
Research Article
Austin J Obstet Gynecol. 2023; 10(2): 1218.
Prognostic Factors for Early-Stage Endometrial Cancer Management in Fertile Patients
Vasilios Tanos¹; Zoe Zervides²; Panayiotis Tanos³*
Professor Obstetrics and Gynaecology, University of Nicosia, Medical School and Aretaeio Hospital, Cyprus MBBS Student, University of Nicosia, Class of 2025, Cyprus Foundation Year Doctor, Antrim Area Hospital, Antrim, Northern Ireland
*Corresponding author: Panayiotis Tanos Foundation Year Doctor, Antrim Area Hospital, Antrim, Northern Ireland. Email: p.tanos@outlook.com
Received: May 23, 2023 Accepted: June 16, 2023 Published: June 23, 2023
Abstract
Introduction: Early-Stage Endometrial Cancer (ES-EC) presents a treatment challenge for women who would like to preserve their genital organs and complete their family planning. Delayed first pregnancy and aging of women in combination with high-definition sonography and cancer awareness is expected to increase the incidence of young women with ES-EC demanding Fertility Sparring Surgery (FSS).
Methods: A thorough literature search was conducted using Medline (OVID), Embase, and Cochrane Library electronic databases in order to identify eligible studies published up to November 2021.
Results: Age is an imperative determinator for FSS in ES-EC, since fertility potential is compromised with age and oocytes carrying higher risk of fragmented DNA. Favourable prognostic factors to FSS include, age younger than 35 years old, absent genetic predisposition to EC, fertile females with normospermic partners, negative histology for cancer cells after hysteroscopic biopsies and progesterone treatment, normal adnexal findings in Trans Vaginal Ultrasound (TVU), absent myometrial carcinomatous invasion in Magnetic Resonance Imaging (MRI) and absence of lympho-vascular involvement in Computerised Tomography Scan. Careful selection of patients based on favourable prognostic factors diminishes the risk of recurrence and allows for the opportunity of reproductive organs preservation until family planning is achieved in a reasonable time frame. Additionally, our review points out the need of hysteroscopic guidance in endometrial sampling for primary EC diagnosis as well as for the endometrial surveillance every 3-6 months follow-ups until pregnancy.
Conclusion: After FSS decision, a strict time frame of one year to achieve pregnancy is essential in order to secure patient’s safety and to reduce the risk of early recurrency in EC.For both endometrial and ovarian cancer, prevention Hysterectomy and Bilateral Salpingo-oophorectomy should preferably be performed by the completion of child bearing age and before the age of 40 years. It is vital that the advantages and risks of FSS must be discussed openly, including the risk of undetectable by imaging occult gynecological cancer which can compromise life expectancy.
Keywords: Endometrial adenocarcinoma; Early-stage endometrial cancer; Conservative treatment; Atypical endometrial hyperplasia; GnRH agonist; Fertility-sparing-surgery
Introduction
According to the World Health Organization, Endometrial Carcinoma (EC) is the most common gynaecological cancer in Europe, with a 5-year prevalence of 34.7% out of 445 805 cases [1]. The incidence of EC is approximately 15,000 newly diagnosed women each year, of which 1600 (10.7%) young women will die of the disease [2]. Four percent of EC occurs in women of reproductive age. In low-risk disease, total hysterectomy and bilateral salpingo-oophorectomy, provide patients up to 93% chance of cure [3]. However, temporal preservation of the uterus in Early-Stage Endometrial Cancer (ES-EC) for reproductive reasons raises primarily medical but also ethical and social dilemmas.
Usually, young age, nulliparity, previous pregnancy loss, infertility, early stage of the disease and the strong wish of the patient for motherhood, oblige the Gynaecologist to review and compromise the standard surgical management for ES-EC. The preservation of the uterus for an uncertain time, even at early stage of EC increases the risk of disease progression and has a bad prognosis for life expectancy. When a young woman with EC has no partner then the decision to preserve the uterus for an unknown time interval is becoming a complex health matter and a big challenge for the treating physicians.
Family planning and the decision to preserve or excise the uterus depends on personal but also societal and cultural beliefs and is of extraordinary importance. A young woman with EC who does not have children nor a uterus, is endangered to social and family isolation. Therefore, patient-specific characteristics are important when considering fertility sparring treatment. Every woman has unique social interests, beliefs, and preferences which should be considered in the shared decision-making process. However, decisions on fertility are equally difficult for both the patient and the treating gynaecologist and therefore, management should involve an experienced, expert, multidisciplinary team.
