
Research Article
J Ophthalmol & Vis Sci. 2023; 8(4): 1087.
Three-Year Efficacy and Safety Outcomes of the Second-Generation Trabecular Micro-Bypass Stents in Conjunction with Cataract Surgery in Ocular Hypertension and Open-Angle Glaucoma
Jiaru Liu, MD¹; Soumaya Bouhout, MD¹; Erika Massicotte, MD, FRCSC¹; Anik Desgroseilliers², MD, FRCSC¹; Frederic Lord, MD, FRCSC¹; Harmanjit Singh, MD, FRCSC1,3
1Department of Ophthalmology, Centre Hospitalier de l’Université de Montréal, Canada
2Department of Ophthalmology, Université de Montréal, Canada
3Department of Ophthalmology, Queen’s University, Canada
*Corresponding author: Harmanjit Singh (HS) 27 Place d’Armes, Kingston, ON, Canada. Tel: (613) 507-4800 ; Fax: (613) 507-4801 Email: hmansingh25@gmail.com
Received: October 25, 2023 Accepted: November 30, 2023 Published: December 07, 2023
Abstract
Purpose: To evaluate the efficacy and safety of the iStent Inject in conjunction with phacoemulsification in patients with open-angle glaucoma.
Design: Prospective, non-randomized, single-center, consecutive case series
Participants: Patients with primary and secondary open angle glaucoma undergoing cataract surgery and iStent Inject
Main outcomes: The primary outcome of efficacy is postoperative reduction in mean Intraocular Pressure (IOP) and number of anti-glaucomatous drop classes. A primary endpoint is the achievement of =20% IOP reduction from baseline. Secondary outcome measures include visual acuity, visual field parameters, cup-to-disk ratio, and average retinal nerve fiber layer thickness.
Results: 150 eyes were included with a mean follow-up of 2.28 years (standard deviation 1.18). IOP significantly decreased at nearly all timepoints and remains significantly reduced from baseline at 36 months. IOP reduction is modest and ranges between 5.6 to 11.7 %, with IOP being stably maintained lower than baseline throughout the observation period. IOP reduction is greatest in the mild glaucoma group. The primary endpoint of an over 20% IOP reduction being achieved in overall 32% of eyes. The burden of glaucoma drops was significantly reduced by 13% at 12 months post-op (p<0.005). There was no statistically significant change in visual field parameters, although they improved after cataract surgery. The rate of additional glaucoma interventions needed were very low at <1%.
Conclusion: The combined cataract surgery and iStent Inject procedure is safe and effective at long-term with a low rate of serious complications. It offers good visual outcomes and reasonable glaucoma control long-term.
Keywords: Istent inject; Efficacy; Safety; MIGS; Cataract surgery
Introduction
Glaucoma, a progressive optic neuropathy, is the second cause of blindness in the world, affecting 76 million people in 2020 and predicted to affect 111.8 million by 2040 [1]. The disease is caused by the death of Retinal Ganglion Cells (RGCs) and the degeneration of their axons that convey visual stimulus to the brain, leading to irreversible loss of the visual field [2].
The only modifiable risk factor for the progression of glaucoma that we know to this day is the Intraocular Pressure (IOP) [3].
The Minimally Invasive Glaucoma Surgery (MIGS) using ab interno trabecular microbypass stents have been developed to optimize the conventional outflow of aqueous humour through the trabecular meshwork. The smaller, second generation iStent Inject (Glaukos) consists of two deployable, heparin-coated titanium stents that are released at two to three clock hours apart in the trabecular meshwork [4].
The increased circumferential spread from this new mechanism of action may allow for better IOP control than its first-generation counterpart at short term [5-7]. It was approved by Health Canada in 2015 as a standalone procedure or in combination with cataract surgery for patients diagnosed with primary open-angle glaucoma, pseudoexfoliative glaucoma or pigmentary glaucoma [5-11]. They are an advantageous option to treat glaucoma as they potentially lessen the burden of anti-glaucomatous medication instillation and avoid surgical complications from more invasive filtering or tube procedures [12,13].
Several studies have already proven the efficacy of the iStent Inject in lowering IOP and the number of drops in patients with mild to moderate glaucoma in a variety of glaucoma subtypes [10,11,14-17]. Although few studies have examined the long-term outcomes of the first generation iStent past 5 years [18-20], there is currently no long-term studies of the outcomes of second generation iStent implanted in patients with different subtypes of glaucoma. This present study offers a 3-year assessment of the efficacy and safety of the iStent Inject in conjunction with phacoemulsification in a large cohort of glaucoma patients.
Methods
Study Design
This study is a prospective, non-randomized, single-center consecutive case series. The objective is to assess the long-term outcomes of efficacy and safety in glaucoma patients receiving the iStent Inject with simultaneous phacoemulsification. This study obtained ethics approvals from Institutional Review Board of Centre Hospitalier de l’Université de Montréal (CHUM).
The main outcome measure is the efficacy of the iStent Inject, which will be based on the short-term and long-term IOP reduction and number of anti-glaucomatous drops used postoperatively. A primary endpoint we will monitor is the achievement of =20% IOP reduction from baseline at each follow-up. The secondary outcome is the safety of the iStent Inject, which comprises preoperative and postoperative visual acuity change, visual field parameters, average retinal nerve fiber layer thickness, and cup-to-disc ratio.
