
Research Article
Phys Med Rehabil Int. 2015;2(2): 1034.
Impact of Short Term OnabotulinumtoxinA Treatment on Dependency and Cost-of-Care in Patients with Spasticity Secondary to Upper Motor Neuron Syndrome
Gryfe P1* and Sharma S2
1Assistive Technology Clinic (ATC) at Sunnybrook Health Sciences Centre and the ATC Spasticity Management Clinic at Baycrest Health Sciences Centre, Toronto, ON
2Sunnybrook Health Science Centre, Department of Medicine, Division of Physiatry, Toronto, ON and ATC Spasticity Management Clinic, Baycrest Centre, Toronto, ON
*Corresponding author: Gryfe P, Clinical and Managing Director, Assistive Technology Clinic Baycrest Site, 3560 Bathurst St Toronto, ON
Received: January 08, 2015; Accepted: February 18, 2015 Published: February 19, 2015
Abstract
Background: Central nervous system injury or neurodegenerative disease with associated upper motor neuron damage often produces spasticity, with patients having physical disability, pain, and limitations in their ability to perform activities of daily living.
Objective: This open-label, non-randomized trial of adult outpatients with spasticity evaluated the impact of a single injection of onabotulinumtoxinA (BOTOX®, Allergan, Inc.) compared to a wait-listed control group on caregiver dependency, cost, and pain scores.
Methods: A convenience sample of 67 patients participated in the study. 42 patients (mean age 50.8 ± 18.85 years) in a multidisciplinary outpatient adult spasticity management clinic received a single injection of onabotulinumtoxinA plus standard care. 25 age-matched (mean age 50.54 ± 18.38 years) wait-listed controls received standard care. At month 1 and 3 follow-up visits, surveys to assess dependency, cost, and pain were administered.
Results: Of the 67 patients, 7 (2 treatment group and 5 wait-listed group) were lost to follow-up. The average dose of onabotulinumtoxinA administered was 420 U (range 100 to 600 U). The 38 onabotulinumtoxinA-treated patients exhibited statistically significant improvements in their degree of dependency (8.23 hour per week reduction in caregiver assistance [p=0.001]), their community cost subscale (decrease of $157 per week [p=0.001]), and pain (5.2 point reduction [p=0.017]). Wait-listed control group subjects exhibited no significant changes.
Conclusions: These findings suggest that a single injection of onabotulinumtoxinA plus standard care produces significant improvements in caregiver dependency, cost, and pain in adults with spasticity due to upper motor neuron syndromes. A larger well-controlled, randomized, study is warranted.
Keywords: Onabotulinumtoxin A; Dependency; Cost; Pain
Abbreviations
ADL: Activities of Daily Living; CP: Cerebral Palsy; CVA: CerebroVascular Accident; MAS: Modified Ashworth Scale; MPQ: McGill Pain Questionnaire; MS: Multiple sclerosis; NPCNA: Northwick Park Community Needs Assessment; NPDS: Northwick Park Dependency Score; PPI: Present Pain Index; PSW: Personal Support Worker; QALY: Quality Adjusted Life Year; SC: Standard care; SCI: Spinal cord injury; SD: Standard deviation; SF-36: Short Form 36-item; SFMPQ: Short Form McGill Pain Questionnaire; SIP: Sickness Impact Profile; TBI: Traumatic brain injury; UMNS: Upper motor neuron syndrome; VAS: Visual Analog Scale
Introduction
Spasticity is characterized by a velocity-dependent increase in tonic stretch reflexes with exaggerated tendon jerks that occurs as a part of the upper motor neuron syndrome (UMNS) [1]. Spasticity may occur secondary to a wide variety of central nervous system injuries or neurodegenerative diseases such as cerebrovascular accident (CVA) or stroke, spinal cord injury (SCI), traumatic brain injury (TBI), multiple sclerosis (MS), cerebral palsy (CP), or anoxicischemic encephalopathy, with the epidemiology of spasticity being dependent upon its origins [1].
It is estimated that spasticity affects more than 12 million people worldwide. Reported prevalence rates range from 17% to 38% among post-stroke survivors [2,3,4,5,], 17% to 53% of those with MS [6,7,8], 40% to 78% of those with SCI [9,10,11,12,13,14]; and up to 34% of those with TBI [1,15].
Once developed, UMNS and spasticity can cause severe problems such as contractures and painful limb deformities that impact patient positioning, movement, skin integrity, seating and mobility, and their ability to perform activities of daily living (ADLs) [1]. As a result, patients with spasticity and UMNS may be highly dependent on caregivers; so much so, that some consider that spasticity as a secondary complication of the UMNS is more of a limiting factor than the actual disease itself. In addition, motor over-activity often interferes with patient and caregiver functions and has an impact on the patient’s overall health-related quality of life [16].
