
Research Article
J Pediatr Endocrinol. 2025; 10(1): 1069.
Measurement of 17-Alpha Hydroxyprogesterone on a State-of-the-Art ABEI-Based Chemiluminescence System – Establishment of Upper Reference Limit
Ijaz A¹*, Sahar R², Javed B², Ahmed F³, Najeeb S², Shahid F² and Hafeez N²
1NUST School of Health Sciences, Islamabad, Pakistan
2Mohi Uddin Islamic Medical College and Hospital, Mirpur, AJ&K, Pakistan
3Milton-Keyenes University Hospital, NHS Trust, Oxford University Hospitals, UK
*Corresponding author: Prof Aamir Ijaz, NUST School of Health Sciences, National University of Science and Technology (NUST), Islamabad, Pakistan Email: aamirijaz@nshs.nust.edu.pk
Received: February 17, 2025; Accepted: March 10, 2025; Published: March 13, 2025;
Abstract
Background: 17 alpha Hydroxyprogesterone (17a-OHP) is the most commonly used test for the screening and diagnosis of congenital adrenal hyperplasia in neonates. Establishment of Upper Reference Limit (URL) of 17a-OHP using an ABEI-Based chemiluminescence autoanalysers has been done for the first time in Pakistan.
Methods and Materials: Out of 187 neonates included in the study, serum specimens of 139 disease-free neonates (<30 days of age) were collected in Mohi Uddin Teaching Hospital Mirpur AJ&K (MOTH) after taking consent from the mothers of the kids. Excluding ten haemolysed specimens, 129 samples were analyzed in the Pathology Laboratory of MOTH using Maglumi X8 (Snibe, Shenzhen, China), an ABEI (N-(aminobutil)-N-(ethyl)-isoluminol)-based system. Three results were declared outliers, rest were used for calculation of URL of 17a-OHP.
Results: The 99th Percentile URL of 17-OHP was found to be 4.7 nmol/L in 126 samples
Conclusion: A cut-off value of 4.7 nmol/L (156 ng/dl) can be used for the exclusion of congenital adrenal hyperplasia in Pakistan Children
Keywords: Congenital adrenal hyperplasia; 17 Hydroxyprogesterone; 21 Hydroxylase deficiency
Introduction
Congenital Adrenal Hyperplasia (CAH) is a complex and often life-threatening disease with a multitude of hormonal imbalances that can result in disease-and treatment-related adverse outcomes [1]. Markedly raised 17-alpha hydroxyprogesteron (17a-OHP) is pathognomonic of congenital adrenal hyperplasia (CAH) in neonates. The steroid 17-a-OHP) is produced by both the adrenal cortex and gonads [2]. Even though 17a-OHP has relatively little progestational activity, it is of intense clinical interest because it is the immediate precursor to 11-desoxycortisol (11-DOC). 11-DOC is produced by 21-hydroxylation of 17-a-OHP (Figure 1), measurement of 17-a-OHP is a useful indirect indicator of 21-hydroxylase activity [3]. A deficiency of 21-hydroxylase (21OH) leads to accumulation of proximal metabolite i.e. 17-OHP, which has an androgenic action. Cortisol deficiency does not occur due to increased ACTH causing adrenal hyperplasia and near normalisation of cortisol [4]. These neonates may present with salt-losing wasting (both genders) or ambiguous genitalia (girls) [5]. Deficiency of 21-OHD accounts for 95 percent of cases of CAH [4]. About 200 gene mutations in CYP21A2 are involved in causation of a continuum of disease phenotypes and the expected residual 21-OHD activity with each genotype [6]. CAH is included in the mandatory newborn screening programmes of many developed countries [7]. Prevalence of 21-OHD has been found to be approximately 6.5 million livebirths [8], an overall prevalence of approximately 1 in 15,000 livebirths [9,10]. Traditionally, radioimmunoassay (RIA) has been used for the measurement of 17a-OHP [11] but RIA has numerous disadvantages and limitations [12]. So chemiluminescence immunoassay (CLIA) has now been made available for estimation of 17a-OHP [13]. CLIA has the biggest advantage of ultra-high analytical sensitivity and ease of automation [14].
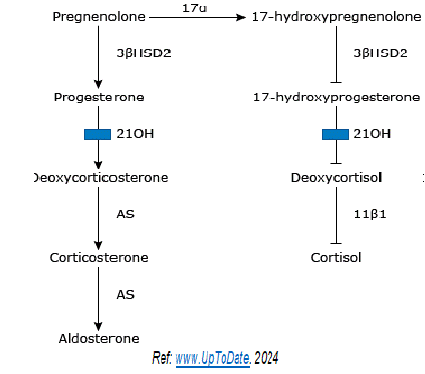
Figure 1: Synthesis Pathway of Cortisol and Aldosterone Synthesis.
