
Research Article
J Pediatri Endocrinol. 2021; 6(1): 1042.
Prevalence and Characterization of Brain and Pituitary Abnormalities in Children with Pituitary Dysfunction
Naji GF1,4*, Poletto E2,4, Brown K3 and Kubicky RA1,4
1Section of Endocrinology and Diabetes, St. Christopher’s Hospital for Children, USA
2Section of Radiology, St. Christopher’s Hospital for Children, USA
3Motion Analysis Lab, St. Christopher’s Hospital for Children, USA
4Department of Pediatrics, Drexel University College of Medicine, USA
*Corresponding author: Ghada Faik Naji, Department of Pediatrics, Drexel University College of Medicine, Section of Endocrinology and Diabetes, St. Christopher’s Hospital for Children, 42356 Piper Creek Ter. Ashburn, VA 20148, USA
Received: February 10, 2021; Accepted: March 09, 2021; Published: March 16, 2021
Abstract
Introduction: Imaging studies help identify structural abnormalities associated with pituitary dysfunction, such as Ectopic Posterior Pituitary (EPP).
Aim: To detect the prevalence of IGHD or CPHD in children with EPP, the association between the location of EPP and pituitary dysfunction; and, to determine the prevalence of brain and pituitary abnormalities detected by MRI.
Methods: A retrospective chart review of MRI reports at St. Christopher’s Hospital for Children (SCHC) from 2006-2018 that were found to have EPP. Pituitary hormone function was evaluated in the majority.
Results: 66 patients, age of (8.31±6.26) included. Of those, 26 patients had EPP. The prevalence rate of documented pituitary dysfunction was higher in patients with EPP (95%).
Of the 26 patients with EPP, age (5.98±5.18 yrs) 20 patients had an endocrine evaluation. Of the 20 children, 14 had CPHD and 4 had IGHD.
Patients with EPP were classified into 3 groups (upper, middle & lower). Of the 21 patients with upper EPP, 17 (100%) were found to have pituitary dysfunction (14 with CPHD, 3 with IGHD). Of the 4 children with middle EPP, 1 had pituitary dysfunction which was IGHD. Diabetes insipidus was not identified in any of the children. Patients with CPHD had higher prevalence of EPP (73.7%) as compared to those with IGHD (21.1%).
Conclusion: Our study supports previous reports that CPHD and IGHD are frequent in patients with EPP. No cases of DI have been reported in children with EPP.
No CPHD was reported in middle/lower but IGHD was found in the middle EPP group.
Keywords: Ectopic posterior pituitary; Diabetes insipidus
Abbreviations
EPP: Ectopic Posterior Pituitary; IGHD: Isolated Growth Hormone Deficiency; CPHD: Combined Pituitary Hormone Deficiencies; DI: Diabetes Insipidus; GH: Growth Hormone; MRI: Magnetic Resonance Imaging
Introduction
The anterior pituitary or adenohypophysis originates from ectoderm and develops from Rathke’s cleft. The neurohypophysis or posterior pituitary is of neuroectodermal origin and develops as a downward extension of the diencephalon (infundibulum).The pituitary (infundibular) stalk connects the median eminence of the hypothalamus to the pituitary gland [1]. The median eminence is where the hypothalamic releasing or inhibiting hormones are released into portal venous capillaries. This network of blood vessels, surrounds the pituitary stalk and penetrates into the anterior pituitary. Structural-functional-hormonal interruption in this area can interfere with the hypothalamic-pituitary axis [1-4].
Imaging studies aid in the detection of structural abnormalities that may be associated with pituitary dysfunction, such as Ectopic Posterior Pituitary (EPP). EPP is a rare developmental anomaly of the hypothalamus that is more commonly detected since the development of Magnetic Resonance Imaging (MRI) as it produces a “bright signal” on T1-weighted images [5-7]. The location of the ectopic lobe can vary, but it is most commonly located along the median eminence at the floor of the third ventricle [5,6,8]. EPP could result from complete or partial defective neural migration during embryogenesis, which could explain the different loci of EPP [1,5,9]. In addition, EPP has been reported in children with mutations in HESX1, SOX3 and LHX4 genes since these genes participate in the evolution of hypothalamic–pituitary axis [10,11,12]. Sometimes the etiology is unknown.
