
Research Article
J Pediatr & Child Health Care. 2021; 6(1): 1038.
Efficacy of Coenzyme Q10 in the Treatment of Cyclic Vomiting Syndrome in Children
Brezin F1, Wiedemann A1,2, Bansept C1, Albuisson E3, Renard E1,2 and Feillet F1,2*
1Reference Center for Inborn Errors of Metabolism of Nancy, France
2Inserm Ngere 1256, 3 UMDS, CHRU Nancy, France
3UMDS, CHRU Nancy, France
*Corresponding author: Francois Feillet, Pediatric Unit, University Children’s Hospital, CHU Brabois, Vandoeuvre Les Nancy, France
Received: February 27, 2021; Accepted: March 18, 2021; Published: March 25, 2021
Abstract
Cyclic Vomiting Syndrome (CVS) is a chronic functional gastrointestinal disorder related to migraine, characterized by episodic nausea and vomiting. The treatment of CVS remains based on tricyclic antidepressants, triptans and antiepileptics. As mitochondriopathy has been involved in the pathophysiology of CVS, Coenzyme Q10 (CoQ10), a mitochondrial cofactor, has been used as the third line treatment in CVS. Considering the excellent safety profile of CoQ10, we decided to use it as the first line treatment in CVS. We retrospectively studied the evolution of 23 CVS patients who were treated for one year by CoQ10 alone. We recorded the characteristics of patients and their CVS history and compared data obtained the year before and the year following the prescription of CoQ10 treatment. We found a significant decrease in the number of vomiting episodes between the year before and the year after the start of CoQ10 (median [IQR]: 18.0 [15.75] vs. 3.00 [5.0]; p <0.001). This decrease persisted with time (2 and 3 years of treatment). The treatment was very efficient in 17/23 patients and did not decrease the number of vomiting episodes in 3 patients. Only one mild side effect related to the drug has been reported.
Conclusions: CoQ10 is an efficient and safe treatment of CVS and should be used as the first line treatment in this episodic syndrome related to migraine.
Keywords: Cyclic vomiting syndrome; Coenzyme Q10; Treatment; Migraine; Children
Introduction
First described in 1806 [1,2], Cyclic Vomiting Syndrome (CVS) is still an underdiagnosed condition which belongs to the larger family of episodic syndromes related to migraines [3]. The latest Rome IV classification [4] defines CVS as stereotypical episodes of vomiting regarding onset (acute) and duration (less than 1 week), with 2 or more episodes in the past 6 months, occurring at least 1 week apart, with return to baseline health between cycles and which cannot be attributed to another condition. Its prevalence is estimated at 1.9% in children [1]. Some authors have evaluated this condition as being responsible for as much as 24 missed-school days per year for these children, or 15% of their childhood time, with an estimated yearly cost of $170,355. First and second-choice prophylactic treatments (Amitriptyline, Cyproheptadine, Propranolol, Pizotifen) are similar to migraine therapies but the safety profile of these drugs can be questionable as CVS is a functional disorder [6-8]. Some relations have been shown between migraine and mitochondrial DNA variants and CoQ10, which is a mitochondrial respiratory chain cofactor and has shown its efficacy (in association with L-carnitine) in the prevention of migraine in adults [9]. Boles published in 1997 an increased prevalence of two mitochondrial DNA polymorphisms similar to those found in migraine in patients with CVS [10] conducing them to try CVS treatment by Coenzyme Q10 (CoQ10) in association with L-carnitine and amitriptyline [11,12]. Considering that, compared to the other drugs used in CVS, CoQ10 has a very favorable safety profile [13] and after a very positive experience (complete disappearance of CVS in a very severe patient only treated by CoQ), we designed this study to evaluate the efficacy of CoQ10 in monotherapy as the first line treatment of CVS.
Methods
Study design
We conducted a monocenter, observational, retrospective study of children diagnosed for CVS and treated by CoQ10 in the reference center for inborn errors of metabolism of Nancy, France.
