
Special Article – Chronic Pain Rehabilitation
Phys Med Rehabil Int. 2018; 5(2): 1141.
Functional Improvement with Continuous Intrathecal Morphine Infusion in Chronic Non-Cancer Pain
Genni Duse* and Giulia Demo
1Pain Therapy Unit, Department of Emergency, S. Antonio Hospital, Padua, Italy
*Corresponding author: Genni Duse, Pain Therapy Unit, Department of Emergency, S. Antonio Hospital,Padua, Italy
Received: February 19, 2018; Accepted: March 16, 2018; Published: March 23, 2018
Abstract
Aim of the Study: To investigate the long-term (24 months FU) results of continuous intrathecal morphine infusion on functional abilities and quality of life in patients suffering from chronic low back and leg non-cancer pain.
Materials and Methods: 44 patients, at with chronic non-cancer pain, unresponsive to multimodal analgesic therapy, were selected after a three week trial period with epidural infusion of morphine*. The sample size as been defined by ethics committee because of a prospectical and observational study. After informed consent and positive trial period, patients underwent to implantation of an intrathecal morphine infusion programmable pump. Pain intensity (VAS: 0-100), functional abilities (Oswestry Disability Index, ODI), and quality of life (Short Form 36 Health Survey, SF-36) were assessed at baseline, 12 and 24 months after the implantation. Adverse side effects and complications were also recorded in order to investigate the safety of the therapy.
Results: 43 patients reached the 12 months follow up (FU) and 39 of them reached the 24 months FU. Back and leg pain significantly decreased from baseline to 12 months FU visits (p<0.001). Performance Functional Status, evaluated with ODI, significantly improved from baseline to each FU visit (p<0.001). For what concerns Quality of Life analysed with SF-36 questionnaire, at 12 months a significant improvement was obtained in Physical Component and Mental Component Scores (p<0.001), we had significant scores also at 24 months (p<0.001).
Conclusions: continuous intrathecal morphine infusion, using implantable programmable pumps, is helpful for pain control, and shows functional activity, patients’ health status and quality of life could also be improved.
Keywords: Intrathecal morphine; Chronic pain; Spinal cord; Pain intensity; Functional status; Quality of life
Introduction
Chronic back and/or lower limbs pain are common in patients suffering from failed back surgery syndrome (FBSS), osteoarthritis and spinal stenosis. This kind of patients is generally difficult to treat, because the severe nociceptive and neuropathic pain presented is often untreatable with conventional therapies [1]. Since these syndromes affect the vertebral column, pain usually increases with the movements and is associated with severe disability, seriously compromising functional abilities and quality of life [2]. For this reasons a multidisciplinary approach is usually required that include, in addition to drugs, physical and psychological therapies.
Furthermore, pharmacological treatment may lead to tolerance, addiction or side effects due to prolonged and high doses administration [3,4].
Intrathecal drug delivery (IDD) by means of an implanted programmable pump may be an effective option for chronic pain, allowing a dramatic reduction of the administered doses and side effects [3-7]. However, in literature, only few studies consider the outcomes in terms of functional abilities and quality of life in patients treated with IDD. Probably this is due to different selection criteria and to different methodological approaches as well as poor use of standardized questionnaires, such as Oswestry Disability Index and SF-36 for functional improvement and quality of life assessment [8].
In our study 44 patients with chronic non-cancer pain, unresponsive to multimodal analgesic therapy, were selected after a three weeks trial period of epidural infusion with morphine. In our previous study we evaluated the impact of the emotional and psychosocial aspects affecting pain perception in 30 patients suffered chronic pain. We observed that the use of a continuous IT morphine infusion reduced intractable chronic pain modifying its perception in terms of intensity and quality. In addition, we found that 87% of patients were able to restore, totally or partially, their daily activities and 92% of patients employed were able to return to full-time employment [9].
However, we didn’t quantify patients’ functional improvement from the pre-treatment baseline state to the follow up. The aim of this data collection is to evaluate functional status and quality of life in patients with FBSS treated with intrathecal morphine in the normal clinical practice.