EPP is usually accompanied by an anterior pituitary gland that is reduced in height and poorly visualized infundibular stalk [9]. EPP was seen in one of 1500 cranial MRIs in patients without any evidence of sellar or parasellar disease [6]. However, patients can have a triad of hypoplasia of the anterior pituitary gland, absent pituitary stalk and EPP bright spot [10]. Patients with EPP may have Isolated Growth Hormone Deficiency (IGHD) or Combined Pituitary Hormone Deficiency (CPHD); Diabetes Insipidus (DI) is not a feature, indicating that despite the presence of ectopic posterior lobe, it is still functioning normally because the upper part of the antidiuretic hormone system remains intact [6,13].
EPP is more common in children with CPHD [14]; furthermore IGHD may progress into CPHD in patients with EPP [15,16]. EPP can be associated with septo-optic dysplasia, Chiari I malformation, agenesis of the corpus callosum, Kallmann syndrome and periventricular heterotopias [5,8].
The objectives of this study are: 1) to detect the prevalence of IGHD or CPHD in children with EPP, 2) to evaluate the association between the location of the EPP and the degree of pituitary dysfunction, 3) to determine the prevalence of brain and pituitary abnormalities detected by MRI in children with documented pituitary dysfunction and to characterize these imaging findings.
Materials and Methods
We conducted a retrospective chart review of all brain/pituitary MRI studies obtained at St. Christopher’s Hospital for Children (SCHC) between January 1, 2006 and December 31, 2018. We reviewed all brain/pituitary studies, and found the cases with EPP, absence of posterior pituitary, and pituitary adenoma. Of the cases we found, we documented if they had septo-optic dysplasia or Chiari I malformation. Of these structural abnormalities, we focused on EPP because there are not many research articles that address the association hormonal or clinical abnormalities in children with EPP.
Pituitary hormone function was evaluated in the majority of the EPP patient population.
Using the Picture Archiving and Communications System (PACS), all brain/pituitary MRI images and bone age radiographs in children with the above-mentioned imaging abnormalities were interpreted by pediatric radiologists. Subsequently, pituitary function was evaluated by pediatric endocrinologists.
The following information was obtained:
• Gender/sex,
• Date of birth,
• Height measurement in centimeters by stadiometer. Height was expressed as standard deviation score (SDS) for sex and chronological age. SDS was calculated using CDC growths along with patient’s height, date of birth and date of exam.
• Skeletal maturation was determined by the bone age radiograph, for children >4 years of age. Using the reference standards of Greulich and Pyle [17].
• Brain and pituitary MRI with and without contrast using T1-weighted sagittal scan.
• Endocrine investigation included: serum TSH and free T4 by Electrochemiluminescence Immunoassay (ECLIA), serum IGF-1 and IGFBP3 by Immunochemiluminometric (ICMA), ACTH stimulation test (diagnosed with adrenal insufficiency when peak cortisol level below 18 mcg/dL) and growth hormone stimulation tests (interpreted as growth hormone deficiency when peak growth hormone level below 10 ng/mL after two pharmacological tests). Peak 1 was interpreted after stimulation with arginine 10% IV (0.5gram/ kg; max dose 30 grams and peak 2 after giving glucagon IM (0.03mg/ kg; max dose 1mg).
In addition to other tests as serum Na, serum osmolality, random urine osmolality and specific gravity (<1.010 defined as diluted urine). Patients were classified into 3 groups (upper, middle and lower) according to EPP location along the pituitary stalk. Results were expressed in mean ± standard deviation, numbers, percentages, frequency and prevalence. Graph Pad-Prism 8 was used for all statistical calculations.
