
Research Article
Phys Med Rehabil Int. 2025; 12(1): 1247.
Efficacy of High-Energy-Density Pulsed Electromagnetic Field Therapy for Rotator Cuff Tendinopathy
Lai CY¹,², Chang CY¹,², Pan KT³, Huang CY¹,², Hsu MC4, Hsu HH4,5, Huang SM6 and Chen LC¹,²*
1Department of Physical Medicine and Rehabilitation, Tri-Service General Hospital, School of Medicine, National Defense Medical Center, Taipei, Taiwan
2School of Medicine, National Defense Medical Center, Taipei, Taiwan
3Graduate Institute of Aerospace and Undersea Medicine, National Defense Medical Center, Taipei, Taiwan
4Department of Physical Therapy, National Defense Medical Center, Taipei, Taiwan
5School of Sports Medicine and Rehabilitation, Beijing Sport University, Beijing, China
6Department of Biochemistry, National Defense Medical Center, Taipei, Taiwan
*Corresponding author: Chen LC, Department of Physical Medicine and Rehabilitation, Tri-Service General Hospital, School of Medicine, National Defense Medical Center, No. 325, Sec. 2, Cheng-gong Rd., Neihu District, Taipei 114, Taiwan Email: clctsgh@yahoo.com.tw
Received: March 19, 2025 Accepted: April 08, 2025 Published: April 11, 2025
Abstract
Objective: This study aimed to evaluate the efficacy of high-energy-density pulsed electromagnetic field (high PEMF) therapy combined with physiotherapy in the treatment of rotator cuff tendinopathy (RCT).
Design: Randomized double-blind clinical trial.
Patients: Twenty-one participants with rotator cuff tendinopathy
Methods: Participants received either high PEMF therapy or sham PEMF therapy, both in combination with physiotherapy. Pain (visual analog scale, VAS), shoulder function (Shoulder Pain and Disability Index, SPADI), and range of motion (ROM) were assessed over a 12-week follow-up period
Results: Within-group analyses showed significant pain VAS reduction in the high PEMF group immediately post-treatment and at 4 weeks, while the sham PEMF group improved immediately and at 12 weeks. Disability scores in SPADI significantly improved in the high PEMF group at all follow-up period, whereas the sham PEMF group improved only at 12 weeks. However, no significant differences were found between the groups in overall outcomes.
Conclusion: High PEMF therapy combined with physiotherapy showed potential for short-term pain reduction and shoulder function improvement in patients with RCT, though not significantly enhancing shoulder mobility. This treatment option is safe, non-invasive, time-saving, and well tolerated, providing a promising alternative for patient care.
Keywords: High-energy-density pulsed electromagnetic field; Physiotherapy; Rotator cuff tendinopathy
Abbreviations
RCT: Rotator Cuff Tendinopathy; PEMF: Pulsed Electromagnetic Field; FDA: Food and Drug Administration; High PEMF: High- Energy-Density Pulsed Electromagnetic Field; VAS: Visual Analog Scale; SPADI: Shoulder Pain and Disability Index; ROM: Range of Motion; aROM: Active ROM; pROM: Passive ROM; IR: Internal Rotation; ER: External Rotation; MCID: Minimal Clinically Important Difference; ESWT: Extracorporeal Shock Wave Therapy.
Introduction
Rotator cuff tendinopathy (RCT) is common among individuals with shoulder pain [1]. Within 1 year of RCT diagnosis, approximately 40–50% of patients experience persistent pain or recurrence, leading to significant disability and reduced quality of life [2]. Therefore, shoulder pain caused by RCT requires careful attention and proper management.
Pulsed electromagnetic field (PEMF) therapy is a conventional treatment that has a long history of use. It is Food and Drug Administration (FDA)-certified for nonunion fracture treatment and has shown positive outcomes in postoperative pain management, swelling reduction, and treatment of arthritis [3]. In studies involving musculoskeletal disorders, PEMF therapy has been shown to inhibit pro-inflammatory cytokines in inflamed or injured tendon cells and promote the production of regenerative factors, thus suppressing pain and facilitating tissue repair [4]. Recent studies evaluating the effectiveness of PEMF therapy for shoulder pain have indicated a lack of significant clinical benefits. Consequently, regular application of the therapy is not recommended [5-7]. Some studies have suggested that this may be because of insufficient magnetic field intensity and improper oscillation frequencies generated by traditional PEMF therapy devices [5].