Inclusion and Exclusion Criteria
We aimed to recruit a total of a hundred patients, followed to at least 36 months post-operatively. Patients were recruited at the ophthalmology department of the Centre Hospitalier de l’Université de Montréal by three glaucoma specialists (H.S., A.D., F.L.).
We included all adult patients over 18 years of age with ocular hypertension and open-angle glaucoma (including primary, pseudoexfoliative or pigmentary) who underwent combined cataract surgery with iStent Inject from January 2016 to January 2019. Patients were either intolerant to topical medications, had inadequate IOP on topical medication only or post Selective Laser Trabeculoplasty (SLT) and had IOP less or equal to 25 mmHg.
We excluded patients with angle closure, as well as those diagnosed with other types of secondary glaucoma (uveitic, neovascular, pupillary block, congenital, post-traumatic angle-recession), those with uncontrolled IOP defined as over 25 mmHg and those with previous glaucoma surgeries within the past 3 years. Patients with corneal opacification with difficult view of the iridocorneal angle and acute or chronic intraocular inflammation were also excluded for this study.
Baseline and Follow-up Examinationss
Written informed consent for the combined procedure as well as for the study were obtained for all patients.
A complete ophthalmologic evaluation was done preoperatively and included Corrected Distance Visual Acuity (CDVA), complete slit-lamp biomicroscopy examination with gonioscopy, IOP measurement using the Goldmann applanation tonometer, central corneal thickness, and dilated fundus examination with assessment of the Cup-to-Disk Ratio (CDR) of the optic nerve are obtained. Visual field imaging is performed using the 24-2 Swedish interactive threshold algorithm (SITA 24-2. Humphrey, Carl Zeiss Meditec, Jena, Germany), and a bilateral optical coherence tomography with retinal nerve fiber layer thickness measurement (Cirrus HD-OCT, Carl Zeiss Meditec, Jena, Germany). There will be no washout period, as to avoid progression in patients with especially an advanced disease [9,21]. Glaucoma severity was graded based on the Canadian Ophthalmology Society clinical practice guidelines on the management of glaucoma in adults [22].
iStent Inject Device and Surgical Procedure
The surgery took place within ninety days following the screening appointment. The surgery was done under local anesthesia. A clear corneal incision was done temporally to easily access the nasal iridocorneal angle. The trabecular micro-bypass stents were implanted before phacoemulsification.
The injector G2-M-IS is preloaded with two iStent Inject® [4]. After proper patient positioning and microscope adjustment, the 23-gauge stainless steel tube connected to the injector was inserted through the temporal clear corneal incision. Under direct visualization with a direct gonioprism, the first stent is released through the nasal trabecular meshwork into the Schlemm’s canal when the surgeon pushes on the release button for the first time.
The surgeon repeats the same steps to deploy the second stent, which will be positioned two or three clock hours away from the first one [4]. The iStent Inject® GTS400 is a surgical-grade non-ferromagnetic gamma-sterilized titanium stent coated with heparin.
This one-piece stent measuring 0.3x0.4 mm features four small lateral lumens that will let the aqueous humor flows from the anterior chamber to the Schlemm’s canal to increase the natural aqueous outflow [4].
Post-Operative Follow-Up
The patients are given an antibiotic drop (moxifloxacin or equivalent) for seven days postoperatively, a corticosteroid drop and a non-steroidal anti-inflammatory drop for at least one month after the surgery (prednisolone acetate 1% or equivalent). There was no washout period to measure the IOP without anti-glaucomatous drops after the surgical procedure. The topical medication is maintained until the target IOP is achieved and then the drops are progressively reduced.
The patients are seen by their attending ophthalmologist one day, one week, one month, three months, six months, nine months and one year after the surgery. They are seen more often if any complication occurs. Thereafter, patients are seen again every 6 months to a year as per the usual glaucoma follow-up regimen. Every visit following the combined procedure, the patients underwent a complete ophthalmic evaluation including: CDVA, IOP measurement, gonioscopy, anterior segment and optic nerve assessment of the cup-to-disk ratio on non-dilated slit-lamp examination. At one-year postoperative, two additional tests are performed: standard automated perimetry using the 24-2 Swedish Interactive Threshold Algorithm (SITA 24-2) and Optical Coherence Tomography (OCT) with Retinal Nerve Fiber Layer (RNFL) thickness measurement. Imaging parameters are analyzed at 12 months, 18 months, 24 months, and 36 months post-op as the changes seen are expected to be more gradual. CDR is assessed at 6 months, 12 months, 18 months, 24 months, and 36 months post-op as the changes are also expected to be of slower onset.
Data Analysis
Statistical analysis was performed using the SPSS software (IBM Corp. Released 2020. IBM SPSS Statistics for macOS, Version 27.0. Armonk, NY: IBM Corp). Paired 2-tailed Student T-tests was used to compare changes in IOP, number of medications and CDVA between each time point. Because the paired T-tests were done in individual pairs, there was no need for a Bonferroni correction for the multiplicity of comparisons. Snellen visual acuities will be converted to logarithm of the minimum angle of resolution (logMAR) values for statistical analysis. A level of p=0.05 will be considered statistically significant.