Results
A total of 66 patients with abnormal brain and pituitary MRI with mean chronologic age of 8.31±6.26 years were included. Of those, 26 patients had an EPP, 21 had absent posterior pituitary and 19 patients with pituitary adenoma. The prevalence rate of EPP was 39% in our patient population.
Our study showed the prevalence rate of documented pituitary dysfunction in children with brain and pituitary abnormalities detected by MRI was 87.5%. The prevalence rate was higher among patients with EPP (95%) as compared to those having absent posterior pituitary and pituitary adenoma, 84.6% and 80%, respectively.
Records of 26 children with EPP were reviewed. Of the 26 patients with EPP, [16 Males (M) and 10 Females (F)], mean chronologic age was 5.98±5.18 years, with height SDS of -3.06±4.77.Only 20 patients underwent laboratory evaluation for pituitary dysfunction at SCHC. The mean chronologic age of those 20 children was 6.18±5.37 years, boys and girls were almost equally affected and height SDS was 2.76±4.69 as shown (Table 1).
Patient Population
Entire Patient Population n=26
Patient Population with SCHC Endocrine Evaluation n=20
Age (yrs) mean ± std
5.98 ± 5.18
6.18 ± 5.37
Height (SDS) mean ± std
1.71
1.93
Gender-male n (%)
16 (61.5)
10 (50)
Table 1: Basic characteristics of EPP patient population.
Patients were classified into 3 groups (upper, middle and lower) according to EPP location along the pituitary (infundibular) stalk, (Figures 1,2 and 3).
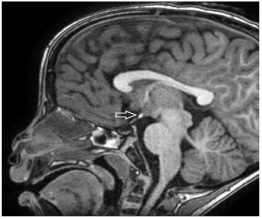
Figure 1: Sagittal T1 weighted magnetic resonance imaging showing upper
EPP.
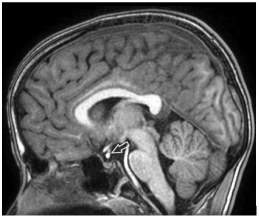
Figure 2: Sagittal T1 weighted magnetic resonance imaging showing middle
EPP.
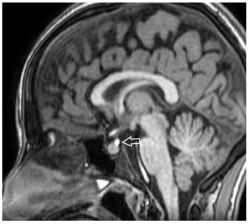
Figure 3: Sagittal T1 weighted magnetic resonance imaging showing lower
EPP.
Group 1 - Upper: at or above optic chiasm
Group 2 - Middle: below optic chiasm, above the insertion of infundibular stalk on adenohypophysis
Group 3 - Lower: At insertion of infundibular stalk on adenohypophysis
Table 2 shows that of 26 patients, 21 children had upper EPP, including 13 males, Mean chronologic age was 5.25±4.74 years, height SDS -3.37±5.09. Four children had middle EEP, including 3 males, Mean age was 7.25±5.62, height SDS of -1.87±0.52. One 16 year old female patient with normal height had lower EPP.
Demographic Data
Group 1 - Upper (n=21)
Group 2 - Middle (n=4)
Group 3 - Lower (n=1)
Total (n=26)
Age (yrs) mean ± std
5.25 ± 4.74
7.25 ± 5.62
16.4 ± 0
5.98 ± 5.18
Height (SDS) mean ± std
1.72
-1.35
0.183 ± 0
1.71
Gender-male n (%)
13 (62)
3 (75)
0 (0)
16 (62)
Table 2: Basic characteristics based on the EPP location.
In the upper EPP group, 17 patients underwent endocrine evaluation, all of whom (100%) had some form of endocrine dysfunction. Fourteen (67%) had CPHD, and three (14%) had IGHD.
In the middle group, 2 patients underwent endocrine evaluation. Only 1 of the 2 patients (50%) had pituitary deficiency, which was isolated GHD. The patient with lower EPP had no pituitary deficiency but did have hyperprolactinemia. One had microcephaly unaccompanied by pituitary dysfunction in middle group and 6 without endocrine evaluation at SCHC (4 in upper and 2 in middle). Diabetes insipidus was not identified in any of the patients, (Table 3). Of note, one out of 14 patients with upper EPP and CPHD had septo-optic dysplasia.