Population
Eligible criteria: Diagnosis for CVS, age under 18 years, treatment by CoQ10 treatment for a planned duration of at least one year. There was no exclusion criterion.
Data collection
We recorded the data of all patients followed for CVS in our center (n=26). 3 patients were excluded because or treatment refusal. Finally, 23 patients were included in the study.
Inclusion was retrospective and data was collected from patients’ medical records from December 1st, 2016 to January 31st, 2019. All data were then anonymized. We collected familial and patient medical history (including personal and maternal migraine history) and yearly auxological data (weight, height and Body Mass Index (BMI)) expressed in z-score. The characteristics of CVS included age of onset, diagnosis and treatment by CoQ10 and the number of episodes per year. The severity of vomiting crises was subjectively evaluated by the parents. The statistical analysis was performed between data of the year before and the year following the prescription of CoQ10 treatment. The long-term efficacy of the treatment has also been evaluated for the patients who have been treated longer than one year. We also collected the safety data and the number of hospitalizations related to vomiting crises before and after CoQ10 treatment.
Objectives
The primary objective was to demonstrate a reduction (of at least 50%) in the number of vomiting crisis under CoQ10 treatment. The secondary objectives were the evaluation of episodes intensity and long-term efficacy for patients treated more than one year. The nutritional status was estimated by yearly weight, height and BMI. The number of hospitalizations before and after treatment was recorded as all the CoQ10 side effects to assess the safety profile of coQ10 in this indication (Table 1).
Statistical analyses
Statistical analysis was performed using IBM SPSS Statistics v22 (IBM Corp.). According to their nature and distributions, variables are expressed with median and Interquartile Range (IQR) or frequencies and percentages. The non-parametric Wilcoxon signed-rank test was used to compare two related samples as before and after the use of CoQ10. Spearman’s Rho was used to study correlations between two parameters. The statistical significance level was set at 5%.
Results
Patients ad CVS description
We identified 26 patients with CVS according to Rome IV definition in our center. 3 patients were not included because of treatment refusal. 23 patients (15 males and 8 females) accepted to be treated by Coenzyme Q10. The 23 patients were referred to our center by their pediatricians or their general practitioners. Before being referred to our center, one patient was seen by a pediatric endocrinologist, one by a pediatric allergist and the last patient by a pediatric gastroenterologist, a child psychiatrist and a psychologist. Median age at onset of CVS was 4.92 years [IQR 6.86], median age at diagnosis was 9.81 years [IQR 6.18] and median age at treatment was 10.00 years [IQR 7.00]. The median delay from the onset of symptoms to treatment is 2.5 years [IQR 4.02].
Past medical history included allergies (pollen, ash, dust mite, n=1), milk hypersensitivity (n=1), mild anorexia nervosa (n=1), mild delay of acquisitions (n=1) and hypospadias (n=1). In one case, CVS was associated with a complex syndrome including Hirschsprung’s disease, facial dysmorphism, psychomotor retardation, asymmetry of the temporal lobes, and thoraco-lumbar scoliosis. This patient was suspected to have a mitochondrial disease or Goldberg-Shprintzen syndrome without genetic proof to date. For 7 patients a trigger of crisis was described. This trigger was stress for 4 patients, infection for 2 patients and fatigue for the 7th patient.
We found a history of migraine in 13/23 patients (57%), and in 12/23 of mothers (52%). In 7 cases children and mothers have migraine symptoms. Two paternal history of migraine was reported in our cohort. One family provides a detailed family tree with multiple occurrence of migraine with maternal transmission which could be compatible with an mtDNA transmission (Figure 1). No significant association was found between personal or familial history of migraine (with and without) and the age of onset of CVS, respectively: personal migraine history (2.78 years [IQR 5.08] vs 7.0 years [IQR 6.33], p=0.08) and maternal migraine history (5.01 years (IQR 6.62] vs 4.34 years [IQR 7.06], p=0.78) or with the number of vomiting crises per year before treatment respectively: personal migraine history (with vs without; 18.0 [IQR 17.25] vs. 18.0 [IQR 12.0], p=0.90) and maternal migraine history (with vs without; 18.0 (IQR 27.0] vs. 18.0 [IQR 15.75], p=0.52).