Results
Baseline Characteristics
In total, 97 patients (150 eyes) were included in the study. Detailed demographic characteristics of the cohort are exhibited in table 1. Patients were on average 72 years old, (range 54-90). Most patients were diagnosed with Primary Open-Angle Glaucoma (POAG) with 68 patients (70%), followed by Pigment Dispersion Glaucoma (PDG) with 10 patients (10%) and Ocular Hypertension (OHT) with 10 patients (10.3%). Over half of the patients had mild disease severity, while 25% had moderate and 23% had advanced. On average, patients were on 2.6 classes of drops pre-operatively (standard deviation of 0.67 drops). The mean pre-op IOP was 17 mmHg (standard deviation of 3.8mmHg). Approximately 20% of patients received prior laser therapy (selective laser trabeculoplasty or argon laser trabeculoplasty). Two eyes (1.3%) had received micropulse cyclophotocoagulation prior to the intervention. Two eyes (1.3%) had prior vitrectomies prior to the iStent Inject implantation. One eye had received a Penetrating Radial Keratoplasty (PRK) before the combined cataract and five had prior refractive surgery.
N patients
97
N eyes
150
Male
59 (60.83%)
Female
38 (39.18%)
Age (mean, range)
72 (54-90)
Glaucoma Type
POAG: 68
OHT: 10
PDG: 10
PXG: 3
NTG: 6Glaucoma severity (n, %)
Mild + HTO: 78 (52.00%)
Moderate: 37 (24.67%)
Severe: 35 (23.33%)Number of glaucoma medication classes (mean, std. dev)
2.64 (0.67)
Average Pre-op IOP (mean, std. dev.)
16.90 (3.77)
Past ocular history
SLT: 28 (18.67%)
ALT: 2 (1.33%)
MP-TSCPC: 2 (1.33%)
Vitrectomy: 2 (1.33%)
Refractive surgery (LASIK or PRK): 7 (4.67%)POAG: Primary Open Angle Glaucoma; OHT: Ocular Hypertension; PDG: Pigmentary Dispersion Glaucoma; PXG: Pseudoexfoliation Glaucoma; NTG: Normal-Tension Glaucoma; SLT: Selective Laser Trabeculoplasty; ALT: Argon Laser Trabeculoplasty; MP-TSCPC: Micropulse Trans-Scleral Cyclophotocoagulation; LASIK: Laser-Assisted in Situ Keratomileusis; PRK: Photorefractive Keratectomy; std. dev.: Standard Deviation.
Table 1: Demographics of included patients.
Primary Outcomes
Summary of the primary outcomes regarding IOP reduction and number of glaucoma drops for all glaucoma groups throughout the three-year follow-up is displayed in Table 2. The progression of IOP and number of glaucoma drops across follow-up timepoints are illustrated in figure 1. Intraocular pressure decreased significantly at all timepoints starting from 1-month post-operative and remained significantly lower than baseline at post-operative month 36 (Figure 1B). There was an average decrease of 1.4 mmHg, with the greatest decrease in IOP at post-op 3 months (1.8 mmHg, p<0.001). There is a decrease in medicated IOP of 10.3%, 10.2% and 8.6% at 1-year, 2-year, and 3-year follow-up. The percentage of patients with high IOP (>20 mmHg) remained under 11% until 36 months following surgery compared to 13% in pre-op.
Follow-up
N eyes VA
VA logMAR (mean, stdev)
Mean difference (p-value)
IOP (mean, stdev)
Mean difference (p-value)
Number of glaucoma classes (mean, stdev)
Mean difference (p-value)
Preop
150
0.33 (0.37)
-
16.98 (3.89)
-
2.54 (1.21)
-
POW1
97
0.16 (0.25)
-0.11 (<0.001)*
18.20 (5.62)
0.76 (0.20)
2.49 (1.20)
0.06 (0.36)
POM1
109
0.17 (0.32)
-0.13 (<0.001)*
16.15 (4.77)
-0.96 (0.07)
2.50 (1.23)
-0.04 (0.64)
POM3
93
0.16 (0.33)
-0.15 (<0.001)*
15.24 (4.25)
-1.83 (<0.001)*
2.45 (1.25)
-0.09 (0.34)
POM6
70
0.21 (0.42)
-0.13 (0.006)*
14.85 (3.71)
-1.57 (<0.001)*
2.44 (1.27)
-0.10 (0.37)
POM12
88
0.16 (0.30)
-0.15 (<0.001)*
15.36 (4.33)
-1.58 (0.005)*
2.26 (1.35)
-0.53 (0.009)*
POM18
97
0.16 (0.37)
-0.13 (<0.001)*
15.30 (3.67)
-1.40 (0.002)*
2.44 (1.15)
-0.02 (0.89)
POM24
70
0.21 (0.47)
-0.10 (0.008)*
15.25 (3.82)
-1.74 (<0.001)*
2.45 (1.30)
0.05 (0.64)
POM36
59
0.32 (0.71)
-0.04 (0.66)
15.51 (3.80)
-1.67 (0.003)*
2.76 (1.21)
0.04 (0.74)
Avg Mean Difference
-
-0.11
-
-1.42
-
-0.08
Paired sample T-tests for Visual Acuity (VA), Intraocular Pressure (IOP), Cup-to-Disc Ratio (CDR) and glaucoma drops. IOP is statistically significant after the immediate post-operative period (3 months) until 36-month follow-up. Visual acuity improves initially due to the removal of cataract and returns to pre-operative levels. IOP and number of glaucoma medications are lower post-operatively with values trending up after 12 months, although IOP is still maintained at lower than pre-op values at 36 months.