Diagnosis
Group 1 - Upper (n=21) n (%)
Group 2 - Middle (n=4) n (%)
Group 3 - Lower (n=1) n (%)
Total (n=26) n (%)
Combined Pituitary Hormone Deficiencies (CPHD)
14 (67)
0 (0)
0 (0)
14 (54)
Isolated GHD (IGHD)
3 (14)
1 (25)
0 (0)
4 (15)
Diabetes Insipidus (DI)
0 (0)
0 (0)
0 (0)
0 (0)
Hyperprolactinemia
0 (0)
0 (0)
1 (100)
1 (4)
(Microcephaly)*
0 (0)
1 (25)
0 (0)
1 (4)
Without SCHC Endocrine Evaluation
4 (19)
2 (50)
0 (0)
6 (23)
*This patient does not have pituitary dysfunction.
Table 3: Frequency of Diagnostic Categories for Groups 1, 2, 3.
Patients with CPHD had higher prevalence of EPP (73.7%) as compared to those with IGHD (21.1%). Hyperprolactinemia prevalence rate was 5.3%.
In upper group, 3 patients with IGHD were reported, 1 male and 2 females, their mean age was 4.5±3.122 years with the mean height of -3.678±2.225 SDS. One patient had an optic nerve hypoplasia and a Chiari I malformation. 2 patients had delayed bone age. All 3 had both GH stimulation peaks <10 ng/mL (suboptimal). In the middle group, one 13 year old female patient was reported with height of -2.245 SDS, GH stimulation peaks <10 ng/mL, had normal bone age, (Table 4).
Upper n= 3
Middle n=1
Age (yrs) Mean ± std
4.5 ± 3.122
13 ± 0
Height (SDS) Mean ± std
-1.453
-2.245
Gender-male n(%)
1 (33.33)
0 (0)
Delayed bone age n (%)
2 (100)*
0 (0)
GH Peak 1 (<10 ng/mL)
3 (100)
1(100)
GH Peak 2 (<10 ng/mL)
3 (100)
1(100)
*Bone age was reported only in 2 patients with upper EPP due to non-reporting bone age in one case (age <4 years).
Table 4: Basic and Hormonal Characteristics for Patients with IGHD in Upper and Middle Group.
Table 5 demonstrates that of 20 patients who underwent endocrine evaluation at SCHC, 10 patients had GH stimulation peak 1<10 ng/mL after arginine injection and 11 patients had GH stimulation peak 2<10 ng/mL following glucagon injection; (one patient had undergone only one pharmacological test with glucagon). Therefore, all patients who underwent GH stimulation test regardless of the stimulating agent had peak GH concentration <10 ng/mL consistent with growth hormone deficiency. Some of those patients had significantly low IGF-1 and IGFBP3 levels for which GH stimulation test was not required. ACTH stimulation test showed adrenal insufficiency in 7 children out of 8 who had low cortisol levels in the GH stimulation test. Thyroid function tests were done in all patients. All patients had normal serum and urine osmolality with the serum sodium levels ranging between 131-143 meq/L.
Results for Patient Population
GH Peak 1
GH Peak 2
GH Stimulation Test Peak Cortisol
ACTH Stimulation Test Peak Cortisol
IGF-1
IGFBP3
Free T4
TSH
n=20
n (%)
n (%)
n (%)
n (%)
n (%)
n (%)
n (%)
n (%)
Low
10 (50)
11 (55)
8 (40)
7 (35)
11 (55)
10 (50)
7 (35)
9 (45)
Normal
0 (0)
0 (0)
3 (15)
1(5)
8 (40)
8 (40)
13 (65)
11 (55)
Not performed
10 (50)
9 (45)
9 (45)
12 (60)
1 (5)
2 (10)
0 (0)
0 (0)
Table 5: Hormonal Characteristics for Entire Patient Population with SCHC Endocrine Evaluation.