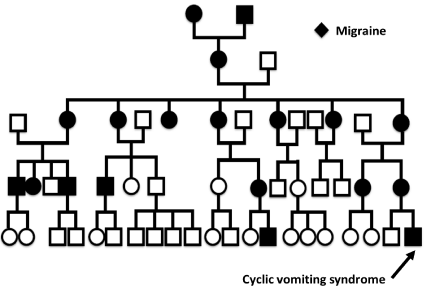
Figure 1: Genealogic tree of one patient with CVS with systematic maternal
inheritance of migraine.
There was no significant relation between the age at onset of CVS and the yearly number of episodes before and after the start of CoQ10 treatment (Spearman’s Rho: p=0.16 and p=0.47 respectively) or the yearly number of hospitalizations before CoQ10 treatment (Spearman’s Rho: p=0.27).
CoQ10 treatment efficacy assessment
23 patients were treated for more than one year and were included in the statistical analysis. CoQ10 median posology on start of treatment was 12.15mg/kg/day [IQR 4.5], divided tid (80%) or bid (20%). The minimum efficient dosage was 5.7mg/kg/day and the maximum dosage was 15.7mg/kg/day. We found a significant decrease in the number of vomiting episodes between the year before and the year after the start of CoQ10 (median [IQR]: 18.0 [15.75] vs. 3.00 [5.0]; p <0.001). The decrease in vomiting crises remains significantly decreased with time as it persists at 2 years (n=11, median [IQR]: 1.0 [2.0]; p <0.003) and 3 years (n=6, median [IQR]: 1.5 [3.0]; p=0.027) of treatment (Figure 2). Median crises’ lowering rate was 78.85% [IQR 44.64] (Figure 3). For 17 patients, the treatment was said very efficient and even “magical” for 7 patients who experienced a complete disappearance of the symptomatology. 17 families (70%) reported a decrease in the intensities of episodes after beginning treatment by CoQ10. Two patients did not respond at all and stopped their treatment at the end of the study.
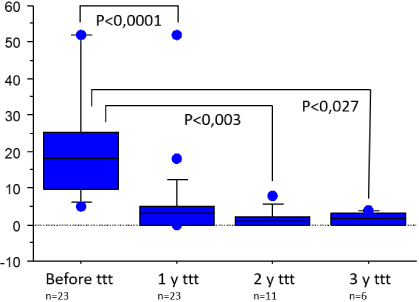
Figure 2: Evolution of the number of vomiting crisis per year before and after
1, 2 and 3 years of treatment by Coenzyme Q10.
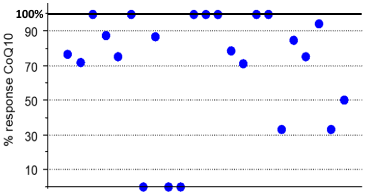
Figure 3: Percentage of decrease of vomiting crises after one year of
treatment for the 23 patients.
For these two patients, CVS was triggered by stress and low dose of amitriptyline was efficient in these two patients once the study the first year of treatment with CoQ10 was achieved. For one patient, the number of crises remained the same, but the intensity of crises diminished significantly and the parents and the child wanted to continue the treatment. Three patients exhibit a mild response (the number of crises decrease of 33% for two patients and of 50% for the other one. Two of these patients wanted to continue the treatment because of the significant decrease of the crisis duration and/or severity. 18/23 patients (78%) described a decrease severity of the remained crisis. 4 patients experienced (during the second year of treatment) an effect ON/OFF of the treatment when they forgot or wanted to stop the treatment. The symptomatology went back rapidly and disappear as soon as they were taking again CoQ10. No correlation between the number of episodes per year before and after treatment was found (Spearman’s Rho=0.012; p=0.63) meaning that COQ10 treatment can be efficient in mild and in severe forms of CVS. We found a significant decrease of the number of hospitalizations for vomiting after treatment (median [IQR]): 0.50 [3] (before treatment) vs. 0.00 [0.00] (after treatment); (p=0.003).