Most available PEMF therapy devices typically offer frequency options of 6–500 Hz and magnetic field intensities below 10 mT. The specific treatment frequencies, number of sessions, and session durations (usually 20–30 min but potentially extending to several hours) vary according to the machine's settings. There are no recommended treatment module settings for musculoskeletal diseases in clinical practice [4,8].
High-energy-density pulse electromagnetic field (high PEMF), in contrast to the traditional PEMF, encompasses a wide electromagnetic wave frequency range (200 kHz to 300 MHz) and features very short pulse durations (approximately 50 μs). With the device's high-voltage (up to 20 kV) and high-current (up to 10 kA) characteristics, each pulse can provide a maximum of approximately 96 J and a magnetic field of 50–150 mT, penetrating body tissues up to 20 cm [9]. High PEMF has achieved treatment success in conditions such as pelvic and lower back pain [10,11]. More recently, it has been used to treat conditions such as rotator cuff tendon and Achilles tendon disorders [5,12]; however, only few related studies are available. Therefore, this study aimed to investigate the efficacy of high PEMF therapy in the treatment of RCT.
Materials and Methods
Design
The study had a randomized controlled trial with a two-parallelgroup design. We included patients with RCT to compare the differences in the effectiveness of high PEMF in the treatment of shoulder pain, mobility, and function. The patients were categorized into those who received high PEMF with physiotherapy (high PEMF group) and those who received sham PEMF with physiotherapy (sham PEMF group). The treatment course extended over 3 weeks, incorporating evaluations at baseline, immediately after treatment, and 4 and 12 weeks after treatment. The study was prospectively registered on the clinicaltrials.gov website (NCT05483517).
Participants and Settings
We included patients who visited the outpatient rehabilitation department of a single medical center in Taipei, Taiwan, between January 31, 2023, and April 8, 2024. Participants who satisfied the enrollment criteria and provided informed consent were included in the study.
The inclusion criteria were as follows: (1) between 20 and 75 years of age; (2) persistent shoulder pain for at least 3 months; (3) positive result in Hawkins–Kennedy, Neer, or Jobe tests; and (4) confirmed RCT by ultrasonography or magnetic resonance imaging (MRI). The exclusion criteria were as follows: (1) complete or fullthickness tear of the rotator cuff discovered via ultrasonography or MRI; (2) previous history of shoulder surgery or severe trauma; (3) cervical radiculopathy-related shoulder pain or referred pain; (4) presence of any of the following systemic diseases: active infection, severe medical condition, cancer, immune-related or rheumatoid arthritis (5) shoulder injections within the last 3 months; and (6) any of the following contraindications for high PEMF: pregnancy or lactation, pacemakers, internal defibrillators and internal metal implants [9]. Of the initial 37 participants selected for the study, 24 provided informed consent and were subsequently enrolled in the study. A randomization sequence was created using Microsoft Excel. Afterward, the participants were allocated to either the high PEMF or sham PEMF group in a 1:1 ratio using block randomization (Figure 1).
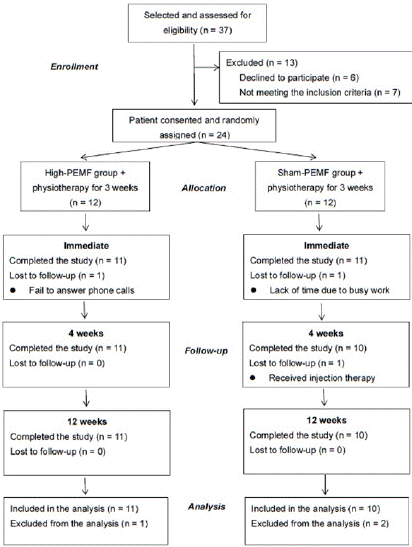
Figure 1: CONSORT diagram of participants’ flowchart throughout the trial.
This flowchart includes the recruitment of participants at the beginning of
the study, exclusions, randomization into different groups, and participants
who completed the post-assessment at the end of the study.
All the participants were evaluated for baseline conditions before any intervention. The treatment course lasted 3 weeks, with evaluations immediately after treatment and at 4 and 12 weeks after treatment to assess improvements in pain, function, and shoulder joint mobility. All measurements were evaluated and recorded by a physiatrist blinded to the group assignments. All participants were instructed not to use other therapies for the treatment of RCT symptoms throughout the study period, except acetaminophen (500 mg, up to 4 g/day) as a rescue medication. Additionally, all the participants were allowed to continue performing previous exercises during the treatment course at home. A research assistant regularly monitored the administration of the medications via phone calls.