Material and Methods
This study was performed by means of a clinical data collection approved by S. Antonio Hospital Institutional Review Board and conforms to the principles outlined in the Declaration of Helsinki. Outpatients from the Pain Unit at S. Antonio Hospital (Padua, Italy) were assessed according to the common clinical practice by multiple pain specialists. All patients had severe predominant nociceptive non-cancer chronic back and/or legs pain, not successfully treated by means of conventional therapies.
All patients had a long history of pain and tried different kinds of systemic drugs (analgesics and opioids rotation) with poor results in pain management or with intolerable side effects due to the prolonged and high doses undertaken. Nobody was eligible to further surgical procedures useful to reduce pain.
In our centre the IT morphine infusion is commonly used when conventional therapies failed.
Patients, after informed consent, are trialed a three weeks percutaneous epidural morphine infusion by an external infusion pump. During the trial period all analgesic systemic drugs were removed in order to detect the real effect of the therapy. Pain intensity was assessed before and after the test with Visual Analogic Scale (VAS, 0-100), where 0 means no pain and 100 worst pain imaginable. The starting morphine dose is 1mg/die and it is gradually increased to 5mg/die. Patients were considered responder to the treatment and eligible to the IT infusion system implantation if the test provided a pain relief =50%. Patients not responder were considered not eligible to permanent intrathecal morphine infusion with IDD.
Patients with a successful trial period underwent the implantation of a permanent IT morphine delivery system a month later.
All the surgical procedures were performed by the same physician (GD) using local anaesthesia with an intravenous sedation, with the patient in the right lateral position, and IT catheter was introduced at L3-L4 level and advanced under fluoroscopy with the tip at T8-T10. The catheter was sutured to the prevertebral fascia and subcutaneously tunnelized up to the left side of the abdomen, where a subcutaneous pocket was created for the pump (Synchromed II 20mL, Medtronic INS, Minneapolis, MN, USA). The initial IT morphine dose was set and scaled according to the effective trial dose for each patient, considering the ratio 1mg epidural: 0.1mg intrathecal, in order to avoid adverse effects secondary to morphine overdose. The administered dose was then modified during every visit in order to obtain a pain relief = 50% [10,11].
Patients were assessed at the baseline and at 6, 12 and 24 months (mo) follow-up visits (FU) with VAS score for pain intensity and with 3 different standardized questionnaires (Oswestry Disability Index, EQ-5D and SF-36) for functional abilities and quality of life.
The Oswestry Disability Index Questionnaire (ODI) evaluates the impact of pain on daily activities. The index has been shown to be sensible in patients’ functional status changes and it well differentiates improvement and non-improvement [12,13]. The Oswestry disability index is based on ten items, each followed by six alternatives [14]. Each question is scored from 0–5, and the sum of the scores is then expressed as a percentage.
The Short Form-36 Health Survey (SF-36) is an indicator of patients’ overall health status. It includes 36 questions that form 8 scales: AF-physical activity (10 items), RP-role limitations due to physical health (4 items) and RE-role limitations due to emotional problems (3 items), BP –bodily pain (2 items), GH-perception of general health (5 items), VT vitality (4 items), SF-social activities (2 items), MH-mental health (5 items) and a single question about changing in health status [15].
The EuroQol (EQ-5D) is a standardised instrument for use as a measure of health outcome that includes five dimensions: mobility, self-care, usual activities, pain, anxiety/depression. For each dimension, the questionnaire investigates whether the person suffers from serious, moderate or no disabilities. The questionnaire also includes a visual analogue scale from 0 to 100 on which the patients indicate the level of perceived health status. Adverse events were also recorded in order to investigate the safety of the therapy [16].
Patient’s satisfaction was evaluated with direct questions with multiple choice: very unsatisfied, little unsatisfied, little satisfied and very satisfied.