In Pakistan, like other countries in the region, no national newborn screening programme exists for any disease. So, CAH has to be diagnosed in symptomatic newborn babies. Snibe (China) offers a series of fully automated chemiluminescence analyzers (Maglumi) for many novel diagnostic markers including 17a-OHP [13]. There is a need to establish reference values using optimum analytical protocol in disease free newborn [15-17]. Since in disease free neonates and infants 17a-OHP is very low, usually below the limit of detection (LOD) of the test, while in CAH the level is very high, we may use 99th Upper Reference Limit (URL) for the diagnosis of CAH. At this level the analytical performance of the test is usually reasonable. It is to be noted that low 17a-OHP is of no clinical significance i.e. no disease can be diagnosed on the basis of decreased 17a-OHP.
Three different statistical methods are used for establishment of reference values i.e. non-parametric method [18], parametric method [19], boot-strap method [20] and robust method [21]. All these methods have their own inherent advantages and limitations [22,23].
Methods and Materials
Venue of the Project
Mohi Uddin Islamic Medical College (MIMC) and Mohi Uddin Teaching Hospital (MOTH) Mirpur AJ&K, Pakistan
Study Duration
Nine Months starting from 15th November 2022 to 15th August 2023.
Reference Individuals
A priori method was used for selection of the reference population of newborn (<30 days of age), free of symptoms, signs and laboratory findings suggestive of any adrenal or other disorder [24].
Exclusion Criteria
1. Neonates requiring admission in hospital for any abnormality like jaundice, failure to thrive or neurological signs.
2. Neonates on any medication.
3. Hemolyzed blood specimen.
Sample Size
A total of 187 neonates were included in the study. Specimens were collected from 139 neonates after excluding the subjects falling in the exclusion criteria; 10 blood specimens were found haemolysed, so 129 specimen were analyzed for 17a-OHP. Three results were declared outliers (upper) based on Dixon`s Method [15]. The sample size was in accordance with the recommended minimum sample size of 120 individuals for establishing reference values without partitioning [25]. Since there was no significant difference in the median of the values (P<0.05) between males and females, partitioning was not carried out. Written informed consent was obtained from mothers of the neonates, not only to collect samples but also to inform about the data usage to calculate reference values.
Permission from Institutional Review Board (IRB)
Proper permission was obtained from IRB of MIMC.
Pre-analytical Factors were addressed carefully before samples were analyzed including:
a. Subject preparation: no abnormal crying or sweating due to weather conditions.
b. Specimen collection: non-fasting condition, no double prick, use of butterfly needles to reduce pain.
c. Specimen handling: early transport to the lab for centrifugation and serum separation.
d. Specimen Transport: Immediate transported of the specimens to the lab.
e. Specimen storage: Stored in an appropriate refrigerator until analyzed.
Laboratory Analyses
a) Chemiluminescence-based autoanalyzer Maglumi X8 by SNIBE (Shenzhen, China) was used for laboratories-based analyses.
b) This is a continuous random access chemiluminescence immunoassay (CLIA) automated system uses nano-magnetic microbeads separation high throughput (180 tests/h) technology, the luminescence substrate being N-(aminobutil)-N-(ethyl)-isoluminol (ABEI).
c) Performance characteristics of the assay is given in Table 1.
Precision
(Intra-assay CV%Analytical Sensitivity
Calibration Traceability
Constant Error
(y-intercept)Proportion Error
(Slope)Passing-Bablok regression equations
Interfering Substances
Hook effect
3.9%
0.5 nmol/L
AMR: 0.5 – 354 nmol/L
Snibe Internal Reference Material
0.4
95% Confidence Interval:
0.1 to 0.50.6
95% Confidence Interval:
0.5 to 0.7y = 0.6x + 0.4
no influence on the assay results.
Haemoglobin (up to 4 mg/mL), Bilirubin (up to 0.5 mg/mL) and Triglyceride (up to 7.5 mg/mL)None
Table 1: Performance Characteristics of 17-Hydroxyprogesterone CLIA Assay [13].
d) Snibe (China) provided reagents and consumables for this project.
Quality Control (QC)
a. Internal QC provided in the reagent kits was run with each batch of analysis.
b. NHS® External Quality Assurance Scheme (EQAS) was participated to ensure accuracy by inter-laboratory comparison.