Interventions
High PEMF: High PEMF treatment was administered using an Electrodynamics Electromagnetic Therapeutic Impulse Generator (PAPIMI Series, Model ASKLIPIOS, Electrodynamics Manufacturing Ltd, Lagoumitzi 61, 117 44 Athens, Greece) (Figure 2), with the following features according to the manufacturer's instructions [9]: (1) a wide electromagnetic wave frequency range (200 kHz to 300 MHz); (2) very short pulse durations (approximately 50 μs); (3) high voltage (up to 20 kV) and high current (up to 10 kA); and (4) each pulse can provide a maximum of approximately 96 J and a magnetic field of 50-150 milliTesla (mT), penetrating body tissues up to 20 cm.

Figure 2: Electrodynamics Electromagnetic Therapeutic Impulse Generator.
The treatment time can range from 1 min to 14 min 48 s, the pulse per second (PPS) can be set between 1 Hz and 8 Hz, and the energy level can be selected as either normal (2/3 of the maximum intensity) or high. A clinical nurse who was aware of the group assignments but was excluded from the subsequent follow-up and result analysis applied this treatment to the patients.
High PEMF group: The patients in this group received a 3-week course of physiotherapy, with sessions conducted twice a week, each lasting approximately 30 min. Under the guidance and supervision of a physical therapist, the sessions included shoulder range of motion (ROM), stretching, and muscle-strengthening exercises. Before each exercise therapy session, the patients received approximately 9 min of high PEMF treatment. The treatment coil was placed over the most painful shoulder area and maintained in position for 9 min. During the session, patients were exposed to normal energy at a frequency of 2 PPS.
Sham PEMF group: Patients in this group underwent a 3-week program of physiotherapy, which was the same as that in the high PEMF group, under the guidance and supervision of the same physical therapist. Before each exercise therapy session, the participants received approximately 9 min of sham PEMF treatment. The sham treatment coil, which had no energy output, was placed over the area of the shoulder where the patient experienced maximum pain and was left in position for 9 min.
Measures
Primary Outcome
Visual Analog Scale: The primary outcome was the average pain score during maximum shoulder movement over the previous week, which was determined using a visual analog scale (VAS) (0–10 cm), with "0" indicating painless and "10" indicating extremely painful. A 1.3-cm reduction in VAS score or a 25% reduction in pain was considered clinically significant [13,14].
Secondary Outcomes
Shoulder Pain and Disability Index: Shoulder function and disability were assessed using the Chinese version of the Shoulder Pain and Disability Index (SPADI). This self-report questionnaire comprises five pain-related questions and eight disability-related questions, addressing the various shoulder issues experienced over the previous week. Each item is scored on a scale of 0 (no pain or normal function) to 10 (maximal pain or disability). The total pain score ranges from 0 to 50 and the disability score ranges from 0 to 80, with higher scores indicating greater pain or disability [15]. A reduction of 8 points on the SPADI was considered clinically significant [16].
ROM: Shoulder active and passive ROM (aROM and pROM) during flexion, abduction in the sitting position, internal rotation (IR) at 90° of abduction, and external rotation (ER) at 90° of abduction of the affected shoulder were measured using a digital goniometer, and the mean of three values was used for analysis [17,18].
Sample Size: The sample size was calculated using the data from a previous study [19]. In addition, the sample size was calculated using STATA software by setting 80% as the power and 0.05% as the significance value. The researcher estimated that at least 11 participants would be required in each group.
Statistical Analysis
Statistical analyses were performed using the IBM SPSS Statistics Version 22. Demographic data were analyzed using the Mann– Whitney U test for continuous variables and the chi-squared test or Fisher’s exact test for categorical variables. Within-group differences were assessed using the Wilcoxon signed-rank test; between-group differences were evaluated using the Mann–Whitney U test. Statistical significance was defined as p < 0.05.