Preliminary evidence in disease progression and life expectancy in patients following temporal uterine preservation for ES-EC, are encouraging and appears to be in general an accepted management option. In a recent systematic review by Schuurman et al. 62.6% of patients with complete remission on hormonal therapy were reported to have a pregnancy wish. Among these patients with complete remission, 36.9% became pregnant [4].
Women with histologically well described type of EC (G1 or G2) and with stage 1 confined to the endometrium, are candidates for progestin therapy. Fertility Sparring Surgery (FSS) could also be considered as a valid option for reproductively aged patients with stage IA type I and G2 EC. Nevertheless, the long-term outcome, survival rate and quality of life in these patients is not yet prospectively investigated.
Four different groups of young patients seem to be involved in ES-EC diagnosis; a) Patients with family history, with 1st degree relatives or multiple 2nd and 3rd degree relatives with EC and/or colon cancer b) Patients with genetic predisposition and inherited risk after testing for Mismatched Repair (MMR) mutated genes c) Women with EC risk factors (PCOD, obese with BMI >30 and >40, endometrial thickness over 20mm) d) Women diagnosed during examination for abnormal uterine bleeding and EC has been reported in endometrial biopsy; Despite FSS availability and preference by many, the lack of evidence-based consensus and guidelines for selected patients, treatment methods and follow-up, complicates the decision-making process [5]. Identification of favourable prognostic factors indicating low risk patients with EC recurrency will select the best candidates with ES-EC for FSS [101-103].
The purpose of this review is to identify the clinical symptomatology, imaging indices, biological, chemical, genetic, and epidemiological factors that can support conservative management of patients with ES-EC (Stage IA Type I and G2); offering a chance for motherhood, prior to hysterectomy. Projected prognostic markers could assist in selecting patients with better prognosis and further guide to a strategy which can minimise the risk of recurrency. Furthermore, results can be used to improve pre-treatment counseling and management decisions for reproductive-age patients at ES-EC.
Methods
Search Strategy
A literature search was conducted using the electronic bibliographic databases Medline (OVID), Embase, and the Cochrane Library to identify eligible studies published in English language. We searched for keywords and equivalent words in the title/abstract and translated the search terms according to the standards of each database. Keywords for endometrial adenocarcinoma, Endometrial Neoplasms, Endometrial Carcinoma, Endometrial Hyperplasia, Conservative Treatment, Gonadotropin-Releasing Hormone, Fertility, Fertility Preservation, and Infertility were combined with terms for fertility-sparing treatments in general and surgery specifically. Reference lists of the included studies and retrieved review articles were searched to identify relevant articles not found in the initial search.
Study Selection
The articles retrieved during the searches were screened for relevance on title/abstract and subsequently full text by the two authors independently. Discrepancies were resolved by consensus after discussion and assessment by both authors. Included articles needed to specify oncological and/or reproductive outcomes after FSS, i.e., endometrial resection, endometrial sampling, absence of myometrial and lymphoglandular invasion, response to progesterone treatment and uterine preservation combined with hormonal therapy in early-stage endometrial cancer.
Data
Published articles on invasive endometrial cancer and absent FSS treatment were excluded from our analysis. The following studies were also excluded: (1) review articles without any new patient data, (2) case reports or small case series with less than 20 patients, (3) letters to editors, commentaries, or (conference) abstracts. Of the articles with duplicate patient information and articles updating prior published series, we included the articles with the most recent and complete data.
Results
The best evidence currently available on oncological and reproductive risk and prognosis after FSS for early-stage endometrial cancer are reported and summarized.
Prognostic Factors
Family History: Patients with first- or second-degree relatives diagnosed with EC and/or colon cancer below the age of 50 have increased cumulative risk of EC by 3.8% and 3% respectively [6,7]. Counselling of family members and investigation of familial pedigree and genetic predisposition is recommended. Furthermore, genetic analysis is offered in all high-risk women and surveillance for carriers of MSH2, MLH1 and MSH6 mutations has been recommended from age 30, 35 and 40 years respectively [8]. These women are counselled to be investigated with frequent annual colonoscopies and TVU scanning measuring endometrial thickness, adnexal morphology and Ca 125 and CEA serum levels [6-8].
