were coincident with the appearance of time-locked EMG burst activity in the triceps surae muscles at both low and high ankle rotation velocities (e.g. low and high ankle extensor muscle stretch velocities). These observations following bTBI are consistent with our previous studies of the development of lower limb spasticity in the rat following closed-head TBI [7] during a similar post-injury time point. Since velocity-dependent ankle torque is accompanied by EMG burst even at the lower test velocities, this spastic pattern can be considered to include tonic and dynamic components of rigidity and spasticity [4].
Bioavailability of dopamine in the MCx and VN following repetitive bTBIs
Although the acute decreases in the dopamine contents of the nigrostriatal system have been reported following TBI in rodents [15,18,19,21,22], TBI-induced changes in the dopamine levels of other brain regions are not consistent. For instance, the dopamine contents in the hypothalamus were significantly increased 1-24 hours following FPI in rats, while those in the cortex was significantly decreased 1h postinjury and persisted up to 2 weeks [29].
MCx: MCx provides significant contributions to the corticospinal and dorsal reticulospinal pathways known to regulate peripheral and central inputs that produce muscle tone [5,30-32]. Following a uni-lateral lesion of the MCx, a significant spasticity of the contralateral hindlimb with increased Hoffmann’s reflex was observed in rats [14]. The present findings are consistent with these and indicate a persisting decreased dopamine expression in the MCx following bTBI (Figure 2B). Accordingly, a decreased role of dopamine in the MCx may contribute to muscle tone dysregulation characteristic of spasticity.
Dopamine projections from the ventral tegmental area to the motor cortex are known to play a key role in motor skill learning and motor cortex synaptic plasticity [33]. D1 and D2 receptor activity influences motor skill acquisition and long-term synaptic potentiation through phospholipase C activation in the primary motor cortex. TBI-induced decreased DOPA expression in the motor cortex could accordingly degrade compensatory cortical synaptic plasticity that could be essential for recovery/rehabilitation following injury. These findings potentially highlight a novel and important role of dopamine in the motor cortex that could contribute to consideration of therapeutic interventions designed to enhance recovery of executive motor function following injury.
VN: Although glutamate, acetylcholine, and GABA are known to be primary neurotransmitters in the vestibular system, dopamine is concluded to be stimulatory to the VN and its descending tract since vestibular-evoked myogenic potentials of peripheral muscles in Parkinson’s disease patients were significantly increased following the administration of L-DOPA, the direct precursor of dopamine [34]. Accordingly, if dopamine functions as an excitatory neurotransmitter, the significant upregulation of dopamine expression in the vestibular nuclei following bTBI observed in the present study (Figure 3B), could contribute to the alteration in the gain of the vestibular reflexes that underlie motor reflex excitability.
Regulation of spasticity via the supraspinal descending tracts
By its definition, spasticity is generated by a spinal reflex. During a muscle stretch, information of a change in the muscle fiber length is sent to an alpha-motor neuron in the spinal cord via a sensory afferent 1a neural fiber, which excites the alpha-motor neuron. This neural signal is then transmitted to the muscle to generate a muscle contraction. However, multiple peripheral and central inputs from segmental and descending fibers compete for the excitability of the presynaptic (afferent input fiber) and the postsynaptic elements (motoneuron) via direct connections or through interneuronal connections. It is believed that spasticity occurs largely due to the maladaptive plasticity of spinal stretch reflex following alterations in the supraspinal descending pathways, such as the corticospinal, cortico-reticulospinal, vestibulospinal, and monoamine spinal tracts [5].
Collectively, via altered dopamine expressions in the MCx and VN, decreased excitability of the cortico-reticular tract (inhibitory) and increased activity of the vestibulospinal tract (excitatory) could significantly contribute to a maladaptive increase in reflex excitability following bTBI.