Table 2: Pre-op and post-op values of visual field index (2A), mean deviation (2B), pattern standard deviation (2C) and retinal nerve fiber layer thickness (2D) for all glaucoma groups. Overall, there was no statistically significant change in visual field parameters or retinal nerve fiber layer thickness at 3 years. VFI: visual field index; MD: mean deviation; PSD: pattern standard deviation; RNFL: retinal nerve fiber layer.
A third of patients demonstrated a 20% reduction in IOP at 1 month. At post-operative month 36, 25% of patients still maintain this significant decrease in IOP (Figure 3. The all-time average percentage of eyes with over 20% IOP decrease is 34%. At one year, over 60% of patients maintain an IOP of 15 mmHg or less (vs 36% at pre-op). At 36 months, 45% of patients still maintain a well-controlled IOP of 15 mmHg or less. The percentage of patients with IOP over 20mmHg remains low at under 11% throughout all time-points starting month 1 (Figure 3).
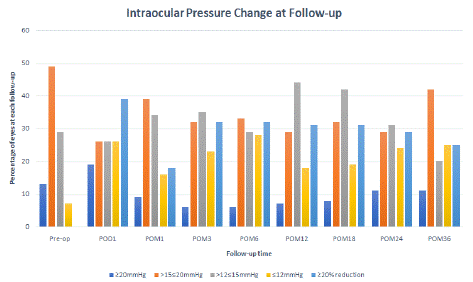
Figure 3: Intraocular pressure (IOP) groups at each follow-up. The percentage of patients with IOP higher than 20mmHg remains controlled at under 20% at long term. The percentage of patients with over 20% reduction in IOP after the immediate post-operative period (<1 month) is stably maintained at around 32%, and wanes slightly at month 36. Over half of the eyes have an IOP of or below 15 mmHg (including =12mmHg).
As seen in Table 2 and Figure 1, the mean number of glaucoma drops were significantly decreased on the first post-operative year compared to pre-op (-0.53 drops or 18.9%, p<0.009) . Although non-significant, there is an increasing trend for necessitating more glaucoma drops thereafter (Figure 1C). Overall, 20% of patients used less drops at follow-up. Compared to prior to surgery, the time point with the most patients using less drops was 12 months post-op (34%).
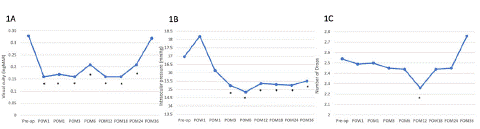
Figure 1: Visual acuity (1A), intraocular pressure (1B) and number of glaucoma drops (1C) after micro-stent insertion at post-operative follow-up of 36 months. Visual acuity improved at most time points after combined cataract and iStent Inject surgery. Intraocular pressure decreased significantly at nearly all timepoints and remained significantly lower than baseline at post-op month 36. The greatest decrease in IOP being at post-op 3 months (1.83 mmHg p<0.001). The number of glaucoma drops decreased significantly at post-op 12 months only, and saw an increasing trend at 3 years post-op.
A subanalysis separating mild, moderate and advanced glaucoma was performed (Supplemental data, table S1). Percentage of IOP reduction was higher in the ocular hypertension and mild glaucoma group compared to moderate and advanced glaucoma groups.
The percentage of eyes with over 20% IOP reduction from baseline was also higher in the milder groups than the entire cohort with on average 34% of eyes achieving these primary endpoint vs 28% from the entire cohort. The overall trend was similar in both analyses, showing statistically significantly reduced IOP throughout follow-ups for the mild groups. Although not significant at all time points, the number of glaucoma drops is still decreased at all follow-ups.
Secondary Outcome Measures
Secondary outcome measures of glaucoma progression include visual acuity (Figure 1), visual field parameters and retinal fiber layer thickness (Table 3 & Figure 2). Supplemental table 2 show the subgroup analyses of these parameters for mild, moderate and advanced glaucoma, respectively. Overall, the Best Corrected Visual Acuity (BCVA) remained significantly improved compared to pre-op values at all time-points after combined iStent Inject and cataract surgery for up to 0.15 logMAR until 2 years post-op. VA returns to pre-op levels, albeit non-significantly.