Effect on growth and side effects of COQ10 treatment
Growth was followed during the study, and we did not see any significant change in weight, height or BMI expressed as z-score with time. These data are presented in the (Supplementary Table 1) and in the (Supplementary Figure 1).
Children
Gender
Age (Year) onset
Age (Year) Diagnosis
Age (Year) CoQ10
Crisis/Year before CoQ10 (n)
Crisis/Year after CoQ10 (n)
Hosp/Y before Ttt (n)
Hosp/Y after Ttt (n)
Lower crisis intensity (n)
CoQ10 dosage (mg/kg/day)
Bid Tid
Side effect Related to drug or not
Hunger
Trigger
ON/OFF effect
A
M
0.25
0.92
1.5
26
6
8
0
Y
14.3
3
N
N
No
Yes
B
F
1.17
2.25
2.25
18
5
0
0
Y
9.4
3
N
N
No
C
F
4.34
5.5
5.5
18
0
0
0
Y
10.0
2
N
N
No
Yes
D
F
10.5
11.17
11.17
24
3
0
0
N
10.2
3
Cutaneous eruption (NR)
Y
No
E
M
2.78
14.27
14.27
8
2
8
0
Y
12.9
3
N
N
Fatigue
F
F
2.50
3.67
3.67
9
0
0
0
Y
15.6
3
N
Y
No
G
M
10.17
14.25
14.25
5
5
0
0
N
15.0
3
N
N
Stress
H
M
3.84
5.15
5.20
15
2
0
0
Y
11
2
N
N
No
I
M
10.75
11.78
11.78
52
52
2
0
N
15.1
3
N
N
Stress
J
F
2.83
5.88
6.03
18
18
4
0
Y
13
3
N
Y
No
K
M
8
11
13
24
0
0
0
Y
15
2
N
N
No
L
M
12.00
13.00
13.00
52
0
1
0
Y
9.8
3
N
Y
No
Yes
M
F
9.58
10.75
13.33
18
0
3
0
Y
9
3
N
N
No
N
M
3.00
5.67
5.67
52
11
0
0
Y
8.6
3
N
N
No
O
M
6.00
11.58
11.58
7
2
5
2
Y
11.4
3
N
N
Stress, infection
Yes
P
M
9.00
9.58
9.58
12
0
1
0
Y
9.4
2
N
N
Infection
Q
M
2.50
15.91
15.91
24
0
1
0
Y
9.8
3
N
Y
No
R
M
0.25
9.81
9.81
6
4
1
0
Y
13.6
3
Tendinous pain (NR)
Y
No
S
F
10.75
16.42
17.58
26
4
2
0
Y
5.45
3
Abdominal pain (R)
N
No
T
M
4.92
9.92
9.92
12
3
0
0
Y
13
3
N
N
Stress
U
M
5.1
6.30
6.30
52
3
0
0
N
14
3
N
Y
Stress
V
M
5
6
6
6
4
0
0
N
15.7
3
N
N
No
W
F
1.5
3.8
3.8
12
6
3
1
Y
15
2
N
N
No
Legend: CoQ10; Coenzyme Q10; Side effect NR: Not Related; R: Related; Hunger: presence of hunger just at the end of vomiting crisis.
Table 1: Summary of patients age, gender, age at onset of symptoms and at treatment, number de vomiting crisis before and one year after CoQ10 treatment, evaluation of the crisis intensity under COQ10, CoQ10 dosage and modality of administration, side effects, presence of hunger at the end of crisis, presence of crisis trigger and ON/OFF effect.