Botulinum toxin type A injection has been used to achieve therapeutic benefits across a wide range of clinical conditions, including that of spasticity associated with UMNS [17,18,19,20,21,22,23]. Ward et al recently reported that the use of botulinum toxin type A (onabotulinumtoxinA) added to standard care as part of a goal-oriented rehabilitation in post-stroke spasticity patients significantly increased passive goal achievement and was associated with higher levels of active function [23]. An evidencebased review and assessment of botulinum toxin type A reported a Level A recommendation for the use of the onabotulinumtoxinA formulation in upper limb spasticity as well as in lower limb spasticity [1]. The primary outcome measures assessed in the evaluated studies were those related to muscle tone using instruments such as the modified Ashworth Scale (MAS), and patient- and/or investigatorreported outcomes such as health-related quality of life and perceived improvements. Few if any studies assessed functional benefits of tone reduction or the degree of residual dependency patients experience with the treatment effect. This may be, in part, due to the notion that the problems experienced by patients with UMNS are so broad and individual that generic standard measures of function may not be sensitive to change [16]. Likewise, few studies have explored the economic impact of botulinum toxin A treatment. Three groups of investigators [24,25,26] explored the economic impacts of therapy; all concluding that further investigation was required to quantify caregiving and/or nursing utilization costs as they are likely to be major drivers of cost.
This study aimed to investigate the impact of a single treatment with onabotulinumtoxinA plus standard care (SC) on caregiver dependency, costs and pain, in patients with moderate-to-severe spasticity due to UMNS.
Methods
Study design
The study was an open-labeled, non-randomized evaluation of onabotulinumtoxinA (BOTOX®, Allergan, Inc.) plus standard care (SC) treatment and a wait-listed plus SC controlled group design that was approved by the Research Ethics Committee of Sunnybrook Health Sciences Centre, a large academic health sciences centre in Toronto, Ontario, Canada and the University of Toronto. The waitlisted plus SC controlled design was an ethical alternative to a placebocontrolled group. At the spasticity clinics in Toronto waiting lists may vary from 4 to 12 months in duration. This design afforded wait-listed patients to participate in the SC control group and compare these changes with those of patients in the onabotulinumtoxinA plus SC treatment group across equivalent time intervals. Inclusion of the wait-listed SC control group reduced possible reactive effects of the experimental procedure, consequently improving external validity for the pre-test/post-test design [27].
Patient recruitment
Patients with moderate-to-severe spasticity due to a UMNS were identified from various interdisciplinary outpatient clinics at Sunnybrook Health Sciences Center. Included patients were required to have spasticity that was interfering with ambulatory function or their ability to perform ADLs, were cognitively intact, able to provide informed consent, and able to speak English or had an English speaking caregiver.
Individuals excluded from participating were those: with pain having no significant effect on function; whose medical condition could be adversely affected by the receipt of botulinum toxin (eg, myasthenia gravis or Eaton-Lambert syndrome); with a fear of needles or pain sensitivity; who had received botulinum toxin injections within the last 4 months, or who had a surgical procedure for spasticity that required general anesthesia.
Potential subjects were contacted by research assistants via telephone. Subjects allocated to the wait-listed control group (CONT) provided verbal consent and were mailed the three questionnaires to complete (baseline data), with a second set of these three questionnaires completed after 1 to 3 months when they were seen in the clinic for the first time. Eligible subjects, or their authorized representative, allocated to the onabotulinumtoxinA treatment group (OBTA) were seen in the clinic where they provided consent after being informed by the clinic physiatrist of the injection procedure, its potential benefits and side effects. OBTA-treatment allocated subjects completed the three questionnaires prior to receiving the first (single) injection and recompleted these questionnaires at 1 to 3 months postinjection. Subjects in both allocation groups (i.e. OBTA or CONT) continued to receive their standard of care which included current oral medications ( ie. Baclofen™, Lioresol™, or Dantrium™) and regular occupational or physical therapy regimens ( ie. 2-3 times per week of OT and/or PT standard range of movement and stretching programs).
A total 215 patients were considered for study inclusion, with 148 being excluded due to not meeting inclusion criteria or other reasons (Figure 1). Of the 67 eligible patients, 41 were allocated to the onabotulinumtoxinA injection plus SC group and 26 were allocated to the wait-listed SC alone control group. The groups were similar with respect to mean age, percentages of males and females, and having an underlying UMNS.
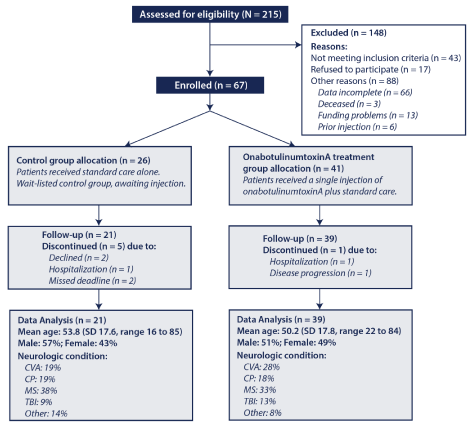
Figure 1: Participant Selection and Progress Flow Diagram. Of the 215 patients considered for the evaluation, 148 were excluded, 67 were enrolled with 26 patients comprising the standard care wait-list control group and 41 receiving a single injection of botulinum toxin type A. SD= Standard Deviation; CVA= CerebroVascular Accident; CP= Cerebral Palsy; MS= Multiple Sclerosis; TBI= Traumatic Brain Injury.