Surveillance for EC in Human Non-Polyposis Colorectal Cancer (HNPCC) (Lynch syndrome) mutation carriers should start at the age of 35 years. However, individual factors need to be taken into consideration and patient tailored screening programs are encouraged to be followed. The decision on the starting age of surveillance should integrate knowledge on the specific mutation, history of family events as well as individual treatment and preferable preventative measures. Screening of the endometrium by annual transvaginal ultrasound as well as annual or biennial biopsy until hysterectomy should be considered in all MMR and HNPCC mutation carriers. Unfortunately, the risk of any occult malignancy during prophylactic surgery for women with HNPCC has been reported to be up to 17% [9]. Therefore, standardization of the order in which protein evaluation, genetic sampling and hysteroscopy are done is pertinent.
Patients with Genetic Predisposition: EC is characterized by various genetic alterations. The most frequent is located at chromosome 10q23 (PTEN gene alteration). PTEN loss is profound in both HNPCC and sporadic EC cases. PTEN gene behaves as a tumour suppressor gene and encodes for a lipid and a protein phosphatase, inducing cell cycle arrest at the G1/S checkpoint and inhibiting growth-factor-stimulated MAPK signaling and focal adhesion formation as well as cell spread and migration, respectively [10].
Approximately 3% of all EC and about 10% of Mismatch Repair Deficient (MMRd) or microsatellite unstable EC are related to germline mutations of one of the MMR genes; MLH1, PMS2, MSH2 and MSH6 [11]. The International Society of Gynecological Pathology recommended using MMR-Immunohistochemistry (IHC) testing for both MMR status and Microsatellite Instability (MSI) in all EC samples, irrespective of patients age [12]. Using IHC the expression of four MMR proteins MLH1, PMS2, MSH6, and MSH2 are assessed and in addition, PMS2 and MSH6 antibodies can be also assessed [13].
The inactivation of MMR genes and MMR protein dysfunction may be the results of germline mutations or spontaneous hypermutation alterations, which may induce MSI. The diagnostic sites of MSI include more than a hundred thousand areas of short tandem repetitive DNA sequences. As recommended by the National Cancer Institute, BAT25 and BAT26 mononucleotide repeats and D5S346, D2S123, and D17S250 dinucleotide repeats are the standard panel sites for MSI testing [14]. MSI-H or dMMR has been specifically detected in HNPCC-associated tumors, including EC [15].
Even though currently there is limited evidence on the benefits of HNPCC-associated EC screening, it requires specialized attention. HNPCC-associated EC has been linked to pre-invasive hyperplasia and particularly concurrent complex atypical hyperplasia. HNPCC-associated EC cases lack additional mutations, suggesting that in the mismatch repair defect context, few additional molecular changes lead from pre-invasive lesions to carcinoma. Therefore, EC patients identified as having an increased risk of HNPCC should be offered genetic counseling and surveillance [16]. For these patients, hysterectomy and bilateral salpingo-oophorectomy should be performed as a preventative measure for endometrial and ovarian cancer. This should preferably be before the age of 40 years, at the completion of childbearing age. Prior to this all advantages and disadvantages of prophylactic surgery must be discussed, including the risk of occult gynecological cancer detection at prophylactic surgery [17].
Lastly, Bokhman et.al. 1983 have characterised EC into 70-80% Estrogen-dependent ENDOMETRIOID EC (EECs) which are linked to unopposed estrogen stimulation in young postmenopausal women. The other 10-20%, are characterised as Non-Endometrioid EC (NEECs) and are associated with a history of atrophic endometrium in older postmenopausal women. NEECs patients usually present with a higher-grade EC and have less favourable outcomes [18-20]. EECs and NEECs are not only differentiated according to clinical and histopathological variables but also according to activation and inactivation of certain genes. EECs have K-ras, Her2/neu and b-Catenin gain-of function as well as microsatellite and PTEN loss-of function. Whereas P53 loss-of function is presented more on NEECs. However, the effect of the aforementioned genes and the benefits of this classification in premenopausal women is unclear [21].
Hence, in patients with evidenced genetic predisposition to EC, FSS is contraindicated.
Clinical Symptoms
Age of EC onset: Even though younger women may not be symptomatic as quickly as older women they tend to have better prognosis than post-menopausal women in EC. Younger women often appear to have lower grade tumors which do not grow deep into the myometrium and which are clinically detected at earlier stages. In contrast, older women often appear to present with more aggressive tumour types and disease advancement and as a result, have a less favorable prognosis.