3 patients experienced side effects during the first year of treatment by COQ10. One patient felt some abdominal pain at the introduction of treatment, then we increased progressively the treatment to reach the maximum dose after 3 weeks without recurrence of the abdominal pain. This side effect has been considered related to CoQ10. The two other side effects described (cutaneous eruption and tendinous pain) spontaneously resolved without any modification of the treatment and were considered as not related to CoQ10.
Discussion
CVS is now well defined and recognized as a feature of migraine and episodic episodes associated to migraine [3]. It is a complex disease and the pathophysiology of CVS remains discussed and is multifactorial. Vomiting comes from the stimulation of the Chemoreceptor Trigger Zone (CTZ) for emesis, also commonly known as the area postrema which is located within the dorsal surface of the medulla oblongata, on the floor the fourth ventricle of the brain. The CTZ contains receptors that detect emetic agents in the blood and relays that information to the vomiting center which is responsible for inducing the vomiting reflex [14]. If the sensibility of CTZ to drugs, toxins or ketone bodies is known, the mechanism by which mild respiratory chain dysfunction could stimulate this CTZ is still unknown. On a clinical point of view, it is interesting to notice that 7/23 children felt that the crisis end suddenly and then, they are very hungry and ate immediately a snack. This clinical pattern seems to us quite specific of CVS as it is quite unusual to be suddenly hungry when vomiting are related to a gastrointestinal disease. This clinical particularity should be confirmed on a larger population. On these 7 patients, 5 were fully responders while two patients have a good response in term of intensity of the crisis, but the number of crisis did not diminish significantly at the end of their crises.
Many drugs have been proposed to treat CVS in the acute phase and on a long term to avoid the recurrences of vomiting crisis. Our study only focuses on the long-term treatment and we will not discuss in this paper the management of the crisis, which is based on antimigraine agents, antiemetics and glucose infusion to avoid the additional effect of ketosis on vomiting. To avoid the recurrence of vomiting crisis, many different drugs have been used (i.e. antidepressants, antiepileptics, antimigraine agents, beta-blockers…) and all these drugs have shown some efficacy in treating CVS patients [7,15,16]. Antidepressants are relevant because anxiety and some psychiatric comorbidities are often found in CVS patients [17,18]. Beta-blockers who modulate the autonomic system are also used. In a recent paper 14/15 patients reported dysautonomic symptoms during CVS episodes, (i.e. palpitations, hypertension, fever, chills or sweat) [18] and beta-blockers are useful to treat this causal part of CVS [19]. CoQ10 has already been used to treat CVS, but mainly in association with other drugs [11].
The rational for the efficacy of CoQ10 in CVS is based on the potential relations between CVS and mitochondrial disease and the link between migraine syndromes and mitochondrial abnormality is supported by multiple facts. The maternal inheritance for migraine and CVS has been demonstrated [20,21] and some mitochondrial DNA (mtDNA) polymorphisms have been associated to migraine and CVS [10]. Histological and biochemical similarities have been found on muscle biopsies from patients with mitochondrial encephalomyopathy or with migraine [22,23]. The pharmacological efficacy of some mitochondrial cofactors (CoQ10, riboflavin, nicotinic acid, L-carnitine) has also been demonstrated in migraine and in CVS [24]. One of our patients has a typical pedigree of migraine related to maternal inheritance (Figure 1) and was fully responsive to CoQ10.