Outcome measures and questionnaires
Muscle tone was assessed using the 0 to 5 point Modified Ashworth Scale (MAS). Muscle strength was evaluated using the 0 to 5 point Oxford Scale (0-5) and passive and active range of motion was quantified with an electro goniometer. Subjects allocated to the OBTA or CONT, each with SC, were asked to complete the Northwick Park Community Needs Assessment, the Short Form McGill Pain, and the Short Form 36-item Health Survey questionnaires at the baseline and follow-up visits.
The Northwick Park Community Needs Assessment (NPCNA) and the Northwick Park Dependency Score (NPDS) have been shown to be simple to use, sensitive, and are reliable and valid in a neurologic setting to assess patient dependency, caregiver burden, and impact on nursing time [28,29]. The NPCNA is derived from the NPDS in that it includes a measure of cost to the community in terms of patient care and considers time need for patient supervision and physical assistance. The NPCNA was developed specifically to show the benefits of therapeutic interventions in terms of reducing costs and is sensitive to use in patients with complex disability [30,31]. In this study the NPCNA was modified to reflect the current costs of community care in the Greater Toronto Area using Canadian dollars and rates for personal support workers and nursing care. This data was utilized to convert the NPDS scores into measures of cost to the community.
The McGill Pain Questionnaire (MPQ) is considered to be "one of the most extensively tested measures of all time" [32]. The MPQ along with the Visual Analog Scale (VAS), another predominant clinical measure of pain, have been used to assess the impact of therapy [33]. The MPQ and VAS and the Present Pain Index (PPI) were merged in 1987 into a shorter and easier to administer McGill Pain Questionnaire called the Short Form McGill Pain Questionnaire (SFMPQ) [34]. The SFMPQ has been shown to correlate highly with the original questionnaire and was shown to have good test-retest reliability [35].
The Short Form 36-item (SF-36), which has been found valid and reliable for clinical use, was utilized instead of the Sickness Impact Profile (SIP) as it may have better retesting and is quicker and easier to complete in subjects with various stages of disability [36].
Statistical analysis
Patients in the OBTA injection group received a mean dose of 422.6 U (range 100 U to 600 U). Mean dose was calculated via an average of the aggregate of dosages for upper and lower extremity spasticity. The mean follow-up period time was 1.89 ± 0.93 months in the OBTA injection group and 2.04 ± 1.16 months in the wait-listed SC alone control group.
Based on the epidemiological study design approach of Hulley et al. [37], the required sample size was calculated at 62. Data was analyzed using SPSS (12.0) for Windows. Descriptive statistics were used to identify baseline characteristics of patients within the treatment group and of those within the control group. Variables identified as continuous were reported using means ± standard deviation (SD). Categorical variables were reported as percentages. Statistical analysis was conducted within subjects (pretest vs. posttest) as well as between subjects (control group versus treatment group). The Shapiro-Wilk test for normality (α, 0.05) showed that variables were not normally distributed. Distribution-free, nonparametric tests were chosen for statistical analysis. Two-related-sample Wilcoxon tests were used to analyze repeated measures for within subject analysis [38]. Mann- Whitney U tests were used for comparison between subjects [38]. All comparisons were made using means and SD. Statistical significance was set at P equal to or less than 0.05. All data was initially assessed for outliers. Outlying responses were carefully examined and determined to be valid cases. Thus, outliers were included throughout analysis, as they could not be reasonably rejected as spurious results.
The study design incorporated repeated measures (preexperimental pre-test/post-test) in which the change in spasticity in the OBTA treatment group served as the independent variable and the primary outcome measure. Level of dependency, cost, pain and health-related quality of life were the dependent variables. The pretest/ post-test design was chosen as an indication of the direction and magnitude of possible treatment effect. Changes within the OBTA plus standard care treatment group were compared to those of the CONT standard care alone group across equivalent time intervals.
Results
As illustrated in Figure 2, patients given the single injection of onabotulinumtoxinA exhibited a significant improvement in muscle tone, with MAS scores decreasing from 3.45 at baseline to 2.25 at follow-up (p=0.001).
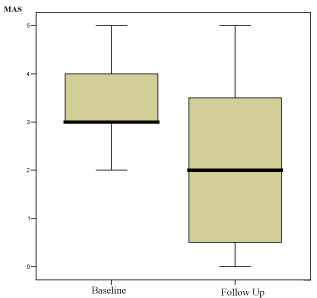
Figure 2: MAS Scores at Baseline and Follow-up in Patients Allocated to
Botulinum Toxin Type A Group.
Patients allocated to the single injection botulinum toxin type A treatment
group exhibited a significant improvement in muscle tone (measured by the
MAS) at follow-up (from 3.45 to 2.25; p=0.001).