Follow-up time
N eyes VFI
VFI (Avg, stdev)
Mean Difference (p-value)
N eyes MD
MD (Avg, stdev)
Mean Difference (p-value)
N eyes PSD
PSD (Avg, stdev)
Mean Difference (p-value)
N eyes RNFL
Avg RNFL, stdev
Mean Difference (p-value)
Preop
150
87.29 (20.72)
-
-6.59 (7.81)
-
4.75 (4.01)
-
71.29 (14.27)
-
POM12
73
87.45 (18.12)
-1.05 (0.09)
83
-5.88 (7.60)
0.21 (0.50)
82
4.95 (4.04)
0.07 (0.71)
68
74.91(14.04)
3.76 (0.004)*
POM18
47
85.23 (20.10)
-0.45 (0.50)
59
-6.02 (7.23)
0.58 (0.18)
59
5.94 (4.40)
0.34 (0.23)
48
73.02 (11.41)
2.31 (0.002)*
POM24
48
90.02 (15.21)
-1.27 (0.20)
61
-6.36 (8.86)
0.36 (0.47)
61
5.31 (4.10)
0.06 (0.84)
57
71.65 (13.42)
-0.35 (0.77)
POM36
38
90.71 (16.03)
-1.58 (0.13)
47
-4.45 (7.17)
0.68 (0.25)
47
5.28 (4.55)
0.54 (0.11)
41
73.10 (11.99)
-0.83 (0.69)
Average mean difference
-
-
-1.09
-
0.46
-
0.25
-
1.22
VFI: Visual Field Index; MD: Mean deviation; PSD: Pattern Stand Deviation; RNFL: Retinal Nerve Fiber Layer.
There is no statistically significant change in visual field parameters at all follow-ups. There is a statistically improved average RNFL thickness at 12 and 18 months, probably due to improved signal strength after cataract removal.
Table 3: Imaging values (Optical Computed Tomography and Visual Field) at follow-up.
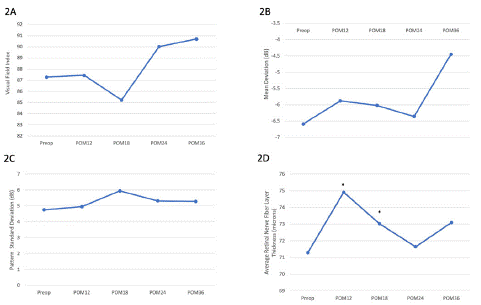
Figure 2: Pre-op and post-op values of visual field index (2A), mean deviation (2B), pattern standard deviation (2C) and retinal nerve fiber layer thickness (2D) for all glaucoma groups. Overall, there was no statistically significant change in visual field parameters or retinal nerve fiber layer thickness at 3 years. VFI: visual field index; MD: mean deviation; PSD: pattern standard deviation; RNFL: retinal nerve fiber layer.
Visual Field Index (VFI), Mean Deviation (MD) and pattern standard deviation remained stable compared to pre-op levels. Average retinal nerve fiber layer thickness significantly improved at post-operative 12- and 18 months, likely in part due to improved signal strength after cataract surgery. Similarly, separate analyses or mild, moderate and advanced group showed relative stability of the visual field and OCT parameters (Supplemental Table 2).
The incidence rate of short-term and long-term complications after combined iStent Inject and cataract surgery in our cohort was low (1 in 150 eye-years at 12 months and 1 in 450 eye-years at 36 months, respectively, Table 4). Complications were rare in the post-operative period and were quickly resolved with appropriate treatment. Two patients required vitrectomy with lens exchange within the first week due to posterior capsule rupture during the cataract surgery. Overall, the rate of additional glaucoma procedures were low during follow-up (6%) Further, three eyes required a placement of an Ahmed tube on average 19 months post-op. Four eyes required a XEN implantation at post-operative year 1 and one eye had received continuous-wave cyclophotocoagulation 3 months post-p. Finally, another eye had a Gonioscopy-Assisted Transluminal Trabeculotomy (GATT) surgery at 2 years follow-up (Table 4).
12 months
13-36 months
Total n of complications/total n of visits (cumulative incidence)
1 in 150 eye-years
1 in 450 eye-years
Elevated pressure (medical therapy or anterior chamber tap) (n)
24
13
Rebound uveitis (n)
6
0
CME (n)
2
0
Additional surgeries/CPC (n)
XEN (4)
CW-CPC (1)
Ahmed tube (1)Ahmed tube (2)
GATT (1)PCO (n)
3
4
Lens subluxation/PPV (n)
2
0
Other (n)
Blowout (1)
ERM (1)Anterior Capsular Fibrosis (1)
CW-CPC: Continuous Wave Cyclophotocoagulation; GATT: Gonioscopy-Assisted Transluminal Trabeculotomy; CME: Cystoid Macular Edema; PCO: Posterior Capsule Opacification; PPV: Pars Plana Vitrectomy; ERM: Epiretinal Membrane.
Table 4: Short-Term (=12 months) and Long-Term (12 months to 36 months) Post-Operative Complications.