Conclusion
In summary, the tonic pattern of spasticity following repetitive bTBIs in rats could be partly due to the down- and up regulation of the dopaminergic system in the MCx and VN respectively. To the best of our knowledge, this is the first study to show the altered dopamine bioavailability in the MCx and VN following repetitive bTBIs. These findings highlight a novel and important role of dopamine in these brain regions, which could contribute to consideration of therapeutic interventions designed to enhance the recovery of executive motor and balance function following TBI. The present study would provide new information for future studies to further investigate the roles of altered dopaminergic system in regulating spasticity.
References
- Shively SB, Perl DP. Traumatic brain injury, shell shock, and posttraumatic stress disorder in the military--past, present, and future. J Head Trauma Rehabil. 2012; 27: 234-239.
- Nakase-Richardson R, McNamee S, Howe LL, Massengale J, Peterson M, Barnett SD, et al. Descriptive characteristics and rehabilitation outcomes in active duty military personnel and veterans with disorders of consciousness with combat- and noncombat-related brain injury. Arch Phys Med Rehabil. 2013; 94: 1861-1869.
- Pasquina P, Kirtley R, Ling G. Moderate-to-severe traumatic brain injury. Semin Neurol. 2014; 34: 572-583.
- Bose P, Parmer R, Thompson FJ. Velocity-dependent ankle torque in rats after contusion injury of the midthoracic spinal cord: time course. J Neurotrauma. 2002; 19: 1231-1249.
- Mukherjee A, Chakravarty A. Spasticity mechanisms - for the clinician. Front Neurol. 2010; 1: 149.
- Sosnoff JJ, Gappmaier E, Frame A, Motl RW. Influence of spasticity on mobility and balance in persons with multiple sclerosis. J Neurol Phys Ther. 2011; 35: 129-132.
- Bose P, Hou J, Nelson R, Nissim N, Parmer R, Keener J, et al. Effects of acute intrathecal baclofen in an animal model of TBI-induced spasticity, cognitive, and balance disabilities. J Neurotraumam. 2013; 30: 1177-1191.
- Taira T, Ueta T, Katayama Y, Kimizuka M, Nemoto A, Mizusawa H, et al. Rate of complications among the recipients of intrathecal baclofen pump in Japan: a multicenter study. Neuromodulation. 2013; 16: 266-272.
- Borrini L, Bensmail D, Thiebaut JB, Hugeron C, Rech C, Jourdan C. Occurrence of adverse events in long-term intrathecal baclofen infusion: a 1-year follow-up study of 158 adults. Arch Phys Med Rehabil. 2014; 95: 1032-1038.
- Calabrò RS, D'Aleo G, Sessa E, Leo A, De Cola MC, Bramanti P. Sexual dysfunction induced by intrathecal baclofen administration: is this the price to pay for severe spasticity management? J Sex Med. 2014; 11: 1807-1815.
- Cardoso AL, Quintaneiro C, Seabra H, Teixeira C. Cardiac arrest due to baclofen withdrawal syndrome. BMJ Case Rep. 2014; pii: bcr2014204322.
- Motta F, Antonello CE. Analysis of complications in 430 consecutive pediatric patients treated with intrathecal baclofen therapy: 14-year experience. J Neurosurg Pediatr. 2014; 13: 301-306.
- Miller DM, Klein CS, Suresh NL, Rymer WZ. Asymmetries in vestibular evoked myogenic potentials in chronic stroke survivors with spastic hypertonia: evidence for a vestibulospinal role. Clin Neurophysiol. 2014; 125: 2070-2078.
- Zong H, Ma F, Zhang L, Lu H, Gong J, Cai M, et al. Hindlimb spasticity after unilateral motor cortex lesion in rats is reduced by contralateral nerve root transfer. Biosci Rep. 2016; 36: pii: e00430.
- Wagner AK, Sokoloski JE, Ren D, Chen X, Khan AS, Zafonte RD, et al. Controlled cortical impact injury affects dopaminergic transmission in the rat striatum. J Neurochem. 2005; 95: 457-465.
- Kobori N, Clifton GL, Dash PK. Enhanced catecholamine synthesis in the prefrontal cortex after traumatic brain injury: implications for prefrontal dysfunction. J Neurotrauma. 2006; 23: 1094-1102.