Statistical Procedures
1. Non-parametric approach was used for calculation of reference values because of following reasons:
a. This is the recommended method by International Federation of Clinical Chemistry (IFCC) [16] and adopted by Clinical and Laboratory Standard Institute (CLSI) of USA [23].
b. The nature of the underlying distribution of the data does not matter.
c. No statistical expertise is required; the values obtained from reference individuals were simply put in rank order by concentration (rank 1 is the lowest, rank 2 is the next lowest, etc.).
d. The 99th percentile value becomes the reference value or cut-off.
e. The 0.90 confidence limit of the upper 99th value was similarly just taken from the data points themselves.
2. Since no statistical difference between the two partitioned groups e.g. males and females were found, so separate reference intervals for different partitions were not needed and data was not collected for each partition.
Results
Initially we included 187 babies of first month of life. We excluded 48 subjects due to presence of one or more of the exclusion criteria (Figure 2). Serum specimens of 139 neonates were collected but 10 specimens were found haemolysed and were not analyzed. After analyses for 17a-OHP, three specimens were rejected being declared outliers. Age and gender distribution of remaining 126 subjects is given in Figure 3. We calculated 99th percentile URL on 126 results of 17a-OHP using following procedure:
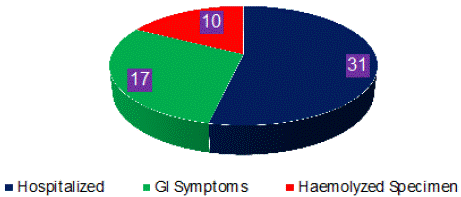
Figure 2: Distribution of Newborn Excluded from Study (n = 58).
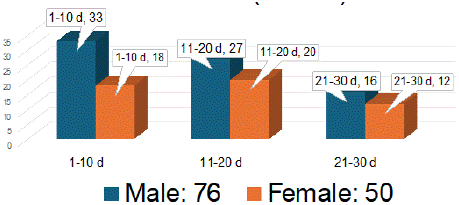
Figure 3: Age and Gender Distribution of Newborn (n = 126).
a. 97.5th Percentile URL = 0.975 x (126 + 1) = 123.82 (i.e. Rank # 124),
b. Original Value of 17a-OHP at 97.5th Percentile URL = 4.7 nmol/L 0.90 Confidence Limits for 97.5th Percentile,
c. Lower 0.90-Confidence Limit: (126+1) – 7 = #120,
d. Value at #120: 4.4,
e. Upper 0.90-Confidence Limit: (126+1) – 1 = #126,
f. Value at #126: 5.2 ng/nmol/L.
Summary
97.5th Percentile URL (0.90 Confidence Limits): 4.7 (4.4 – 5.2) nmol/L,
Traditional Units: 155 ng/dL,
Conversion Factor (nmol/L to ng/dL): 33; (ng/dl to nmol/L): 0.03.
Discussion
In the present study we have established 4.7 nmol/L (156 ng/dl) as the 97.5th percentile URL for 17a-OHP. Unlike traditional literature we have not reported middle 95th percentile values i.e. 2.5th percentile (lower) and 97.5th (upper) values. The logic behind is two- fold: a) There is no clinical importance of lower (2.5th percentile) value as there is no known disease with lower 17a-OHP; b) In CAH, 17a-OHP is unmistakably high (may be thousands of holds) [2], so we may take the highest possible level found in disease-free neonates as our cut-off values. Calculating and reporting two values is a futile exercise and causes unnecessary cognitive burden. Cardiac troponins are examples of analytes reported as 99th percentile URL because in 50% of the reference population without any cardiac disease troponins are almost undetectable [26]. Our reported value is strikingly similar to the upper reference value determined on Abbott Architect (4.2 nmol/L) [27].
Classical variety of CAH can present in multiple ways e.g. enlarged clitoris or partial fusion of labia in XX babies; these morphological anomalies are collectively called ambiguous genitalia. In XX and XY it can also present as salt-losing enteropathy and failure to thrive [2]. Yet another classical CAH presentation is ‘heterosexual precocious puberty’, in this variety an XX child develops as a boy with male-like genitalia and facial hair growth at a very early age [28] (Figure 4). These features are due to very high 17a-OHP right from the birth that remains undetected until early childhood. The instrument we used for analysis of 17a-OHP is the state-of-the-art random access fully automated system (Maglumi X8). This system is the ultimate development of time-honored ELISA plate readers, that required immense time and manpower resources with many potential sources of errors [29]. Lapic et al (2021) has evaluated 17a-OHP on CLIA based Maglumi 800 along with six other parameters and found it acceptable as compared to currently used system (Diasorin) [13]. Although CLIA cannot replace the ‘Gold Standard’ Liquid Chromatography- Mass Spectrometry, its ease of performance makes it the system of choice for the modern diagnostic labs [30].