As summarized in Table 1, at baseline there were no significant differences (all p>0.16) between the patients allocated to the OBTA injection and the CONT plus SC, groups in mean NPCNA dependency scores (measured as caregiver burden in hours per week) and cost to the community (measured as treatment costs per week). At baseline, the two groups did not differ in mean SFMPQ pain scores.
Wait-List Control Group
Botulinum toxin type A treatment group
Baseline
Follow-up
(2.04 ± 1.16 months)
Baseline to Follow-up Difference Score
Baseline
Follow-up
(1.89 ± 0.93 months
Baseline to Follow-up Difference Score
NPCNA Caregiver Hours per week
Mean ± SD
47.83 ± 39.01
50.41 ± 39.69
2.58 ± 9.80
70.77 ± 60.71
62.53 ± 59.02
-8.23 ± 10.89
N
21
21
21
38
38
38
Test Statistics-Between groups (treatment vs. control) at Baseline and Follow-Up
Baseline
Mann-Whitney U 317.5; Z = -1.29; Asymp Sig (2 tailed) = 0.19
Follow-up
Mann-Whitney U 149.5; Z= -3.99; Asymp Sig (2 tailed) < 0.000
Wilcoxon Signed Rank test-within group (baseline to follow-up)
Z
-1.52
-4.41
Asymp. Sig. (2-tailed)
0.13
<0.000
NPCNA Community Cost (weekly cost of care in dollars)
Mean ± SD
1020 ± 915
1077 ± 897
57.52 ± 228
1396 ± 1144
1238 ± 1082
-157 ± 301
N
21
21
21
38
38
38
Test Statistics-Between groups (treatment vs. control) at Baseline and Follow-up
Baseline
Mann-Whitney U 311.5; Z = -1.39; Asymp Sig (2-tailed) = 0.164
Follow-up
Mann-Whitney U 213.5; Z = -3.34; Asymp Sig (2-tailed) = 0.001
Wilcoxon Signed Rank test-within group (baseline to follow-up)
Z
-1.26
-3.42
Asymp. Sig. (2-tailed)
0.21
0.001
SFMPQ Pain Score
Mean ± SD
14.19± 13.708
12.41 ± 13.29
-2.20 ± 7.21
16.90 ± 18.32
10.75 ± 12.12
-5.2 ± 8.70
N
17
16
16
28
27
26
Test Statistics-Between groups (treatment vs. control) at Baseline and Follow-up
Baseline
Mann-Whitney U 155.50; Z = -0.38; Asymp Sig (2-tailed) = 0.70
Follow-up
Mann-Whitney U 127.00; Z = -0.77; Asymp Sig (2-tailed) = 0.44
Wilcoxon Signed Rank test-within group (baseline to follow-up)
Z
-1.16
-2.39
Asymp. Sig. (2-tailed)
0.25
0.02
SF-36 Quality of Life (Physical Subscale)
Mean ± SD
33.15 ± 9.55
33.75 ± 9.20
0.22 ± 6.55
31.91 ± 10.68
34.41 ± 9.55
2.08 ± 7.64
N
17
16
16
28
27
26
Test Statistics-Between Groups (treatment vs. control) at Baseline and Follow-up
Baseline
Mann-Whitney U 203.00; Z = -0.82; Asymp Sig (2-tailed) = 0.41
Follow-up
Mann-Whitney U 172.00; Z = -0.93; Asymp Sig (2-tailed) = 0.35
Wilcoxon Signed Rank test-baseline to follow-up
Z
-0.21
-1.28
Asymp. Sig. (2-tailed)
0.84
0.20
SF-36 Quality of Life (Mental Subscale)
Mean ± SD
49.85 ± 18.19
50.78 ± 11.06
-0.01 ± 9.84
49.86 ± 11.16
51.49 ± 13.14
2.88 ± 15.96
N
17
16
16
28
27
26
Test Statistics-Between Groups (treatment vs. control) at Baseline and Follow-up
Baseline
Mann-Whitney U 235; Z = -0.07; Asymp Sig (2-tailed) = 0.94
Follow-up
Mann-Whitney U 193; Z = -0.40; Asymp Sig (2-tailed) = 0.69
Wilcoxon Signed Rank test-baseline to follow-up
Z
-0.05
-0.59
Asymp. Sig. (2-tailed)
0.96
0.55
Table 1: Dependency, Costs of Care, Pain and Health-Related Quality of Life Scores at Baseline.
Difference scores (ie, from post-test to pre-test) were calculated to determine the amount of change in each category. Those allocated to the OBTA treatment group had a decrease in caregiver burden hours of 8.23 per week (from 70.77 to 62.53) as well as decrease in weekly community cost of $157.71 (CND) (from $1396.03 to $1238.32 (CND) per week) and a decrease in pain score of 5.2 points (from 16.9 to 10.75) (Table 1). Using nonparametric analysis to compare difference scores between the OBTA and CONT groups, Mann-Whitney U tests revealed statistically significant between-groups differences in caregiver hours (-8.23 hours versus +2.58 hours; Mann Whitney U 149.5; Z= -3.99; p<0.000) and in community costs per week (-157.71 versus +57.52; Mann Whitney U 213.5; Z =-3.34; p=0.001).