Results
Clinical and Demographic Characteristics of Study Participants
Twenty-one participants ultimately completed the study, with one participant in the high PEMF group and two participants in the sham PEMF group lost to follow-up and were all excluded from the analysis because of the reasons listed in Figure 1. Baseline characteristics are listed in Table 1, with no significant differences found between the groups. No adverse effects resulting from high PEMF or physiotherapy, as observed during the follow-up period.
high PEMF (n=11)
Sham PEMF (n=10)
P valuea
Sex (%)
0.67
male
6
4
female
5
6
DM (%)
0
1
-
HTN (%)
1
3
0.311
Smoking (%)
0
0
-
Drinking (%)
1
0
-
Age (year)
62.73 (7.51)
56.10 (10.55)
0.121
Height (cm)
162.64 (9.06)
163.90 (7.78)
0.916
Weight (kg)
62.00 (5.87)
63.05 (6.79)
0.671
BMI (kg/m2)
23.49 (2.11)
23.47 (2.01)
0.888
Duration (weeks)
13.91 (3.70)
19.80 (14.91)
0.506
Table 1: Baseline demographic and clinical characteristics of study subjects.
Primary Outcome
Pain VAS: Figure 3 shows the baseline values and changes in the VAS scores over different weeks. No significant differences were observed between the groups at all follow-up periods (All p < 0.05). Within the groups, there was statistical significance immediately after treatment and at 4 weeks after treatment in the high PEMF group (p = 0.023 and p = 0.032, respectively), whereas statistical significance was found immediately and 12 weeks after treatment in the sham PEMF group (p = 0.036 and p = 0.031, respectively). A minimal clinically important difference (MCID) was observed at 4 and 12 weeks after treatment in the high PEMF group and immediately after and 12 weeks after treatment in the sham PEMF group. The actual values are listed in the supplementary material (Table 2).
high PEMF (n=11)
Mean (SD)p valuea
Sham (n=10)
Mean (SD)p valuea
p valueb
Pain VAS
Baseline
5.91 (2.51)
5.90 (1.91)
1.000c
Immediately
4.73 (2.37)
0.023
4.60 (2.27)
0.036
0.746
4 weeks
3.55 (1.69)
0.032
4.90 (1.52)
0.205
0.141
12 weeks
3.72 (2.61)
0.073
3.50 (2.27)
0.031
0.618
SPADI
Pain score
Baseline
26.46 (8.34)
27.30 (11.39)
1.000c
Immediately
19.10 (11.95)
0.036
22.90 (10.02)
0.169
0.548
4 weeks
24.10 (22.26)
0.386
33.90 (30.22)
0.953
0.359
12 weeks
15.55 (9.69)
0.008
13.90 (13.32)
0.018
0.573
Disability score
Baseline
31.82 (15.48)
38.50 (21.60)
0.481c
Immediately
20.10 (14.39)
0.008
29.50 (18.04)
0.169
0.944
4 weeks
17.18 (12.49)
0.003
26.30 (14.06)
0.114
0.972
12 weeks
17.73 (13.06)
0.003
16.90 (21.14)
0.022
0.621
high PEMF: High-Energy Density Pulse Electromagnetic Field; SD: Standard Deviation; VAS: Visual Analog Scale; aWilcoxon Signed-Rank test (each time-points versus baseline); bMann–Whitney U Test (mean difference, intergroup); cMann–Whitney U Test (mean, intergroup).
Table 2: Comparison of outcome variables (VAS and SPADI) between both groups.
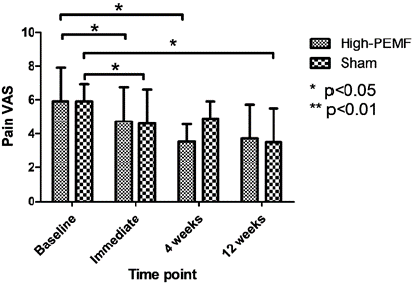
Figure 3: Comparison of pain VAS between both groups.
Secondary Outcomes
SPADI: Figure 4 shows the baseline values, changes in SPADI subsets over different weeks. No significant differences were observed between the groups at all follow-up periods for any SPADI subset (All p < 0.05). Within the groups, pain scores in SPADI significantly improved immediately after treatment and at 12 weeks after treatment (p = 0.036 and p = 0.008, respectively) in the high PEMF group, whereas statistical significance was found in the sham PEMF group only at 12 weeks (p = 0.018). Disability scores in SPADI significantly improved immediately, at 4 weeks, and at 12 weeks after treatment in the high PEMF group (p = 0.008, p = 0.003, and p = 0.003, respectively), whereas the sham PEMF group showed statistical significance only at 12 weeks (p = 0.022). The actual values are listed in the supplementary material (Table 2).