Statistical analysis
Collected data were analyzed to assess the baseline characteristics and changes from baseline to FU. The software used for statistical analysis was STATA/SE 12.1 (StataCorp, College Station, TX, USA). All new generated variables and descriptive and analytical procedures developed in STATA were saved in a file of code “Stata Do-file”. All statistical tests are to be considered 2-tailed and a p-value of 0.05 was considered significant. In case of Quality of Life missing data, the Last-Observation-Carried- Forward (LOCF) method was used.
Continuous data were expressed as mean and standard deviation, median with 1st-3rd quartile and range while categorical variables were expressed as absolute number and percentage. Percentages will refer to patients with available information (N).
Comparisons between baseline and follow-up data (12 or 24 months) and between the two follow-up data were evaluated with Student t-test for paired samples, if the distribution was Gaussian, or with Wilcoxon sign rank test otherwise.
Normality was assessed by a Shapiro–Wilk test. Homoscedasticity was evaluated by an F test for the homogeneity of variances. All 2-tailed p value <0.05 was considered statistically significant.
Results
44 patients (33 females and 11 males) with nociceptive prevalent pain, mean age of 70.6 + 12 years (range: 40 – 87 years) with severe intractable chronic pain have been implanted after positive response to trial test and followed up to 24 mo FU.
Pain areas were back and legs in 46% of patients, back and one leg in 36% or legs in 18%. The primary etiology of pain was FBSS in 40.9% of cases, spondylodiscartrosis 20.5%, spinal stenosis in 18.2% and osteoarthritis in 13.6%. Time from pain onset to implant was 69.0 + 56.8 (range: 5 months – 25 years) and all patients had already made opioids rotation and at the baseline the pharmacological treatment was: morphine sulfate (55% of cases), buprenorphine (27%), oxycodone (27%) and fentanyl (9%).
Furthermore 36% were treated with anticonvulsants, 18% wit NSAIDs and 9% with antidepressants (Table 1) 64% of the patients also underwent previous treatments as: rehabilitation (20.5%), nerve blocks (6.8%), TENS (6.8 %), eperidurolysis (4.6%) and magnetic therapy (2.3%).
Baseline
FU 6 mo
FU 12 mo
FU 24 mo
P-value
ODI (0-100)
57.0±12.5
41.0±14.1
37.9±18.7
37.3±18.6
0.001*
EQ5D index (-1-1)
0.13±0.31
0.30±0.31
0.43±0.37
0.41±0.52
0.204*
EQ VAS (0-100)
30.7±23.6
49.6±25.0
52.5±30.1
50.9±24.1
0.027*
Table 1: Mean ± sd scores for ODI, EQ5D index and EQ VAS at baseline, 6, 12 and 24mos Fu. Generalized liner model for repeated measures.
Table 2 shows mean±SD, median value (1st – 3rd quartile) and range of VAS score at baseline: VAS score at baseline was 87.9±17.9 for the back and 85±18.2 for the lower limbs.
Table 3 shows mean±SD, median value (1st – 3rd quartile) and range of QoL score at baseline (Oswestry score, EQ-5D index and vas, SF36 specific and general scores).
All patients implanted underwent a trial test period and had a positive response with an average epidural morphine dose of 1.6± 0.6 mg/day. After the trial with epidural morphine infusion (average dose 1.6±0.6 mg/day), patients underwent the implantation of IDD system (Synchromed II 20mL, Medtronic Inc., Minneapolis, MN) and the mean morphine daily dosage at implant was 1.79.4 ± 95.1 μg (range 100 – 600 μg).
All patients performed at least one Follow Up visit (FU), 43 at FU12 and 39 at FU24.
Figure 1 shows the median VAS scores (0-100) at baseline and after 6, 12, 24 months from pump implant for Back and Lower limbs.
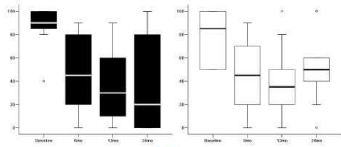
Figure 1: Median VAS scores (0-100) at baseline, 6, 12 and 24 mo FU visits
for back (left panel) and legs (right panel).