Using a 2-related sample Wilcoxon Signed Rank test comparing pre-test and post-test data (ie, mean of 1.89 months in OBTA group), those given onabotulinumtoxinA had a significant within group improvement from baseline in SFMPQ pain scores (Z=-2.39; p=0.017) and in caregiver hours (Z=-4.41; p<0.000) and community cost of care (Z=-3.42; p=0.001). None of the pre-test to post-test changes (mean of 2.04 months) in the CONT group was significant.
A post-hoc subgroup analysis was conducted on the OBTA treatment group to examine the relative baseline to follow-up improvements in each outcome measure by initial neurologic diagnosis (ie, CVA, CP, MS, TBI, or other). Due to the limited number of patients in the CONT group a similar sub-analysis was not performed. Among those given a single injection of onabotulinumtoxinA, the summarized data showed that those with MS experienced the most severe pain at baseline and the greatest improvement at follow-up (baseline 26.38 to 18.38 at follow-up). Substantial decreases in community costs per week were noted across the neurologic etiology groups (ranging from -$64 (CND) in those with CP to -$448(CND) per week in those with "other" conditions). Caregiver hours decreased in all groups, with the weekly number of hours decreasing by 3.86 hours per week in patients with CVA to -16.06 hours per week in those with TBI.
With regards to health related quality of life scores in the SF- 36 improvements in mental and physical quality of life measures were not significant (p> .05):. In fact, in the physical subscale scores ranged from slight worsening (-0.47, CP patients) to a 4.86 point improvement in those with TBI. Similarly, health-related quality of life improvements in the mental subscale ranged from -0.60 in those with CP and MS to a +3.98 and +7.86 improvement in those with TBI and CVA, respectively. These results may be attributable to limitations inherent to the study instrument. Patients regularly expressed dissatisfaction with SF-36 health survey, stating that the questions were not applicable to them. Patients often expressed difficulty in selecting responses stating that outside of their conditions they were content with their quality of life. Researchers involved in this study feel that an instrument that quantitatively assesses patients’ perceptions of and satisfaction with the efficacy of their treatment is of greater relevance, given the aforementioned mentioned patient concern.
Discussion
The findings of this open-label, non-randomized analyses indicate that a single injection of onabotulinumtoxinA at an average dose of 420 U was associated with significant reductions, as compared to before the injection, in muscle tone (as measured using the MAS), pain, degree of caregiver dependency, and costs in adults with upper motor neuron syndrome-related spasticity. OnabotulinumtoxinA treated patients as well as the age-matched, wait-listed control group received standard care; however, the wait-listed control group experienced no significant changes in the parameters of pain, caregiver dependency, or cost.
Improvements in muscle tone, pain and reductions in caregiver dependency with the use of onabotulinumtoxinA injection(s) as observed in our analyses have the potential to result in substantial cost savings. These findings support the data and conclusions from other reported studies using onabotulinumtoxinA to reduce spasticity, decrease pain, improve patient functioning, and reduce caregiver dependency, which together should impact the overall costs.
In one study by Ward and colleagues, patients within the onabotulinumtoxinA treatment group experienced significantly greater improvements in caregiver burden, measured as the number of care hours and community cost per week, than their counterparts in the control group [26]. In our analysis, the reduction of approximately 8.23 hours per week of caregiver assistance using hourly rates of $19 (CND) per hour for personal support workers and rates of $50 (CND) per hour for registered nursing care is projected to save between $156 (CND) (all personal support workers) to $411.50 (CND) (all registered nursing hours) per week or $8,164 to $21,372 (CND) annually. Even when factoring in the cost of onabotulinumtoxinA injections, which are approximately $360 (CND)per 100 U with administration every 3 months or approximately $5,760 (CND) for a patient who receives 400 U four times per year, the overall cost savings are substantial, ranging from 30% to 73% savings in the cost of care.
Lundstrom and colleagues followed a sample of patients with stroke requiring hospitalization for 1 year during which all direct costs were identified [39]. The investigators noted that the level of costs for patients with stroke was correlated with the presence of spasticity and the degree of disability. The mean annual direct cost for stroke patients with spasticity was 4-times higher as compared to those without spasticity. Specifically, the direct costs were $84,195 for the 1-year period in those with spasticity as compared to $21,385 in those without spasticity (p<0.001). [39]
In a study looking at resource utilization costs, and health-related quality of life in patients with multiple sclerosis and different levels of spasticity by Svensson and colleagues, the total costs increased with the severity of spasticity Overall total costs were 2.4 times greater in those with severe spasticity than in those with mild spasticity [40].
In contrast, in a study by Shaw et al. of 333 adults with post stroke spasticity, the addition of botulinum toxin type A to an upper limb therapy programme for the treatment of upper limb spasticity due to stroke was not estimated to be cost-effective at levels of willingness to pay for a QALY set by NHS decision-makers. However, they did report that botulinum toxin type A may have a role to play in improving the ability of some patients to undertake some basic upper limb functional tasks and may reduce pain at 12 months [41].