CoQ10 is a mitochondrial electron-transport chain cofactor acting as a carrier of high-energy electrons from complex I and II to complex III [25]. Physiologically, complex II has a saturated activity whereas complex I’s activity is linked to the mitochondrial membrane concentration of CoQ10 [26]. Consequently, an increase of CoQ10’s concentration could improve the respiratory chain function [26]. This could be the mechanism by which exogenous intake of CoQ10 can improve symptoms in mitochondrial myopathies or cardiomyopathies [26]. Other studies have shown that CoQ10 is efficient in adult’s migraine [9,25,27,28] and two studies showed that CoQ10, in association with other treatments, was efficient in CVS [11,12]. Our study demonstrates that CoQ10, used in monotherapy, can be efficient at a posology of 10-15mg/kg/day with a significant decrease of CVS episodes per year under treatment by CoQ10 with a median response rate of 78.85% [IQR 44.64]. In our experience, some patients have a better response at 15mg/kg/day and when the treatment is given three times a day (tid) compared to twice a day (bid). The dosage and the modalities of administration must be adapted to each case. Considering the potential difficulty to give the treatment during the school time in children, some of our patients were taking CoQ10 bid during the school days and tid the other days. This beneficial effect continues over time as we can see in our 6 patients who are presently treated for more than 3 years. Today, mitochondrial cofactors are not recommended as the first line treatment in CVS. In a recent review CoQ10 is only recommended in the mild forms while antidepressant agents, ondansetron and phenotiazines are recommended in the severe forms of CVS [29]. In comparison, the Consensus Statement on Diagnosis and Management of CVS established a classification of standard treatment of CVS by response rates defined by the percentage reduction in numbers of episodes following treatment [30]. The efficiency has been established for, at least 50% reduction of the number of vomiting episodes. Considering this criterion, 83% of our patient had a 50% or more reduction of crisis while the drugs usually used to treat CVS (i.e. pizotifen, amitriptyline, propranolol and cyproheptadine) had respectively 100%, 81%, 65% and 61% of response rate over 50% of reduction of number of episodes [3]. In our study, CoQ10 has 83% of response rate over 50% and 74% obtained a reduction of 70% and more (Figure 2).
This means that CoQ10 is, at least, as efficient as most standard treatments with a much better safety profile. For the safety data, we only report one benign side effect related to CoQ10 introduction. In adult, side effects were reported in 34% of patients taking amitriptyline for CVS, even if these adverse events did not result in discontinuation of therapy [6]. A retrospective study showed that 26% of patients responding to conventional CVS prophylactic therapy with tricyclic antidepressants, antiepileptic medications such as topiramate, and/ or mitochondrial supplements had side effects including behavioral changes, nightmares, and increased somnolence8. In our study, we only notice one adverse event related to CoQ10 during 40 years of treatment for the whole population. One patient presented some abdominal pain at the initiation of treatment. A more progressive introduction of CoQ10 allowed to reach the full dosage and to continue the treatment. The two other adverse events (cutaneous eruption and tendinous pain) were not estimated related to the drug, and resolved while the treatment was maintained.
On the whole population, we did not find variation of auxological data because at baseline, most of these children did not present any sign of malnutrition. However, one of our patients who had the more severe CVS (1 crisis per week) improved his BMI from -2.4SD to -0.3SD after one year of treatment indicating that or some individuals, the nutritional aspect of CVS can be a real task which must be taken into account.
CVS has a financial burden including the cost of drugs but also the cost of hospitalization and of the parents unworked days because of CVS crisis of their children. In our study, we found a significant decrease in the number of hospitalizations before and after treatment; the same benefit has been observed for the scholar absenteeism for children and for the unworked days for the parents, even if the methodology of this retrospective study does not allow a precise quantification of these parameters. Currently, the cost of CoQ10 as a medical drug is higher than the cost of other standard treatments of CVS but the better safety profile and the spared cost of repeated hospitalizations, of the school and of professional absenteeism should be taken into account to determine the cost/benefit ratio of this treatment. On another hand, this molecule can be bought on internet at a very low price; 120 softgels (100mg/softgel) for less than 10$.