In the first part of the analysis, VAS values, Quality of Life and Functional Activities scores were compared between baseline and 12-months follow up in order to show any possible improvement. Pain intensity and quality of life, revealed a significant improvement from baseline to 12 mo FU VAS score for back and leg pain shows a p-value <0.001: Table 4 shows the median (1st – 3rd quartile) VAS value at baseline and after 12 months from pump implant; Table 5 shows the mean±SD quality of life score (Oswestry score, EQ-5D index and vas, SF36 specific and general scores) at baseline and after 12 months from pump implant.
In the second time of the analysis VAS values, quality of life and Functional Activities scores were compared between baseline and 24-months follow up.
Table 6 and Table 7 that show mean±SD, median value (1st – 3rd quartile) and range of VAS values, QoL score (Oswestry score, EQ- 5D index and vas, SF36 specific and general scores) at baseline and after 24 months.
Ziconotide has been used in four patients in order to optimize pain management according to patients’ specific needs (in one patient before the 6 mo FU and in three patients after the 18 mo FU).
In the 39 patients who received morphine for all period of the study, the administered dose significantly increased from the implant (179.4 ± 95.1μg/day) to 24 mo FU (896.1 + 303.7 μg/day). ODI score significantly improved from baseline to each FU visit (p<0.001) as well as EQ VAS (p=0.027). EQ5D index score improved not significantly (Figure 2).
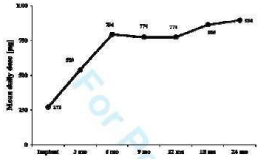
Figure 2: Average IT morphine daily dose at the implant and at the FU visits
(8 patients, p<0.001).
Leg pain variation from baseline to 24mos FU was correlated with the variation of the Oswestry score in order to assess a possible difference between patients’ pain perception and the real functional improvement provided by the administered therapy. A statistical positive correlation (ρ=0.772 and ρ=0.711) between improvements in VAS score for lower limbs and improvements in Oswestry score was found at 6 mo FU and at 12 mo FU (p=0.009 and p=0.021) but not significant at 24 month follow up (Figure 3).
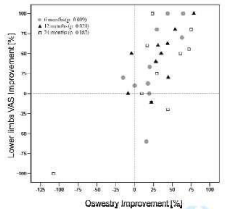
Figure 3: Rate of improvement in Oswestry score correlated to the rate of
improvement in lower limbs VAS score at 6 mo FU (p=0.772, p=0.009), at 12
mo FU (p=0.711, p=0.021) and at 24 mo FU (p=0.455, p=0.187).
In the SF-36 questionnaire, a significant improvement was showed in Physical Component Summary score (p<0.05), Role Physical (p<0.05) and Body Pain (p<0.05) (Figure 4).
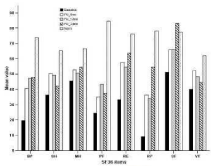
Figure 4: SF-36 mean scores at the baseline and at FU visits for the
assessed patients compared to normal populations Italian population results
(IQoLA group study (1995), 2031 persons).
Only 1 adverse event occurred: it was related to dislocation of the catheter which was replaced without any other complications.
All patients were satisfied, and 82% of them were really satisfied.
Discussion
Pain reduction is an important outcome measure as well as the improvement of functional abilities. The goal of IT morphine therapy is not only pain reduction but also the improvement of quality of life. Chronic pain related to spine disease dramatically limits patients’ ability and frequently leads to lost of autonomy. The collected data show that IT morphine infusion reduces pain intensity and increases patients’ functional ability and quality of life. Our results suggest that the IT morphine outcomes are optimized within the first year of the treatment and then maintained till 24 mo FU.
Results up to 2 years follow up show that pain influences patients’ functionality during the study period and, back pain improved more than leg pain. These findings may suggest different interpretations. While leg pain, dramatically improved during the first year of IT administration, it seems worsened during the second year. The first hypothesis could be that implanted patients with pain relief increases their daily activities and this can resume pain. A significant positive correlation was found between changes in VAS score for leg pain and changes in Oswestry score only till 12mos FU but not at 24mos FU, showing that pain relief is directly correlated by an improvement of pain impact on activities of daily living only on short term FU. The second hypothesis could be related to patients’ expectations that after a significant starting improvement of the outcomes (especially functional abilities) does not accept the steady state or does not accept that subsequent improvements become progressively less significant.