Discussion
The second generation iStent Inject is a relatively new procedure that has since been widely used as a stand-alone procedure or in conjunction with cataract surgery for its minimally invasive advantages and low risk profile. Both generations of the iStent spare important ocular tissues that allow for the success of future filtering or tube surgeries23. First-generation combined iStent and cataract surgery studies have demonstrated a mild, but significant mean IOP decrease ranging from 7 to 27%, and reduction in medication burden with glaucoma drop decrease of up to 50% [14,18,24]. The implantation of two first-generation iStents along with cataract surgery reduces IOP by 14% at 1 year [25], and achieves an even higher decrease at 5-year follow-up with a 40% reduction [25,26]. This rate is significantly higher with the addition of a prostaglandin, with an over 20% IOP reduction from baseline in 63 to 100% of patients [24,27,28]. Studies with two or even three first-generation iStents implanted did not show a significant difference in IOP control without medication at 1 year, but better medication-free IOP in those with two or more iStent compared to one iStent at long-term (48 months) [29].
Since the availability of the smaller, more easily deployable iStent Inject, several studies have examined their short-term efficacy and safety profile and show promising results in the first year of its implantation combined with cataract surgery. Ex-vivo studies of the iStent demonstrate a negligible resistance of the outflow of the first and second-generation micro-bypass stents that allows for significant pressure decrease [30]. In fact, a single iStent resulted in a pressure reduction of 6.0±2.2 mmHg, and the addition of a second stent lowered the pressure by another 2.9±1.1 mmHg. The implantation of the two iStents at 60 to 90 degree apart recruit up to 5 to 6 clock hours of arcs of flow, and improves aqueous drainage by accessing more collector channels [31]. The stents, with their shorter inlet length, were technically more efficient to deploy and allows for a significantly dilated Schlemm canal that still maintains its optimal major diameter at post-operative 12 months [32].
Salimi et al. reported an 18% decrease in IOP and a 56% reduction in glaucoma drops in a cohort of 118 eyes with mild, moderate or advanced open-angle glaucoma at one year [10]. Samuelson and colleagues also demonstrated a favourable efficacy and safety profile for mild to moderate open-angle glaucoma at two years, with 75.8% of eyes with combined iStents and cataract surgery and 61.9% of the cataract surgery only group achieving a clinically significant reduction of medication-free IOP of =20% from baseline [11]. Comparative studies also demonstrate higher IOP reduction in the second-generation than the first generation iStent in the immediate post-operative period [5]. A similar multi-surgeon study of a variety of glaucoma types of including mild to advanced glaucoma showed promising results in terms of IOP response at 1 and 2 year post-op (23% and 16%, respectively) [8,9]. This current study provides an extended observation period of the combined procedure in mild, moderate and advanced glaucoma with follow-up up to 36 months post-op. The findings in our cohort echo previous studies in its efficacy and safety in different stages of glaucoma, at an intermediate to long-term follow-up, albeit showing a decrease in efficacy with more glaucoma drops being added at 36 months, as is expected with the progression of glaucoma. We report a lower percentage of IOP reduction in our cohort but demonstrate a relatively stable reduction in IOP from baseline throughout follow-ups. The reduction in IOP is higher in the mild and moderate glaucoma groups, corroborating previous literature demonstrating a more conservative IOP reduction in patients with advanced glaucoma [20,21,29].
Our cohort also reported a more moderate decrease in hypotensive drops than previous literature but offers a longer follow-up at three years post-op [8-11,17]. This might in part be explained by different practice patterns of surgeons who have a preference with tapering glaucoma drops at different time points. The one-year visit is commonly viewed as a notable post-operative follow-up where imaging tests are performed more consistently. Therefore, there might be an inclination in tapering medication more proactively at this follow-up based on the reassurance of tests, leading to a significant and more realistic reduction in drops. Also, we did not perform a pre-operative medication washout period, which could explain a less significant reduction in hypotensive drops such as reported in Samuelson’s study [11]. The fluctuation in terms of VFI and MD, as well as improvement in average RNFL thickness were probably secondary to an improved signal strength after cataract surgery and development of posterior capsular opacification at later follow-ups.
There are a few limitations to our study. First, the lack of a control arm limited the comparison between the effect of the iStent Inject itself versus that of cataract surgery alone. Studies on the impact of cataract surgery alone on IOP ranged from 9 to 20% of reduction in IOP, but found that the effect was short-lived, and seemed to wane after 24 months [33-35]. In our study, the percentage of IOP reduction was maintained at 36 months, which suggests the additive efficacy of the iStent Inject to that of cataract surgery alone.
Second, as previously mentioned, there was no pre-operative washout period of the hypotensive medication, which could decrease the reported IOP reduction and the reduction in glaucoma drops. The study being conducted by multiple surgeons entails different practice patterns that may affect the placement of the stents, the rate with which glaucoma drops are tapered, the follow-up period and the availability of data.
The fluctuation of visual field parameters and visual acuity parameters is also difficult to control due to multiple factors contributing to the values recorded at each timepoint, such as reliability, presence of dry eyes, posterior capsular opacification, and signal strength. Lastly, several patients were lost to follow-up or were discharged from our service back to their responding ophthalmologist or optometrist with adequate control of their glaucoma post-operatively, leading to a potential bias of more patients with inadequate IOP being followed for longer periods. The onset of a global pandemic during our study period also limited the availability for follow-up of many patients, leading to a smaller cohort at 36 months post-operatively. The decreasing follow-up rates in the setting of a public health crisis could also explain the less significant reduction in glaucoma drops previously described in the literature, as patients may not be weaned off drops for lack of regular follow-up.