Mitochondriopathy cannot resume the pathophysiology of CVS, in our study, 2 patients were completely unresponsive, both of them have an important anxious personality and amitriptyline has been prescribed at the end of the study and was very efficient in these two cases. The third patient for whom the number of crisis did not decrease was nevertheless a partial responder as the intensity of crisis significantly decrease to parent’s view with CoQ10 treatment. Considering that CVS is a multifactorial disease, each patient should have a psychologic evaluation to evaluate the level of anxiety and dysautonomic signs during the crisis should be systematically screened to determine the optimal prophylactic treatment. We think that, considering the level of efficacy associated to the excellent safety profile, CoQ10 should be the first line treatment in patients in whom the initial evaluation did not find anxiety or clear dysautonomic signs.
Conclusion
CVS is a potentially severe and disabling condition with a significant impact on quality of life. It can alter child’s nutritional status and be responsible for school absenteeism. We demonstrate the efficacy of CoQ10 as monotherapy in the prophylactic treatment of CVS, which supports the hypothesis for a mitochondrial origin to this syndrome. The favorable safety profile of CoQ10 makes it a good candidate for the first line treatment of CVS.
Ethics
This study was approved by the Ethics Comity of CHRU Nancy and registered at the French data protection authority (Commission Nationale Informatique et Libertés, N°CNIL R2017-04). We obtained written consent from all patients and families. This study is registered to ClinicalTrial.gov (N° NCT03295760).
References
- Cuvellier JC, Lepine A. [Childhood periodic syndromes]. Revue neurologique. 2010; 166: 574-583.
- Pareek N, Fleisher DR, Abell T. Cyclic vomiting syndrome: what a gastroenterologist needs to know. The American journal of gastroenterology. 2007; 102: 2832-2840.
- Headache Classification Committee of the International Headache S. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia: an international journal of headache. 2013; 33: 629-808.
- Hyams JS, Di Lorenzo C, Saps M, Shulman RJ, Staiano A, van Tilburg M. Functional Disorders: Children and Adolescents. Gastroenterology. 2016.
- Li BU, Balint JP. Cyclic vomiting syndrome: evolution in our understanding of a brain-gut disorder. Advances in pediatrics. 2000; 47: 117-160.
- Hejazi RA, Reddymasu SC, Namin F, Lavenbarg T, Foran P, McCallum RW. Efficacy of tricyclic antidepressant therapy in adults with cyclic vomiting syndrome: a two-year follow-up study. Journal of clinical gastroenterology. 2010; 44: 18-21.
- Brezin F, Wiedemann A, Feillet F. [Cyclic vomiting syndrome in children]. Archives de pediatrie: organe officiel de la Societe francaise de pediatrie. 2017; 24: 1129-1136.
- Kumar N, Bashar Q, Reddy N, Sengupta J, Ananthakrishnan A, Schroeder A, et al. Cyclic Vomiting Syndrome (CVS): is there a difference based on onset of symptoms--pediatric versus adult? BMC gastroenterology. 2012; 12: 52.
- Hajihashemi P, Askari G, Khorvash F, Reza Maracy M, Nourian M. The effects of concurrent Coenzyme Q10, L-carnitine supplementation in migraine prophylaxis: A randomized, placebo-controlled, double-blind trial. Cephalalgia: an international journal of headache. 2019: 39: 648-654.
- Zaki EA, Freilinger T, Klopstock T, Baldwin EE, Heisner KR, Adams K, et al. Two common mitochondrial DNA polymorphisms are highly associated with migraine headache and cyclic vomiting syndrome. Cephalalgia: an international journal of headache. 2009; 29: 719-728.
- Boles RG, Lovett-Barr MR, Preston A, Li BU, Adams K. Treatment of cyclic vomiting syndrome with co-enzyme Q10 and amitriptyline, a retrospective study. BMC neurology. 2010; 10: 10.
- Boles RG. High degree of efficacy in the treatment of cyclic vomiting syndrome with combined co-enzyme Q10, L-carnitine and amitriptyline, a case series. BMC neurology. 2011; 11: 102.
- Baggio E, Gandini R, Plancher AC, Passeri M, Carmosino G. Italian multicenter study on the safety and efficacy of coenzyme Q10 as adjunctive therapy in heart failure. CoQ10 Drug Surveillance Investigators. Molecular aspects of medicine. 1994; 15: s287-294.