- Yan HQ, Ma X, Chen X, Li Y, Shao L, Dixon CE. Delayed increase of tyrosine hydroxylase expression in rat nigrostriatal system after traumatic brain injury. Brain Res. 2007; 1134: 171-179.
- Wagner AK, Drewencki LL, Chen X, Santos FR, Khan AS, Harun R, et al. Chronic methylphenidate treatment enhances striatal dopamine neurotransmission after experimental traumatic brain injury. J Neurochem. 2009; 108: 986-997.
- Shin SS, Bray ER, Zhang CQ, Dixon CE. Traumatic brain injury reduces striatal tyrosine hydroxylase activity and potassium-evoked dopamine release in rats. Brain Res. 2011; 1369: 208-215.
- van Bregt DR, Thomas TC, Hinzman JM, Cao T, Liu M, Bing G, et al. Substantia nigra vulnerability after a single moderate diffuse brain injury in the rat. Exp Neurol. 2012; 234: 8-19.
- Chen YH, Huang EY, Kuo TT, Ma HI, Hoffer BJ, Tsui PF, et al. Dopamine release impairment in striatum after different levels of cerebral cortical fluid percussion injury. Cell Transplant. 2015; 24: 2113-2128.
- Xu X, Cao S, Chao H, Liu Y, Ji J. Sex-related differences in striatal dopaminergic system after traumatic brain injury. Brain Res Bull. 2016; 124: 214-221.
- Chen YH, Huang EY, Kuo TT, Hoffer BJ, Miller J, Chou YC, et al. Dopamine release in the nucleus accumbens is altered following traumatic brain injury. Neuroscience. 2017; 348: 180-190.
- Liu M, Bachstetter AD, Cass WA, Lifshitz J, Bing G. Pioglitazone attenuates neuroinflammation and promotes dopaminergic neuronal survival in the nigrostriatal system of rats after diffuse brain injury. J Neurotrauma. 2017; 34: 414-422.
- Yang Z, Lin F, Robertson CS, Wang KK. Dual vulnerability of TDP-43 to calpain and caspase-3 proteolysis after neurotoxic conditions and traumatic brain injury. J Cereb Blood Flow Metab. 2014; 34: 1444-1452.
- Thompson FJ, Browd CR, Carvalho PM, Hsiao J. Velocity-dependent ankle torque in the normal rat. Neuroreport. 1996; 7: 2273-2276.
- Bose PK, Hou J, Parmer R, Reier PJ, Thompson FJ. Altered patterns of reflex excitability, balance, and locomotion following spinal cord injury and locomotor training. Front Physiol. 2012; 3: 258.
- Hou J, Nelson R, Nissim N, Parmer R, Thompson FJ, Bose P. Effect of combined treadmill training and magnetic stimulation on spasticity and gait impairments after cervical spinal cord injury. J Neurotrauma. 2014; 31: 1088-1106.
- McIntosh TK, Yu T, Gennarelli TA. Alterations in regional brain catecholamine concentrations after experimental brain injury in the rat. J Neurochem. 1994; 63: 1426-1433.
- Engberg I, Lundberg A, Ryall RW. Reticulospinal inhibition of interneurones. J Physiol. 1968; 194: 225-236.
- Woolsey CN. “Discussion on experimental hypertonia in the monkey: interruption of pyramidal or pyramidal-extrapyramidal cortical projections.” Transactions of the American Neurological Association. 1971; 96: 162–168.
- Andrews C, Knowles L, Hancock J. Control of the tonic vibration reflex by the brain stem reticular formation in the cat. J Neurol Sci. 1973; 18: 217-226.
- Rioult-Pedotti MS, Pekanovic A, Atiemo CO, Marshall J, Luft AR. Dopamine Promotes Motor Cortex Plasticity and Motor Skill Learning via PLC Activation. PLoS One. 2015; 10: e0124986.
- Pötter-Nerger M, Reich MM, Colebatch JG, Deuschl G, Volkmann J. Differential effect of dopa and subthalamic stimulation on vestibular activity in Parkinson's disease. Mov Disord. 2012; 27: 1268-1275.