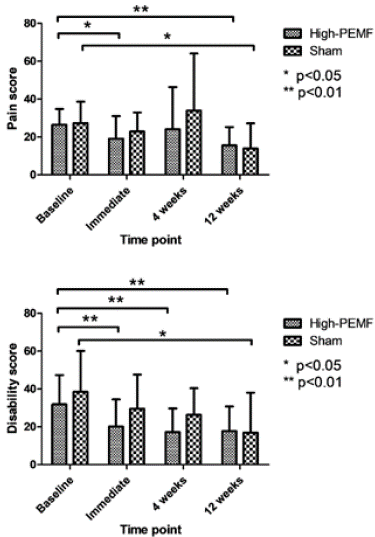
Figure 4: Comparison of SPADI between both groups.
Active and Passive ROM of the Shoulder Joint
The baseline values and changes in shoulder aROM and pROM at different weeks are listed in the supplementary material (Table 3). No significant differences were observed between groups at baseline or during the follow-up period. Within the groups, only statistical significance was found for the aROM of IR at 4 and 12 weeks after treatment (p = 0.026 and p = 0.003, respectively) and the pROM of IR at 12 weeks (p = 0.021) in the high PEMF group (Figure 5).
high PEMF (n=11)
Mean (SD)p valuea
Sham (n=10)
Mean (SD)p valuea
p valueb
Flex-aROM
Baseline
143.74 (20.18)
154.11 (23.47)
0.341c
Immediately
147.76 (19.81)
0.093
152.53 (24.93)
0.917
0.228
4 weeks
144.89 (21.66)
0.721
149.79 (24.70)
0.051
0.218
12 weeks
148.25 (22.54)
0.203
159.29 (25.29)
0.674
0.359
Flex-pROM
Baseline
157.89 (22.46)
168.77 (13.65)
0.218 c
Immediate
157.18 (22.57)
0.779
168.68 (11.53)
0.917
0.720
4 weeks
160.18 (23.16)
0.401
167.47 (11.93)
0.753
0.315
12 weeks
158.89 (22.03)
0.575
171.61 (10.41)
0.528
0.887
Abd-aROM
Baseline
142.51 (24.13)
153.79 (26.34)
0.257 c
Immediately
147.16 (24.78)
0.790
154.22 (29.54)
0.735
0.724
4 weeks
152.93 (27.24)
0.203
153.00 (28.30)
0.917
0.156
12 weeks
149.74 (25.48)
0.575
163.34 (27.59)
0.249
0.943
Abd-pROM
Baseline
159.67 (20.51)
172.80 (12.53)
0.192 c
Immediately
162.75 (26.13)
0.401
172.80 (15.44)
0.715
0.608
4 weeks
166.04 (22.49)
0.091
170.30 (16.67)
0.893
0.187
12 weeks
163.10 (22.21)
0.327
174.93 (14.30)
0.500
0.856
IR-aROM
Baseline
60.75 (18.71)
73.03 (15.41)
0.078 c
Immediately
68.46 (18.68)
0.062
73.33 (14.33)
0.674
0.231
4 weeks
71.19 (15.56)
0.026
80.94 (7.29)
0.051
0.888
12 weeks
76.66 (12.36)
0.003
81.79 (11.29)
0.173
0.360
IR-pROM
Baseline
72.89 (14.38)
80.22 (14.12)
0.251 c
Immediately
77.63 (13.74)
0.374
82.09 (11.22)
0.600
0.618
4 weeks
75.63 (13.96)
0.260
86.38 (5.26)
0.176
0.943
12 weeks
81.01 (11.26)
0.021
87.07 (3.71)
0.091
0.357
ER-aROM
Baseline
59.66 (20.24)
62.24 (22.82)
0.833 c
Immediately
62.83 (20.64)
0.929
65.89 (19.72)
0.233
0.438
4 weeks
63.19 (20.84)
0.534
65.58 (21.96)
0.484
0.972
12 weeks
58.18 (21.04)
0.450
69.50 (16.02)
0.214
0.439
ER-pROM
Baseline
68.05 (19.77)
66.31 (21.66)
0.888 c
Immediately
68.57 (18.78)
0.878
74.23 (19.61)
0.063
0.112
4 weeks
69.97 (21.43)
0.386
72.68 (19.96)
0.123
0.778
12 weeks
68.28 (20.30)
0.799
78.12 (13.90)
0.086
0.193
Abd: abduction; aROM: active range of motion; ER: external rotation; Ext: Extension; Flex: Flexion; high PEMF: High-Energy Density Pulse Electromagnetic Field; IR: Internal Rotation; pROM: Passive Range of Motion; SD: Standard Deviation; VAS: Visual Analog Scale; a Wilcoxon Signed-Rank test (each time-points versus baseline); b Mann–Whitney U Test (mean difference, intergroup);cMann–Whitney U Test (Mean, intergroup).