Discussion
In this retrospective study, we describe a total of 66 patients with abnormal brain and pituitary MRI. Of those, 26 patients had an EPP, 21 had absent posterior pituitary and 19 patients with pituitary adenoma. The prevalence rate of EPP was 39%.
Our data demonstrates that the prevalence of pituitary dysfunction in children with MRI structural abnormalities inclusive of EPP was 87.5%, while a previous study showed MRI abnormalities together with EPP were observed in 48.65% of patients with IGHD and 93.5% with CPHD [14].
The location of the ectopic lobe along the pituitary stalk can differ. In our study, EPP is commonly located along the upper third of pituitary stalk at the median eminence level in the floor of the third ventricle which was similar to that noted by Mahomed et al. [5] and Ginat et al. [8]. Chen et al. suggested EPP is not always at the median eminence, but can be located at different levels of the pituitary stalk which corresponds with the findings of our study [18].
Ectopic posterior pituitary appearing at the level of median eminence or along the pituitary stalk has been reported in idiopathic GH deficiency [12].
Our data support that the presentation of EPP can vary with the location of EPP; patients with EPP that is located along the upper third of pituitary stalk at the median eminence level, presented with more severe pituitary dysfunction as the upper most EPP is closest to the hypothalamus. Chen et al. [18] and Cerbone et al. [19] documented that the posterior pituitary location along the stalk is a significant protective factor for the severity of the hormonal abnormality, with a greater number of hormonal deficiencies present when the posterior lobe is located at the median eminence or in the hypothalamic region, as our study demonstrates.
The GH Research Society stated that in the patients with an initial diagnosis of IGHD, particularly those with ectopic posterior pituitary, or other developmental abnormalities, the clinician should be aware of the risk of the development of CPHD [20].
Interestingly, the risk of progression from IGHD to CPHD is higher in children with EPP.
Iorgi et al. [21] reported a prospective study with 2 years of follow-up that 61% of young adults with childhood onset IGHD associated with EPP developed additional anterior pituitary hormonal deficiencies.
In our study, the prevalence of CPHD in children with EPP was 73.7%, which is comparable to that reported by Jagtap et al; they found the prevalence of CPHD was 58.1% and 18.1% in those with and without EPP, respectively. [14] This is in contrast to Dutta et al; who reported 80% prevalence of CPHD amongst those with orthotopic posterior pituitary as compared to 50% in those with EPP [12]. In this study, patients with EPP had a higher prevalence of CPHD (73.7%) as compared to IGHD (21.1%), which was similar to that explained by both Abrahams et al. [22] and Bozzola et al. [23] who reported a higher prevalence of EPP in CPHD than IGHD but in contrary to what has been reported by Dutta et al. [24].
Bozzola et al. reported a higher prevalence of EPP in CPHD (76% at prepubertal and 93% at pubertal age) when compared to the groups of IGHD (16% in prepubertal children, 0% in children with normal onset puberty and 11% in those with delayed onset of puberty) [23]. In comparable to that seen by Abrahams et al; they found EPP in 87% of subjects with CPHD and 10% of those with IGHD [22].
Dutta et al; has elucidated EPP in 50% of patients with CPHD and in 87.5% in those with IGHD [24]. Our study demonstrated one out of 14 patients with EPP and CPHD had septo-optic dysplasia in compares to Jagtap et al; they found four out of 15 patients with EPP and CPHD had evidence of septo-optic dysplasia [14]. In our study hyperprolactinemia was seen in one patient through 20 children with EPP who had endocrine evaluation at SCHC. Jagtap et al. observed hyperprolactinemia in four patients out of 31 patients with EPP [14].
Our results revealed that no cases of DI have been reported in children with EPP, indicating that despite the presence of ectopic posterior lobe, it is still functioning normally because the upper part of the antidiuretic hormone system remains intact. This corresponds with the findings of prior studies [6,13].