In a separate analysis of the Shaw data, Shackley et al. estimated that the incremental cost per quality adjusted life year (QALY) gained with botulinum toxin type A plus an upper limb therapy program as compared to an upper limb therapy program alone did not exceed 0.39. It appears that this conclusion did not acknowledge the assumptions that botulinum toxin type A plus therapy may have some clinical benefit and may have a role to play in improving the ability of some patients to undertake basic upper limb functional tasks and reduce pain at 12 months [42].
In another review of the impact of post-stroke spasticity on caregiver burden, Zorowitz and colleagues reported that stroke survivors with spasticity were more dependent. Consequently, the reliance on caregivers results in these individuals experiencing poorer physical and emotional health. Additionally, the caregivers of those with stroke often manifest emotional feelings of confinement and other issues as compared to the general population [43].
Several analyses utilizing botulinum toxin type A treatments support its efficacy in reducing pain by decreasing muscle tone and thereby increasing patient functioning. Brashear et al reported improvement in pain in post stroke patients with affected upper limbs after a single treatment of fixed dose of OnabotulinumtoxinA [19]. Studies by Dunne and Sheean have also reported significant reductions in pain in patients receiving onabotulinumtoxinA injections [44, 45]. Ward et al reported that adding onabotulinumtoxinA treatment to standard care in the open label phase of a randomized placebo controlled study significantly improved the outcome in passive functional goals and that patients receiving onabotulinumtoxinA treatment reported significant improvements in pain on the Short Form McGill Pain Questionnaire (p=0.017) [46].
Although the advantages of using OnabotulinumtoxinA for spasticity management are well documented, as with any invasive procedure, patients and care providers need to weigh the potential benefits of treatment with the demands and risks that are associated with treatment. According to all the literature reviewed no serious side effects associated with OnabotulinumtoxinA for spasticity management or its long-term use have been reported. Potential side effects could include dry mouth and difficulty in swallowing and OnabotulinumtoxinA has been contra- indicated for patients with Myasthenia Gravis and Eaton-Lambert syndrome [47]. OnabotulinumtoxinA has been given to patients as young as 2 and as old as 82 and has been shown to be safe in both populations, both of which are outside the boundaries defined for this study [44, 48]. The most serious side effects shown in our study were soreness and redness at the site of injection, or muscle weakness caused by the injection, which is the desired result.
Confounders
The authors recognize that there were a number of confounders inherent in this study not the least of which were the cohort effects and the fact that the control and treatment groups were not matched with regards to the etiology of spasticity, age or sex. In analysis, subjects were stratified into subgroups according to their etiology then the relation between the predictor and outcome was examined separately in each subgroup. In the treatment group comparisons were first made within subjects to minimize the influences of extraneous variable on the dependent variable (controlling for between subject variability), thereby increasing the likelihood of producing significant results.
Additional limitations include the fact that the Pre-test/post test design is limited as causality cannot adequately be inferred nor were the investigators able to control for placebo effects. These limitations can be resolved by doing a randomized placebo controlled trial of at least 3 cycles of treatment.
Conclusion
Results from our cohort study seem to support the data from other similar type studies that reveal that even a single treatment of onabotulinumtoxinA demonstrates the potential for improving patients’ comfort by reducing muscle spasticity and pain and reducing the burden of care. Combining this treatment with standard care also has the potential to reduce the cost of care over a short period. Despite improvements in pain and caregiver burden, the study’s findings suggest that patients receiving treatment did not experience significant improvements in their quality of life. These results may be attributable to limitations inherent to the study instrument.
Due to limitations in time and resources, data was only collected for patients at baseline and post injection follow up. It would be interesting to study whether improvements indicated in this study across pain; care hours and community costs are sustained over a longer study period, tracking subsequent injection-follow-up cycles.
The authors recognize that there are a number of intrinsic limitations to the study, particularly that this study considers patients with various forms of UMN syndromes, across a large spectrum of disability. As a result the authors conclude that a larger randomized controlled study is warranted and that future investigations should measure and control for degree of disability matching the etiology of spasticity, severity, dose of onabotulinumtoxinA and site of involvement.
With the great necessity for more effective programs to meet the needs confronting our clients, health professionals need to rely on evaluative outcomes as a source of knowledge and direction. OnabotulinumtoxinA has virtually revolutionized the way we treat hyper-tonicity in patients with UMNS and spasticity and as a result more attention should be given to botulinum toxin treatment as an adjunct to physical and occupational therapy for enhancing function, preventing further disability, and when considering relief of pain and care giver burden.
Acknowledgements
This study was supported by a grant from Human Resources and Development Canada (HRDC). The authors wish to thank Allergan, Inc., for supporting the costs of production and also Susan Ruffalo (MedWrite International, Inc.) for her assistance in editing the manuscript.
References
- Esquenazi A, Albanese A, Chancellor MB, Elovic E, Segal KR, Simpson DM, Smith CP . Evidence-based review and assessment of botulinum neurotoxin for the treatment of adult spasticity in the upper motor neuron syndrome. Toxicon. 2013; 67: 115-128.