Table 3: Comparison of changes of shoulder range of motion between both groups.
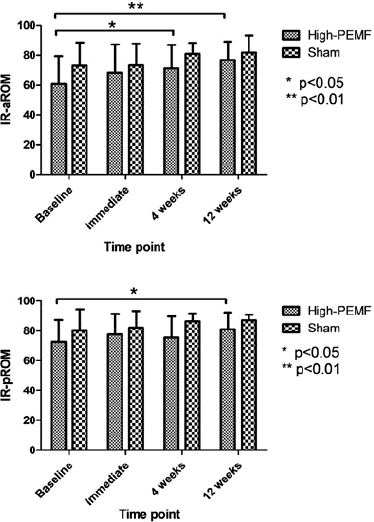
Figure 5: Comparison of changes of shoulder IR between both groups.
Discussion
This is the first study to combine high PEMF and exercise to evaluate the efficacy of high PEMF therapy for the treatment of patients with RCT. The most significant findings were pain reduction and functional improvement compared to baseline immediately and at 4 weeks after treatment in the high PEMF group, despite the lack of intergroup differences. In other words, there is a potential short-term (within 1 month) benefits of pain and function improvements with the use of high PEMF as an additional therapy for patients with RCT.
There is paucity of literature evaluating the efficacy of PEMF in the treatment of RCT [20]. The most recent systematic review by Pieters et al. [7] included four systematic review articles but the papers had a total sample size of only 230. The review concluded that there was no evidence to support the use of PEMF therapy for the treatment of RCT. Moreover, the results were obtained from the conventional PEMF, which has a low energy output, low and narrow frequency range, and a low magnetic field. Besides, according to previous studies, for PEMF therapy to be effective, the field strength needs to be greater than 10 mT [19,21]. Theoretically, a PEMF with a higher energy, higher and wider frequency, and larger magnetic field is expected to offer better benefits. Nevertheless, only one randomized control trial found using high PEMF (the article used the term “electromagnetic transduction therapy” instead) for patients with RCT [5]. The study compared extracorporeal shock wave therapy (ESWT) combined with high PEMF to ESWT combined with sham PEMF and reported a favorable synergistic effect, indicating that high PEMF significantly improved the outcomes of ESWT [5]. In our study, we opted to combine high PEMF with exercise because no previous studies had done so; besides, exercise is a more common and widely used treatment for RCT compared to ESWT.
The primary outcome of our study was pain reduction, which is usually the main concern for patients with RCT. Short-term pain relief was achieved with high PEMF therapy and maintained pain reduction that exceeded the MCID to ongoing self-exercise during the follow-up period. Notably, there was a discrepancy in pain improvement in pain VAS and pain scores in SPADI at 4 weeks after treatment in the high PEMF group; this may be related to the difference in the questions asked in both measures. The pain VAS measures the average pain score during maximum shoulder activity over the previous week; the SPADI pain score evaluated pain in five different situations and then sums them up. Another reason might be that in the high PEMF group at 4 weeks after treatment, there was one paradoxical exception where a participant reported an increased pain score of 29 in the SPADI but an improvement in pain from 3 to 1 on the VAS. This discrepancy resulted in worse pain scores on the SPADI. If we excluded this participant, the average reduction in pain scores of SPADI was 7.2, which was almost equivalent to the reduction observed immediately after treatment.
Regarding functional improvement, we mainly attributed the short-term reduction of SPADI disability scores to high PEMF therapy, whereas the continued improvement up to 12 weeks after treatment in both groups was mostly attributed to continuous self exercises. It is reasonable to expect that relief in shoulder pain may cause patients to be more willing to actively engage in shoulder activities, leading to functional improvements. Exercise, supported by extensive RCT evidence, is as effective as surgery and superior to no treatment or placebo in improving pain, function, and ROM, with benefits increasing over time and potentially maximized when combined with another conservative treatment [20,22]. Moreover, a systematic review comparing supervised physiotherapy with home exercise programs for patients with subacromial impingement syndrome found that both approaches were equally effective in the conservative treatment of this condition [23]. This could explain the continued improvement in pain and function observed at 12 weeks after treatment in both groups. Our patients received only 3 weeks of one-on-one face-to-face physiotherapy, comprising six sessions, and thereafter engaged in unsupervised self-exercise during the follow-up period.