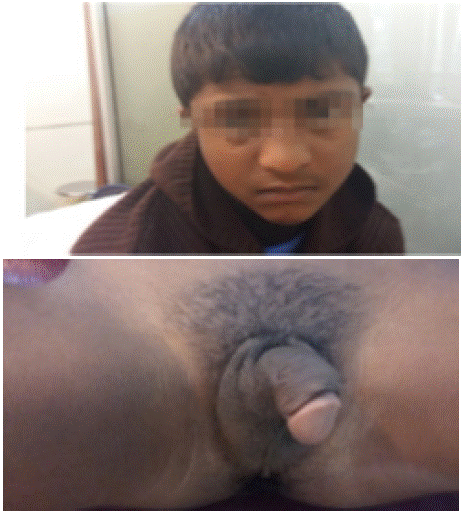
Figure 4: Patient of Heterosexual Precocious Puberty at 6 years of age
(XX Child with very high 17 alpha hydroxyprogesterone) -A presentation of
classical CAH (Please see Ref No 28).
(Note: Explicit permission of the child and parents has been obtained for
publication of these pictures)
In XY boys classical CAH can present as precocious puberty with markedly raised 17a-OHP2. 17a-OHP is also the investigation of choice in non-classical forms of CAH presenting in adolescence girls as hirsutism and acne. In this situation, sometimes the basal level of 17a-OHP is not above the URL and ACTH stimulation has to be resorted [31].
17a-OHP can also be used as a supportive test in CAH due to enzyme defects other than 21-hydroxylase e.g. 17-hydroxylase deficiency [32].
Conclusion
We report upper reference limit for 17 hydroxyprogesterone as 4.7 nmol/L (156 ng/dL) to be used as a cut-off value for the diagnosis of congenital adrenal hyperplasia in newborn.
Recommendation
Facilities for estimation of 17-OHP on a state-of-the-art fully automated ABEI-based chemiluminescence system be made available in hospital labs, so that diagnosis and monitoring of congenital adrenal hyperplasia is carried out effectively by our physicians.
Acknowledgement
We are greatly indebted to SNIBE (Shenzhen-China) for providing reagent and consumables for this project and Pakistan Society of Chemical Pathologists (PSCP) for funding this project.
Ethics Statement
• Present article reports portion of the data of a project carried out to facilitate the users of fully automated ABEI-based Chemiluminescence System Maglumi X8.
• By no means we report any comparison of performance of this system with any other system.
• No conflict of interest is declared by the authors, since no comparison of instruments was involved.
• This study was reviewed and approved by the Institutional Review Board of Mohi Uddin Islamic Medical College Mirpur AJ&K, Pakistan.
• Written informed consent of the volunteers was obtained for the study
Author Contributions
AI, SU and FA were involved in study design, interpreting data, statistical analysis, creating tables and figures, and writing of the manuscript. RS, BJ, FS, NH were involved in dealing with the mothers of the subjects, collecting data and supervised the work. All authors contributed to the article and approved the submitted version.
References
- Merke DP, Auchus RJ. Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency. N Engl J Med. 2020; 383: 1248-1261.
- Merke DP. Clinical manifestations and diagnosis of classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency in infants and children. 2024.
- Bertholf RL, Cooper M, Winter WE. The Adrenal Cortex. In: Rifai N, Horvath AR and Wittwer CT, eds. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. 6th Edition. Elsevier. Philadelphia, US. 2018: 1530-1572.
- Speiser PW, Arlt W, Auchus RJ, Baskin LS, Conway GS, Merke DP, et al. Congenital Adrenal Hyperplasia Due to Steroid 21-Hydroxylase Deficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2018; 103: 4043.
- Honour JW. 17-Hydroxyprogesterone in children, adolescents and adults. Annals of Clinical Biochemistry. 2014; 51: 424-440.
- New MI, Abraham M, Gonzalez B, et al. Genotype-phenotype correlation in 1,507 families with congenital adrenal hyperplasia owing to 21-hydroxylase deficiency. Proc Natl Acad Sci USA. 2013; 110: 2611–2616.
- Speiser PW, Arlt W, Auchus RJ, Baskin LS, Conway GS, Merke DP, et al. Congenital Adrenal Hyperplasia Due to Steroid 21-Hydroxylase Deficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2018; 103: 4043-4088.
- Kaye CI, Committee on Genetics, Accurso F, La Franchi S, Lane PA, Hope N, et al. Newborn screening fact sheets. Pediatrics. 2006; 118: e934.
- Pang, S, Clark, A. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency: Newborn screening and its relationship to the diagnosis and treatment of the disorder. Screening. 1993; 2: 105.