- Lundström E, Terént A, Borg J. Prevalence of disabling spasticity 1 year after first-ever stroke. Eur J Neurol. 2008; 15: 533-539.
- Sommerfeld DK, Eek EU, Svensson AK, Holmqvist LW, von Arbin MH. Spasticity after stroke: its occurrence and association with motor impairments and activity limitations. Stroke. 2004; 35: 134-139.
- Watkins CL, Leathley MJ, Gregson JM, Moore AP, Smith TL, Sharma AK. Prevalence of spasticity post stroke. Clin Rehabil 2002;16: 515-522.
- Welmer AK, von Arbin M, Widén Holmqvist L, Sommerfeld DK. Spasticity and its association with functioning and health-related quality of life 18 months after stroke. Cerebrovasc Dis. 2006; 21: 247-253.
- Barnes MP, Kent RM, Semlyen JK, McMullen KM. Spasticity in multiple sclerosis. Neurorehabil Neural Repair. 2003; 17: 66-70.
- Goodin DS. A questionnaire to assess neurological impairment in multiple sclerosis. Mult Scler. 1998; 4: 444-451.
- Rizzo MA, Hadjimichael OC, Preiningerova J, Vollmer TL. Prevalence and treatment of spasticity reported by multiple sclerosis patients. Mult Scler. 2004; 10: 589-595.
- Anson CA, Shepherd C. Incidence of secondary complications in spinal cord injury. Int J Rehabil Res. 1996; 19: 55-66.
- Johnson RL, Gerhart KA, McCray J, Menconi JC, Whiteneck GG. Secondary conditions following spinal cord injury in a population-based sample. Spinal Cord. 1998; 36: 45-50.
- Maynard FM, Karunas RS, Waring 3rd, WP. Epidemiology of spasticity following traumatic spinal cord injury. Arch Phys Med Rehabil 1990; 71: 566-569.
- Noreau L, Proulx P, Gagnon L, Drolet M, Laramée MT. Secondary impairments after spinal cord injury: a population-based study. Am J Phys Med Rehabil. 2000; 79: 526-535.
- Sköld C, Levi R, Seiger A. Spasticity after traumatic spinal cord injury: nature, severity, and location. Arch Phys Med Rehabil. 1999; 80: 1548-1557.
- Walter JS, Sacks J, Othman R, Rankin AZ, Nemchausky B, Chintam R, et al. A database of self-reported secondary medical problems among VA spinal cord injury patients: its role in clinical care and management. J Rehabil Res Dev. 2002; 39: 53-61.
- Wedekind C, Lippert-Grüner M. Long-term outcome in severe traumatic brain injury is significantly influenced by brainstem involvement. Brain Inj. 2005; 19: 681-684.
- Sheean G. Botulinum toxin treatment of adult spasticity. Expert Rev Neurother. 2003; 3: 773-785.
- Ashford S, Turner-Stokes I. Management of shoulder and proximal upper limb spasticity using botulinum toxin and concurrent therapy interventions: A preliminary analysis of goals and outcomes. Disabil Rehabil 2009;31: 220-226.
- Bakheit AMO, Fedorova NV, Skoromets AA, Timerbaeva SL, Bhakta BB, Coxon L. The beneficial antispasticity benefit of botulinum toxin type A is maintained after repeated treatment cycles. J Neurol Neurosurg Psychiatry 2004; 75:1558-1561.
- Brashear A, Gordon MF, Elovic E, Kassicieh VD, Marciniak C, Do M, Lee CH. Intramuscular injection of botulinum toxin for the treatment of wrist and finger spasticity after a stroke. N Engl J Med. 2002; 347: 395-400.
- Elovic E, Brashear A, Kaelin D, Liu J, Millis SR, Barron R, Turkel C. Repeated treatment with botulinum toxin type A produce sustained decreases in the limitations associated with focal upper-limb poststroke spasticity for caregivers and patients. Arch Phys Med Rehabil 2008; 89:799-806.
- Kaňovský P, Slawek J, Denes Z, Platz T, Comes G, Grafe S, et al. Efficacy and safety of treatment with incobotulinum toxin A (botulinum neurotoxin type A free from complexing proteins; NT 201) in post-stroke upper limb spasticity. J Rehabil Med. 2011; 43: 486-492.
- Turner-Stokes L, Baguley IJ, De Graaf S, Katrak P, Davies L, McCrory P, Hughes A. Goal attainment scaling in the evaluation of treatment of upper limb spasticity with botulinum toxin: A secondary analysis from a double-blind placebo-controlled randomized clinical trial J Rehabil Med 2010;42: 81-89.
- Ward AB, Wissel J, Borg J, Ertzgaard P, Herrmann C, Kulkarni J, et al. Functional goal achievement in post-stroke spasticity patients: the BOTOX® Economic Spasticity Trial (BEST). J Rehabil Med. 2014; 46: 504-513.