The poorest result in our study was shoulder mobility, with almost no significant findings in both groups except for aROM of IR at 4 and 12 weeks after -treatment. Because our participants had RCT rather than frozen shoulder, where significant limitations in both aROM and pROM are common, they already had a relatively good ROM, which was sufficient to perform activities of daily living [24]. This limited the potential for improvement and may have made it difficult to observe significant changes, especially in pROM. The improvement in aROM of the IR may be related to pain improvement, as well as the positioning used IR measurement. We measured the IR angle at 90° abduction, which is similar to the Hawkins–Kennedy test position, which can induce shoulder pain.
Limitations
This study had some limitations. First, the study had a relatively small sample size and was conducted at a single medical centre in Taipei, Taiwan. This limits the applicability of our results to a wider population. Furthermore, the average age of participants in our study was higher than that in other studies (average, 50–55 years) [5,7]. In particular, the high PEMF group had eight participants aged over 60 years, with the oldest being 72; this may have affected the efficacy of high PEMF therapy. Second, although statistical significance and MCID were found for shoulder pain and function in the high PEMF group, the placebo effect in the sham PEMF group could not be neglected because there were no intergroup differences.
However, in addition to the previously mentioned benefits, the advantage of high PEMF therapy lies in its shorter treatment time, with the longest sessions not exceeding 15 min, compared to the conventional PEMF therapy, in which the shortest sessions are 20–30 minutes and often extend to hours. Third, similar to the conventional PEMF therapy, high PEMF therapy has variable machine settings for treatment frequency, number of sessions, and session duration. An optimal treatment parameter for RCT has not been established. In this study, the general settings recommended in the device manual were selected. Therefore, it is possible that different settings (e.g., high energy, 3 Hz, 9 min or more) might yield different results, which warrants further investigation in future studies. Fourth, the specific mechanisms underlying the observed clinical improvements were not evaluated. Thus, future research in this area, including cytokine analysis, is warranted.
Conclusion
In conclusion, high PEMF therapy combined with physiotherapy appears to be safe and demonstrates potential efficacy in pain reduction and shoulder function improvement, but not shoulder mobility, in patients with RCT in the short term. Therefore, high PEMF therapy offers patients a non-invasive, time-saving, and welltolerated treatment option.
Funding Statement
This work was supported by the Medical Affairs Bureau, Ministry of National Defense (Grant No. MND-MAB-D-113086), the National Science and Technology Council (Grant No. MOST- 112–2314-B-016-026), and the Tri-Service General Hospital, Taiwan, Republic of China (Grant No. TSGH-D-113134).
References
- Lewis J, McCreesh K, Roy JS, Ginn K. Rotator Cuff Tendinopathy: Navigating the Diagnosis-Management Conundrum. J Orthop Sports Phys Ther. 2015; 45: 923-937.
- van der Windt DA, Koes BW, Boeke AJ, Devillé W, De Jong BA, Bouter LM. Shoulder disorders in general practice: prognostic indicators of outcome. Br J Gen Pract. 1996; 46: 519-253.
- Gaynor JS, Hagberg S, Gurfein BT. Veterinary applications of pulsed electromagnetic field therapy. Res Vet Sci. 2018; 119: 1-8.
- Hu H, Yang W, Zeng Q, Chen W, Zhu Y, Liu W, et al. Promising application of Pulsed Electromagnetic Fields (PEMFs) in musculoskeletal disorders. Biomed Pharmacother. 2020; 131: 110767.
- Klüter T, Krath A, Stukenberg M, Gollwitzer H, Harrasser N, Knobloch K, et al. Electromagnetic transduction therapy and shockwave therapy in 86 patients with rotator cuff tendinopathy: A prospective randomized controlled trial. Electromagn Biol Med. 2018; 37: 175-183.
- Page MJ, Green S, Mrocki MA, Surace SJ, Deitch J, McBain B, et al. Electrotherapy modalities for rotator cuff disease. Cochrane Database Syst Rev. 2016; 2016: Cd012225.
- Pieters L, Lewis J, Kuppens K, Jochems J, Bruijstens T, Joossens L, et al. An Update of Systematic Reviews Examining the Effectiveness of Conservative Physical Therapy Interventions for Subacromial Shoulder Pain. J Orthop Sports Phys Ther. 2020; 50: 131-141.