- Therrell BL. Newborn screening for congenital adrenal hyperplasia. Endocrinol Metab Clin North Am. 2001; 30: 15.
- Chong H, Cheah SH, Ragavan N, Joganligam VT. Production and characterizations of monoclonal antibodies against 17 alpha hydroxyprogesterone. Hybridoma (Larchmt). 2006; 1: 34-40.
- The limitations of radioimmunoassay (RIA), Trends in Analytical Chemistry. 1982; 1: 128-131.
- Lapic I, Kralik Oguic S, Rogic D. Preliminary evaluation of eight less frequent endocrine assays designed for MAGLUMI 800 chemiluminescence immunoanalyzer. Scand J Clin Lab Invest. 2021; 81: 332-338.
- Feldkamp CS. Immunochemical Techniques. In: Kaplan LA and Pesce AJ, eds. Clinical Chemistry: Theory, Analysis, Correlation. 5th Edition. MOSBY (Elsevier). 2010: 162-179.
- Horowitz G, Jones GRD. Establishment and use of reference intervals. In: Rifai N, Horvath AR and Wittwer CT, eds. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. 6th Edition. Elsevier. Philadelphia, US. 2018: 170-194.
- The IFCC recommendations for determining reference intervals: strengths and limitations. J Lab Med. 2009; 33: 45–51.
- Kendal MG, Buckland WR. A dictionary of statistical terms, 5th edition. London: Longman; 1990.
- Linnet K. Non-parametric estimation of reference intervals by simple and boot-strap procedures. Clin Chem. 2000; 46: 867-869.
- Kim T, Choi H, Lee SM. Parametric and non-parametric estimation of reference intervals for routine laboratory tests: an analysis of health check-up data for 260 889 young men in the South Korean military. BMJ Open. 2022; 12: e062617.
- Linnet K. Nonparametric estimation of reference intervals by simple and bootstrap-based procedures. Clin Chem. 2000; 46: 867–869.
- Horn PS, Pesce AJ. Reference Intervals: a user’s guide, Washington, DC: AACC Press. 2005.
- Harris EK, Boyd JC. Statistical bases of reference values in laboratory medicine. New York: Marcel Dekker. 1995.
- Clinical and Laboratory Standard Institute. Defining, establishing and verifying reference intervals in the clinical laboratory. CLSI document C28-A3c. Wayne Pa: Clinical and Laboratory Standard Institute. 2010.
- Karbasy K, Ariadne P, Gaglione S, Nieuwesteeg M, Adeli K. Advances in Pediatric Reference Intervals for Biochemical Markers: Establishment of the Caliper Database in Healthy Children and Adolescents. J Med Biochem. 2015; 34: 23-30.
- Bjerner J. Age-dependent biochemical quantities: an approach for calculating reference intervals. Scan J Clin Lab Invest. 2007; 67: 707-722.
- Bahadur K, Ijaz A, Salahuddin M, Alam A. Determination of highly sensitive cardiac troponin I 99th percentile upper reference limits in a healthy Pakistani population. Pak J Med Sci. 2020; 36: 1303-1307.
- Konforte D, Shea JL, Kyriakopoulou L, Colantonio D, Cohen AH, Shaw J, et al. Complex biological pattern of fertility hormones in children and adolescents: a study of healthy children from the CALIPER cohort and establishment of pediatric reference intervals. Clin Chem. 2013; 59: 1215-1227.
- Haroon ZH, Ijaz A, Haroon MA, Ahmed S, Pervez MA, Asif N. Hetero sexual precocious puberty: Case report and literature review. Case Reports in Internal Medicine. 2017; 4: 12-16.
- Kricka LJ, Park JY. Principles of Immunochemical Techniques. In: Burtis CA, and Bruns DE, eds. Tietz Fundamentals of Clinical Chemistry and Molecular Diagnostics. 6th Edition. SAUNDERS (Elsevier). Philadelphia, US. 2018: 361-363.
- Debeljak Ž, Markovic I, Pavela J, Lukic I, Mandic D, Mandic S, et al. Analytical bias of automated immunoassays for six serum steroid hormones assessed by LC-MS/MS. Biochem Med (Zagreb). 2020; 30: 030701.
- Haroon ZH, Rana Z, Muhammad Asif M, Farhan Javed Dar FJ and Ramzan S. Congenital Adrenal Hyperplasia. In ‘Chemical Pathology for the Beginners’ First Edition. Azeem Academy Lahore. 230.
- Chormanski D, Muzio MR. 17-Hydroxylase Deficiency. [Updated 2023 Jan 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024.