Maghnie et al. had reported that evidence of posterior pituitary hyperintensity does not rule out the diagnosis of central DI, as release of stored antidiuretic hormone may be impaired in some cases of autosomal dominant DI, as well as in some idiopathic forms [25].
We further elucidate that patients with IGHD and upper EPP were both shorter and younger than those with middle EPP. This is the first study to report such finding. Our study has some limitations, first; overall small sample size of subjects and most notably small number of patients in middle and lower EPP groups make the statistically analysis unsuccessful to establishing the significant difference between groups (p-values). This lowers the power of the study.
Second; we don’t report changes in hormone deficiency over time, so we don’t know if patients with combined deficiency started as isolated, or if the isolated patients will be become combined (this is not prospective). Third; lacking of endocrine evaluation of six patients with EPP.
Conclusion
Imaging studies aid in the detection of structural abnormalities that may be associated with pituitary dysfunction, such as EPP. Therefore, EPP represent a useful predictor of anterior pituitary development, anatomy, structure and function.
There is a well-defended connection between the location of EPP and the magnitude of pituitary dysfunction. Our study supports previous reports that CPHD and IGHD are frequent in patients with EPP. Similarly, our data further demonstrate that no cases of DI have been reported in children with EPP.
In our study, EPP is most commonly located along the upper third of pituitary stalk at the median eminence level, with a higher prevalence of CPHD and IGHD compared with middle and lower EPP, a finding similar to prior studies. No CPHD was reported in middle/lower but IGHD was found in the middle EPP group.
This study also elucidates that patients with IGHD with upper EPP were shorter and younger than those with middle EPP. More studies in a large cohort of patients may be helpful to reveal the correlation between the location EPP and the patient’s linear growth. Furthermore, more longitudinal studies required to identify the risk of progression from IGHD to CPHD.
References
- Lahiri AK, Sundareyan R, Jenkins D, Nilak A. MRI of ectopic posterior pituitary gland with dysgenesis of pituitary stalk in a patient with hypogonadotropic hypogonadism. J Radiol Case Rep. 2018; 13: 764-766.
- Wang C-Z, Guo L-L, Han B-Y, Su X, Guo Q-H, Mu Y-M. Pituitary Stalk Interruption Syndrome: From Clinical Findings to Pathogenesis. J Neuroendocrinol. 2017; 29.
- Yin W, Gore AC. The hypothalamic median eminence and its role in reproductive aging. Ann NY Acad Sci. 2010; 1204: 113-122.
- Bep-Shlomo A, Melmed S. Nonpituitary. Hypothalamic Regulation of Anterior Pituitary Function. Editor. In: Melmed S. The Pituitary. 3rd edition. Amsterdam. Elsevier/Academic Press; 2011: 21-41.
- Mahomed N, Motshudi T. The ectopic posterior pituitary gland. S Afr J Surg. 2013; 51: 148.
- Moshkin O, Albrecht S, Bilbao J, Kovacs K. Nonpituitary Tumor of the Sella Region. In: Melmed S, editor. The Pituitary. 3rd edition. Amsterdam. Elsevier/ Academic Press; 2011: 655-668.
- Kucharczyk J, Kucharczyk W, Berry I, Groot Jde, Kelly W, Norman D, et al. Histochemical characterization and functional significance of the hyperintense signal on MR images of the posterior pituitary.Am J Roentgenol. 1989; 152: 153-157.
- Ginat DT, Meyers SP. Intracranial Lesions with High Signal Intensity on T1- weighted MR Images: Differential Diagnosis. RadioGraphics. 2012; 32: 499- 516.
- Saleem SN, Said A-HM, Lee DH. Lesions of the Hypothalamus: MR Imaging Diagnostic Features. RadioGraphics. 2007; 27: 1087-1108.
- Parks J, Felner E. Hypopituitarism. Editors. In: Kliegman R, Stanton B, St Geme J, Schor N. Nelson Textbook of Pediatrics. 20th edition. Philadelphia, Pa Elsevier. 2016: 2637-2644.