Conclusion
In our study, we demonstrated the intermediate-to-long-term efficacy and safety of the combined iStent Inject and cataract surgery in patients with different types of mild, moderate and advanced glaucoma. IOP reduction is more conservative than previous literature with shorter observation periods, but remains controlled at 3 years, and is more prominent in those with ocular hypertension and mild glaucoma.
The number of hypotensive drops generally remain stable or lower, with more medication needed at year 3 to maintain the same percentage of IOP reduction, as is expected with the progression of glaucoma. The safety profile is very reassuring and congruent with prior literature as well. Due to moderate efficacy of the iStent Inject in advanced glaucoma, a cost-effective analysis of the surgery in this subgroup of patients would provide valuable insight in the decision of the appropriate management plan. Therefore, the combined iStent Inject and cataract surgery could be considered a treatment of choice in those with mild to moderate glaucoma unresponsive to topical medication and laser, and a reasonable option for those with advanced glaucoma.
Author Statements
Funding
The authors have no funding to declare.
Conflict Interest
The authors have no financial interests to declare.
References
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040 A systematic review and meta-analysis. Ophthalmology. 2014; 121: 2081-90.
- Agostinone J, Di Polo A. Retinal ganglion cell dendrite pathology and synapse loss: implications for glaucoma. Prog Brain Res. 2015; 220: 199-216.
- Canadian ophthalmology society. Canadian Ophthalmological Society evidence-based clinical practice guidelines for the management of glaucoma in the adult eye. 2009; 44: s7-93.
- Guedes RAP, Gravino DM, Lake JC, Guedes VMP, Chaoubah A. Intermediate Results of iStent or iStent inject Implantation Combined with Cataract Surgery in a Real-World Setting : A Longitudinal Retrospective Study. Ophthalmol Ther. 2019; 8: 87-100.
- Shalaby WS, Lam SS, Arbabi A, Myers JS, Moster MR. iStent versus iStent inject implantation combined with phacoemulsification in open angle glaucoma. Indian J Ophthalmol. 2021; 69: 2488-2495.
- Manning D. Real-world Case Series of iStent or iStent inject Trabecular Micro-Bypass Stents Combined with Cataract Surgery. Ophthalmol Ther. 2019; 8: 549-61.
- Clement CI, Howes F, Ioannidis AS, Shiu M, Manning D. One-year outcomes following implantation of stents in conjunction with cataract surgery for various types of glaucoma or ocular hypertension: multicenter, multi-surgeon study. Clin Ophthalmol. 2019; 13: 491-9.
- Clement C, Howes F, Ioannidis AS, Shiu M, Manning D, Lusthaus J, et al. Two-year Multicenter Outcomes of iStent inject Trabecular Micro-Bypass Stents Combined with phacoemulsification in Various Types of Glaucoma and Ocular hypertension. Clin Ophthalmol. 2020; 14: 3507-17.
- Salimi A, Lapointe J, Harasymowycz P. One-year outcomes of second-generation trabecular micro-bypass stents (iStent inject) implantation with cataract surgery in different glaucoma subtypes and severities. Ophthalmol Ther. 2019; 8: 563-75.
- Samuelson TW, Sarkisian SR, Lubeck DM, Stiles MC, Duh YJ, Romo EA, et al. Prospective, randomized, controlled pivotal trial of an ab interno implanted trabecular micro-bypass in primary open-angle glaucoma and cataract two-year results. Ophthalmology. 2019; 126: 811-21.
- Robin A, Grover DS. Compliance and adherence in glaucoma management. Indian J Ophthalmol. 2011; 59: S93-6.
- Zahid S, Musch DC, Niziol LM, Lichter PR, Collaborative Initial Glaucoma Treatment Study Group. Risk of endophthalmitis and other long-term complications of trabeculectomy in the collaborative initial glaucoma treatment study (CIGTS). Am J Ophthalmol. 2013; 155: 674-680.e1.
- Arriola-Villalobos P, Martínez-de-la-Casa JM, Díaz-Valle D, Fernández-Pérez C, García-Sánchez J, García-Feijoó J. Combined iStent trabecular micro-bypass stent implantation and phacoemulsification for coexistent open-angle glaucoma and cataract: a long-term study. Br J Ophthalmol. 2012; 96: 645-9.
- Voskanyan L, García-Feijoó J, Belda JI, Fea A, Jünemann A, Baudouin C, et al. Prospective, unmasked evaluation of the iStent inject system for open-angle glaucoma: synergy trial. Adv Ther. 2014; 31: 189-201.
- Donnenfeld ED, Solomon KD, Voskanyan L, Chang DF, Samuelson TW, Ahmed II, et al. A prospective 3-year follow-up trial of implantation of two trabecular microbypass stents in open-angle glaucoma. Clin Ophthalmol. 2015; 9: 2057-65.
- Neuhann R, Neuhann T. Second-generation trabecular micro-bypass stent implantation: retrospective analysis after 12- and 24-month follow-up. Eye Vis (Lond). 2020; 7: 1.