- Ryang We S, Koog YH, Jeong KI, Wi H. Effects of pulsed electromagnetic field on knee osteoarthritis: a systematic review. Rheumatology (Oxford). 2013; 52: 815-824.
- Pappas P. PAPIMI® Central Europe 0110 Manual (e). In. MTG Medizinisch- Technische Geräte GmbH. 1999.
- Jorgensen WA, Frome BM, Wallach C. Electrochemical therapy of pelvic pain: effects of pulsed electromagnetic fields (PEMF) on tissue trauma. Eur J Surg Suppl. 1994: 83-86.
- Krath A, Klüter T, Stukenberg M, Zielhardt P, Gollwitzer H, Harrasser N, et al. Electromagnetic transduction therapy in non-specific low back pain: A prospective randomised controlled trial. J Orthop. 2017; 14: 410-415.
- Gerdesmeyer L, Saxena A, Klueter T, Harrasser N, Fullem B, Krath A. Electromagnetic Transduction Therapy for Achilles Tendinopathy: A Preliminary Report on a New Technology. J Foot Ankle Surg. 2017; 56: 964- 967.
- Spadoni GF, Stratford PW, Solomon PE, Wishart LR. The evaluation of change in pain intensity: a comparison of the P4 and single-item numeric pain rating scales. J Orthop Sports Phys Ther. 2004; 34: 187-193.
- Bijur PE, Chang AK, Esses D, Gallagher EJ. Identifying the minimum clinically significant difference in acute pain in the elderly. Ann Emerg Med. 2010; 56: 517-521.e1.
- Breckenridge JD, McAuley JH. Shoulder Pain and Disability Index (SPADI). J Physiother. 2011; 57: 197.
- Lin CL, Chen YW, Wu CW, Liou TH, Huang SW. Effect of Hypertonic Dextrose Injection on Pain and Shoulder Disability in Patients with Chronic Supraspinatus Tendinosis: A Randomized Double-Blind Controlled Study. Arch Phys Med Rehabil. 2022; 103: 237-244.
- Lin CL, Yang MT, Lee YH, Chen YW, Vitoonpong T, Huang SW. Comparison of Clinical and Ultrasound Imaging Outcomes Between Corticosteroid and Hypertonic Dextrose Injections for Chronic Supraspinatus Tendinopathy. Orthop J Sports Med. 2022; 10: 23259671221129603.
- Tozzo MC, Ansanello W, Martins J, Zatiti SCA, de Oliveira AS. Inclinometer Reliability for Shoulder Ranges of Motion in Individuals With Subacromial Impingement Syndrome. J Manipulative Physiol Ther. 2021; 44: 236-243.
- Wuschech H, von Hehn U, Mikus E, Funk RH. Effects of PEMF on patients with osteoarthritis: Results of a prospective, placebo-controlled, double-blind study. Bioelectromagnetics. 2015; 36: 576-585.
- Haik MN, Alburquerque-Sendín F, Moreira RF, Pires ED, Camargo PR. Effectiveness of physical therapy treatment of clearly defined subacromial pain: a systematic review of randomised controlled trials. Br J Sports Med. 2016; 50: 1124-1134.
- Galace de Freitas D, Marcondes FB, Monteiro RL, Rosa SG, Maria de Moraes Barros Fucs P, Fukuda TY. Pulsed electromagnetic field and exercises in patients with shoulder impingement syndrome: a randomized, double-blind, placebo-controlled clinical trial. Arch Phys Med Rehabil. 2014; 95: 345-352.
- Cooper K, Alexander L, Brandie D, Brown VT, Greig L, Harrison I, et al. Exercise therapy for tendinopathy: a mixed-methods evidence synthesis exploring feasibility, acceptability and effectiveness. Health Technol Assess. 2023; 27: 1-389.
- Gutiérrez-Espinoza H, Araya-Quintanilla F, Cereceda-Muriel C, Álvarez- Bueno C, Martínez-Vizcaíno V, Cavero-Redondo I. Effect of supervised physiotherapy versus home exercise program in patients with subacromial impingement syndrome: A systematic review and meta-analysis. Phys Ther Sport. 2020; 41: 34-42.
- Oosterwijk AM, Nieuwenhuis MK, van der Schans CP, Mouton LJ. Shoulder and elbow range of motion for the performance of activities of daily living: A systematic review. Physiother Theory Pract. 2018; 34: 505-528.