Our analysis also confirmed that the IT infusion system is a safe treatment able to reduce, indirectly, the opioid-related side effects. No side effects were seen up to 24mos FU due to the drugs at the administered doses.
However our data collection is still ongoing so further analysis will be provided to investigate the long term therapy outcome on a larger population.
Conclusions
We consider that, when conventional therapies fail, IT morphine administration may be an affective option for the management of severe, non-malignant chronic pain with disabilities. Furthermore a multidisciplinary approach with appropriate patients selection, correct clinical diagnosis and a trial infusion period could reduce post implant failure and improve results. Additional methods to investigate patient’s satisfaction should be developed.
References
- Boswell MV, Trescot AM, Datta S, Schultz DM, Hansen HC, Abdi S, et al. Interventional Techniques: Evidence-based Practice Guidelines in the Management of Chronic Spinal Pain. Pain Physician. 2007; 10: 7-111.
- Freburger JK, Holmes GM, Agans RP, Jackman AM, Darter JD, Wallace AS, et al. The rising prevalence of chronic low back pain. Arch Intern Med. 2009; 169: 251-258.
- Kumar K, Kelly M, Pirlot T. Continuous intrathecal morphine treatment for chronic pain of nonmalignant etiology: long-term benefits and efficacy. Surg Neurol. 2001; 55: 79-86; discussion 86-88.
- Anderson VC, Burchiel KJ, Cooke B. A prospective randomized trial of intrathecal injection vs. epidural infusion in the selection of patients for continuous intrathecal opioid therapy. Neuromodulation. 2003; 6: 142-152.
- Doleys DM, Coleton M, Tutak U. Use of intraspinal infusion therapy with noncancer pain patients: follow up and comparison of workers compensation vs. non-workers compensation patients. Neuromodulation. 1998; 1: 149-159.
- Winkelmuller M, Winkelmuller W. Long term effects of continuous intrathecal opioid treatment in chronic pain of nonmalignant etiology. J Neurosurg. 1996; 85: 458-467.
- Deer T, Chapple I, Classen A, Javery K, Stoker V, Tonder L, et al. Intrathecal drug delivery for treatment of chronic low back pain: Report from the National Outcomes Registry for low back pain. Pain Med. 2004; 5: 6-13.
- Thimineur MA, Kravitz E, Vodapally MS. Intrathecal opioid treatment for chronic nonmalignant pain: a 3-year prospective study. Pain. 2004; 109: 242- 249.
- Duse G, Davià G, White PF. Improvement in psychosocial outcomes in chronic pain patients receiving intrathecal morphine infusions. Anesthesia and Analgesia. 2009; 109: 1981-1986.
- Corrado PE, Varga CA, Concannon DM. Is intrathecal drug therapy an effective treatment for chronic pain? Am J Pain. Manage. 2005; 15: 33–37.
- Koulosakis A, Kuchta J, Bayarassou A, Sturm V. Intrathecal opioids for intractable pain syndromes. Acta Neurochir Suppl. 2007; 97: 43-48.
- Beurskens AJ, de Vet HC, Köke AJ. Responsiveness of functional status in low back pain: a comparison of different instruments. Pain. 1996; 65: 71–76.
- Taylor SJ, Taylor AE, Foy MA, Fogg AJ. Responsiveness of common outcome measures for patients with low back pain. Spine. 1999; 24: 1805–1812
- Fairbank JC, Davies JB, Couper J, O‘Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980; 66: 271–273.
- Apolone G, Mosconi P. The Italian SF-36 Health Survey: translation, validation and norming. J Clin Epidemiol. 1998; 51: 1025-1036.
- EuroQOL Group. EuroQOL – A new facility for the measurement of healthrelated quality of life. Health Pol. 1990; 16: 199-208.
*Consensus Conference -2012 Intrathecal Trialing Consensus Deer, et al. Neuromodulation. 2012; 15: 420-435.