- Ferguson TJ, Mechels KB, Dockter Z, Bleeker A, Ibach M, Schweitzer J, et al. iStent Trabecular Microbypass Stent Implantation with Phacoemulsi fication in Patients with Open-Angle Glaucoma: 6-year Outcomes. Clin Ophthalmol. 2020; 14: 1859-66.
- Ziaei H, Au L. Manchester iStent study: long-term 7-year outcomes. Eye (Lond). 2021; 35: 2277-82.
- Salimi A, Watt H, Harasymowycz P. Long-term outcomes of two first-generation trabecular micro-bypass stents (iStent) with phacoemulsification in primary open-angle glaucoma: eight-year results. Eye Vis (Lond). 2021; 8: 43.
- Ansari E. 5-year outcomes of single iStent (G1) trabecular microbypass implantation with phacoemulsification in moderately advanced primary open angle glaucoma. PLOS ONE. 2021; 16: e0257015.
- Canadian Ophthalmological Society Glaucoma Clinical Practice Guideline Expert Committee. Canadian Ophthalmological Society evidence-based clinical practice guidelines for the management of glaucoma in the adult eye. Can J Ophthalmol. 2009; 4: S7-93.
- Saheb H, Ike II, Ahmed K. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol. 2012; 23: 96-104.
- Ahmed IIK, Katz LJ, Chang DF, Donnenfeld ED, Solomon KD, Voskanyan L, et al. Prospective evaluation of microinvasive glaucoma surgery with trabecular microbypass stents and prostaglandin in open-angle glaucoma. J Cataract Refract Surg. 2014; 40: 1295-300.
- Buffet J, Brasnu E, Baudouin C, Labbé A. Efficacy of 2 trabecular micro-bypass stents during phacoemulsification for mild to advanced primary open-angle glaucoma controlled with topical Hypotensive Medications. J Glaucoma. 2017; 26: 1149-54.
- Saheb H, Donnenfeld ED, Solomon KD, Voskanyan L, Chang DF, Samuelson TW, et al. Five-year outcomes prospective study of two first-generation trabecular micro-bypass stents (iStent®) in open-angle glaucoma five-year outcomes prospective study of two first-generation trabecular micro- bypass stents (iStent®) in open-angle glaucom. Curr Eye Res. 2021; 46: 224-31.
- Chang DF, Donnenfeld ED, Katz LJ, Voskanyan L, Ahmed II, Samuelson TW, et al. Efficacy of two trabecular micro-bypass stents combined with topical travoprost in open-angle glaucoma not controlled on two preoperative medications: 3-year follow-up. Clin Ophthalmol. 2017; 11: 523-8.
- Berdahl J, Voskanyan L, Myers JS, Katz LJ, Samuelson TW. iStent inject trabecular micro-bypass stents with topical prostaglandin as standalone treatment for open-angle glaucoma: 4-year outcomes. Clin Exp Ophthalmol. 2020; 48: 767-74.
- Katz LJ, Erb C, Carceller Guillamet A, Fea AM, Voskanyan L, Giamporcaro JE, et al. Long-term titrated IOP control with one, two, or three trabecular micro-bypass stents in open- angle glaucoma subjects on topical hypotensive medication: 42-month outcomes. Clin Ophthalmol. 2018; 12: 255-62.
- Hunter KS, Fjield T, Heitzmann H, Shandas R, Kahook MY. Characterization of micro-invasive trabecular bypass stents by ex vivo perfusion and computational flow modeling. Clin Ophthalmol. 2014; 8: 499-506.
- Bahler CK, Hann CR, Fjield T, Haffner D, Heitzmann HAL, Fautsch MP. Second-generation Trabecular Meshwork Bypass Stent (iStent inject) Increases Outflow Facility in Cultured Human Anterior Segments. Am J Ophthalmol. 2012; 153: 1206-13.
- Gillmann K, Mansouri K, Ambresin A, Bravetti GE, Mermoud A. A Prospective Analysis of iStent inject® Microstent Implantation: surgical Outcomes, Endothelial Cell Density and Device Position at 12-month. J Glaucoma. 2020; 29: 639-47.
- Jimenez-Roman J, Lazcano-Gomez G, Martínez-Baez K, Turati M, Gulías-Cañizo R, Hernández-Zimbrón LF, et al. Effect of phacoemulsification on intraocular pressure in patients with primary open angle glaucoma and pseudoexfoliation glaucoma. Int J Ophthalmol. 2017; 10: 1374-8.
- Poley BJ, Lindstrom RL, Samuelson TW. Schulze RJMp. Intraocular pressure reduction after phacoemulsification with intraocular lens implantation in glaucomatous and nonglaucomatous eyes Evaluation of a causal relationship between the natural lens and open-angle glaucoma. JCRS. 2009; 35: 1946-55.
- Armstrong JJ, Wasiuta T, Kiatos E, Malvankar-Mehta M, Hutnik CML. The effects of phacoemulsification on intraocular pressure and topical medication use in patients with glaucoma: A systematic review and meta-analysis of 3-year data. J Glaucoma. 2017; 26: 511-22.