- Miller AD, Leslie RA. The area postrema and vomiting. Frontiers in neuroendocrinology. 1994; 15: 301-320.
- Li BUK. Managing cyclic vomiting syndrome in children: beyond the guidelines. European journal of pediatrics. 2018; 177: 1435-1442.
- Lagman-Bartolome AM, Lay C. Pediatric migraine variants: a review of epidemiology, diagnosis, treatment, and outcome. Current neurology and neuroscience reports. 2015; 15: 34.
- Andersen JM, Sugerman KS, Lockhart JR, Weinberg WA. Effective prophylactic therapy for cyclic vomiting syndrome in children using amitriptyline or cyproheptadine. Pediatrics. 1997; 100: 977-981.
- Redon S, Mareau C, Guedj E, Donnet A. Cyclic Vomiting Syndrome in Adults and Children: A Hypothesis. Headache. 2017; 57: 943-951.
- Forbes D, Withers G. Prophylactic therapy in cyclic vomiting syndrome. Journal of pediatric gastroenterology and nutrition. 1995; 21: S57-59.
- Boles RG, Adams K, Ito M, Li BU. Maternal inheritance in cyclic vomiting syndrome with neuromuscular disease. American journal of medical genetics Part A. 2003; 120A: 474-482.
- Lemos C, Alonso I, Barros J, Sequeiros J, Pereira-Monteiro J, Mendonca D, et al. Assessing risk factors for migraine: differences in gender transmission. PloS One. 2012; 7: e50626.
- Montagna P, Sacquegna T, Martinelli P, Cortelli P, Bresolin N, Moggio M, et al. Mitochondrial abnormalities in migraine. Preliminary findings. Headache. 1988; 28: 477-480.
- Uncini A, Lodi R, Di Muzio A, Silvestri G, Servidei S, Lugaresi A, et al. Abnormal brain and muscle energy metabolism shown by 31P-MRS in familial hemiplegic migraine. Journal of the neurological sciences. 1995; 129: 214-222.
- Boehnke C, Reuter U, Flach U, Schuh-Hofer S, Einhaupl KM, Arnold G. Highdose riboflavin treatment is efficacious in migraine prophylaxis: an open study in a tertiary care centre. European journal of neurology. 2004; 11: 475-477.
- Rozen TD, Oshinsky ML, Gebeline CA, Bradley KC, Young WB, Shechter AL, et al. Open label trial of coenzyme Q10 as a migraine preventive. Cephalalgia: an international journal of headache. 2002; 22: 137-141.
- Littarru GP, Tiano L. Bioenergetic and antioxidant properties of coenzyme Q10: recent developments. Molecular biotechnology. 2007; 37: 31-37.
- Sandor PS, Di Clemente L, Coppola G, Saenger U, Fumal A, Magis D, et al. Efficacy of coenzyme Q10 in migraine prophylaxis: a randomized controlled trial. Neurology. 2005; 64: 713-715.
- Bianchi A, Salomone S, Caraci F, Pizza V, Bernardini R, D’Amato CC. Role of magnesium, coenzyme Q10, riboflavin, and vitamin B12 in migraine prophylaxis. Vitamins and hormones. 2004; 69: 297-312.
- Bhandari S, Jha P, Lisdahl KM, Hillard CJ, Venkatesan T. Recent Trends in Cyclic Vomiting Syndrome - Associated Hospitalizations with Liberalization of Cannabis Use in the State of Colorado. Internal medicine journal. 2019; 49: 649-655.
- Li BU, Lefevre F, Chelimsky GG, Boles RG, Nelson SP, Lewis DW, et al. North American Society for Pediatric Gastroenterology, Hepatology and Nutrition consensus statement on the diagnosis and management of cyclic vomiting syndrome. Journal of pediatric gastroenterology and nutrition. 2008; 47: 379-393.