- Radensky PW, Archer JW, Dournaux SF, O'Brien CF. The estimated cost of managing focal spasticity: a physician practice patterns survey. Neurorehabil Neural Repair. 2001; 15: 57-68.
- Wallesch CW, Maes E, Lecomte P, Bartels C. Cost effectiveness of botulinum toxin type A injection in patients with spasticity following stroke: A German perspective. Euro J Neurol 1997; 54, S53-S57.
- Ward A, Roberts G, Warner J, Gillard S. Cost-effectiveness of botulinum toxin type a in the treatment of post-stroke spasticity. J Rehabil Med. 2005; 37: 252-257.
- Hursey KG, Rains JC, Penzien DB, Nash JM, Nicholson RA. Behavioral headache research: methodologic considerations and research design alternatives. Headache. 2005; 45: 466-478.
- Hatfield A, Hunt S, Wade DT. The Northwick Park Dependency Score and its relationship to nursing hours in neurological rehabilitation. J Rehabil Med. 2003; 35: 116-120.
- Turner-Stokes, L. Measurement of Outcome in Rehabilitation: Basket of Measures. The British Society of Rehabilitation Medicine; 2000.
- Nyein K, Turner-Stokes L, Robinson I. The Northwick Park Care Needs Assessment (NPCNA): a measure of community care needs: sensitivity to change during rehabilitation. Clin Rehabil. 1999; 13: 482-491.
- Turner-Stokes L. Outcome measures for inpatient neurorehabilitation settings. Neuropsycholog Rehabil 1999; 9: 329-343.
- Graham C, Bond SS, Gerkovich MM, Cook MR. Use of the McGill pain questionnaire in the assessment of cancer pain: replicability and consistency. Pain. 1980; 8: 377-387.
- Marques AP, Rhoden L, de Oliveira Siqueira J, João SM. Pain evaluation of patients with fibromyalgia, osteoarthritis, and low back pain. Rev Hosp Clin Fac Med Sao Paulo. 2001; 56: 5-10.
- Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987; 30: 191-197.
- Grafton K, Foster N, Wright CC. Test-retest reliability of the short form McGill pain questionnaire: Assessment of intraclass correlation coefficients and limits of agreement in patients with osteoarthritis. Clin J Pain 2005; 21: 73-82.
- Ho AK, Robbins AO, Walters SJ, Kaptoge S, Sahakian BJ, Barker RA. Health-related quality of life in Huntington's disease: a comparison of two generic instruments, SF-36 and SIP. Mov Disord. 2004; 19: 1341-1348.
- Hulley SB, Cummings SR, Browner WS, Grady D, Hearst N, Newman TB (Eds.). Designing clinical research: an epidemiological approach (second edition ed.), 2001, Philadelphia: Lippincott Williams & Wilkins, Table 6D, pg. 90.
- Daniel WW. Biostatistics. Eighth ed. New Jersey: John Wiley & Sons; 2005.
- Lundström E, Smits A, Borg J, Terént A. Four-fold increase in direct costs of stroke survivors with spasticity compared with stroke survivors without spasticity: the first year after the event. Stroke. 2010; 41: 319-324.
- Svensson J, Borg S, Nilsson P. Costs and quality of life in multiple sclerosis patients with spasticity. Acta Neurol Scand. 2014; 129: 13-20.
- Shaw L, Rodgers H, Price C, van Wijck F, Shackley P, Steen N, et al. BoTULS: A multicenter randomized controlled trial to evaluate the clinical effectiveness and cost-effectiveness of treating upper limb spasticity due to stroke with botulinum toxin types A. Health Tech Assess 2010;14: 26.
- Shackley P, Shaw L, Price C, van Wijck F, Barnes M, Graham L, et al. Cost-effectiveness of treating upper limb spasticity due to stroke with botulinum toxin type A: results from the botulinum toxin for the upper limb after stroke (BoTULS) trial. Toxins (Basel). 2012; 4: 1415-1426.
- Zorowitz RD, Gillard PJ, Brainin M. Poststroke spasticity: sequelae and burden on stroke survivors and caregivers. Neurology. 2013; 80: S45-52.
- Dunne JW, Heye N, Dunne SL. Treatment of chronic limb spasticity with botulinum toxin A. J Neurol Neurosurg Psychiatry. 1995; 58: 232-235.
- Sheean G. Botulinum toxin for the treatment of musculoskeletal pain and spasm. Curr Pain Headache Rep. 2002; 6: 460-469.
- Ward AB, Aguilar M, Zegers B, Gedin S, Kanovsky P, Molteni F, et al. Use of botulinum toxin type A in management of adult spasticity--a European consensus statement. J Rehabil Med 2003; 35: 98-99.
- Brin, MF, Aoki, KR. Botulinum Toxin Type A: Pharmacology. Spasticity: A we move Self-Study CME Activity, Etiology, Evaluation, Management and the Role of Botulinum Toxin. ; 2002. p. 110-124.
- Ubhi T. Treatment of paediatric cerebral palsy with Dysport. Hosp Med. 2000; 61: 718-721.