- Omer A, Haddad D, Pisinski L, Krauthamer AV. The Missing Link: A Case of Absent Pituitary Infundibulum and Ectopic Neurohypophysis in a Pediatric Patient with Heterotaxy Syndrome. J Radiol Case Rep. 2017; 11: 28-34.
- di Iorgi N, Secco A, Napoli F, Calandra E, Rossi A, Maghnie M. Developmental Abnormalities of the Posterior Pituitary Gland. Endocr Dev. 2009; 14: 83-94.
- Bonneville JF, Bonneville F. The Ectopic Posterior Lobe. In: MRI of the Pituitary Gland. Springer, Cham. 2016.
- Jagtap VS, Acharya SV, Sarathi V, Lila AR, Budyal SR, Kasaliwal R, et al. Ectopic posterior pituitary and stalk abnormality predicts severity and coexisting hormone deficiencies in patients with congenital growth hormone deficiency. Pituitary. 2012; 15: 243-250.
- Bozzola M, Mengarda F, Sartirana P, Tato L, Chaussain J. Long-term followup evaluation of magnetic resonance imaging in the prognosis of permanent GH deficiency. Eur J Endocrinol. 2000; 143: 493-496.
- Maghnie M, Strigazzi C, Tinelli C, Autelli M, Cisternino M, Loche S, et al. Growth Hormone (GH) Deficiency (GHD) of Childhood Onset: Reassessment of GH Status and Evaluation of the Predictive Criteria for Permanent GHD in Young Adults. J Clin Endocrinol Metab. 1999; 84: 1324-1328.
- Greulich W, Pyle S. Radiographic atlas of skeletal development of the hand and wrist. 2nd editionn. Stanford University Press. 1959.
- Chen S, Leger J, Garel C, Hassan M, Czernichow P. Growth Hormone Deficiency with Ectopic Neurohypophysis: Anatomical Variations and Relationship between the Visibility of the Pituitary Stalk Asserted by Magnetic Resonance Imaging and Anterior Pituitary Function. J Clin Endocrinol Metab. 1999; 84: 2408-2413.
- Cerbone M, Dattani MT. Progression from isolated growth hormone deficiency to combined pituitary hormone deficiency. Growth Horm IGF Res. 2017; 37: 19-25.
- Society GR. Consensus Guidelines for the Diagnosis and Treatment of Growth Hormone (GH) Deficiency in Childhood and Adolescence: Summary Statement of the GH Research Society1. J Clin Endocrinol Metab. 2000; 85: 3990-3993.
- di Iorgi N, Secco A, Napoli F, Calandra E, Rossi A, Maghnie M. Deterioration of Growth Hormone (GH) Response and Anterior Pituitary Function in Young Adults with Childhood-Onset GH Deficiency and Ectopic Posterior Pituitary: A Two-Year Prospective Follow-Up Study.J Clin Endocrinol Metab. 2007; 92: 3875-3884.
- Abrahams JJ, Trefelner E, Boulware SD. Idiopathic growth hormone deficiency: MR findings in 35 patients.Am J Neuroradiol. 1991; 12: 155-160.
- Bozzola M, Adamsbaum C, Biscaldi I, Zecca M, Cisternino M, Genovese E, et al. Role of magnetic resonance imaging in the diagnosis and prognosis of growth hormone deficiency. Clin Endocrinol (Oxf). 1996; 45: 21-26.
- Dutta P, Bhansali A, Singh P, Rajput R, Khandelwal N, Bhadada S. Congenital Hypopituitarism: Clinico-Radiological Correlation.JPediatr Endocrinol Metab. 2009; 22: 921-928.
- Maghnie M, Villa A, Arico M, Larizza D, Pezzottz S, Beluffi G, et al. Correlation between magnetic resonance imaging of posterior pituitary and neurohypophyseal function in children with diabetes insipidus. J Clin Endocrinol Metab. 1992; 74: 795-800.