In a sample of young patients with EC and a median age of 46 years old, Parc et al., 2000 verified 34% women with microsatellite instability. The microsatellite positive group showed an absence of hMLH1, hMLH2 expression for 57% and 19% respectively as well as 23.8% normal protein expression [22].
Obesity: The risk of EC is increased 5 times when BMI is over 30 and 20 times when BMI is over 40 as compared to general female population with normal BMI. Additionally, in metabolic syndrome when obesity is combined with diabetes and high blood pressure, has been linked with a less favourable prognosis [23]. Factors increasing the obstetrical risks might also be added and considered prior to FSS.
Diabetes Mellitus: A recent meta-analysis showed diabetic women at a 72% increased risk of EC compared to those without diabetes [24]. Currently the most advocated mechanisms are hyper-glycemia, -lipidemia, -insulinemia, disorders of leptin and adiponectin and abnormal fat metabolism. T-cells and macrophages attack these adipose cells and cause chronic inflammation, leading to an increase in Inflammatory cytokines (IL6, TNF-a/b), adipokines (visfatin, leptin) and inflammatory mediators C-reactive protein as well as protease inhibitor (plasminogen activator inhibitor-1) which can all lead to proliferation, invasion and even metastasis of the primary tumour. Most of these inflammatory mediators further increase aromatase which accelerates estrogen synthesis by inhibiting the synthesis of Sex Hormone Binding Globulins (SHBG) [25].
Blood Pressure: High blood pressure, increasing levels of diastolic blood pressure and in particular, systolic blood pressure as well as a history of hypertension have been associated with increased risk of EC in several studies, but the results have not been consistent. However, a meta-analysis of 19 case-control and 6 cohort studies suggested that women with hypertension may have a 61% increase in the relative risk of developing EC [26]. The associations remained positive, statistically significant and had heterogeneity in almost all subgroup analyses. Confounding factors adjusted for were smoking (p=0.02), BMI (p=0.003), the use of oral contraceptives (p=0.02), hormone replacement therapy (p=0.08), parity (p=0.03), and menopausal age (p=0.07) [27].
Polycystic Ovarian Disease: Patients with Polycystic Ovarian Syndrome (PCOS) who have period irregularities, unopposed estrogens and do not receive any treatment, are reported to have a 2.7-fold increased risk for developing EC. A major contributing factor for this increased risk of malignancy is prolonged exposure of the endometrium to unopposed estrogen that results from anovulation. Ding et al. 2018 reported a statistically significant increased risk of EC for women with PCOS, but no association risk between PCOS and ovarian or breast cancer. The incidence of EC was reported to be 226 in women with PCOS in comparison to 15 per 100,000 person-years in the control groups [28].
Other than unopposed estrogen exposure to the endometrium, there more molecular mechanisms linked to increase risk of EC in women with PCOS; insulin resistance as well as endometrial overexpression of insulin like growth factor-1, insulin like growth factor binding protein-1, PTEN genes, sterol regulatory binding protein-1 and lastly endometrial overexpression of adiponectin [29]. Nair et al. 2013 explain that derangements in adipocyte, lipid and fatty acid metabolism, increased EC risk, either through inflammation promotion or through fatty acids release from cancer-associated adipocytes which are used in cancer cells for intracellular energy production [30].
Shafiee et.al. 2020, demonstrated that although lipid compounds mechanisms have been linked to EC, plasma concentrations of LDL low density lipoproteins and high density lipoproteis HLD do not directly correlate to EC and therefore cannot currently be used as biomarkers for EC in PCOS. However, women with PCOS and monoacylglycerol 24:0 and capric acid metabolites showed comparable changes in tissue to women with ES-EC and lower BMI, which If validated and correlated with plasma results in future studies, it could be used as possible biomarkers for ES-EC in women with PCOS [31].
Imaging
Ultrasound scanning, Computed Tomography (CT), and Magnetic Resonance Imaging (MRI) are extensively used to rule out endometrial thickening, myometrial involvement and extrauterine disease and in order to eliminate the need of definitive surgery, enabling the option of FSS.
Transvaginal Ultrasound (TVU): TVU is considered the first line screening modality to follow up high risk women for ES-EC in peri- and post- menopausal women as well as women in reproductive age. The maximum Endometrial Thickness (ET) measured by TVU provides a reliable index of EC risk. More specifically, ET measurements over 15mm has a fivefold increase in EC risk. When ET is over 20mm, this risk increases by 20 times more than the risk in general population [32]. In premenopausal patients who undergo selective estrogen receptor modulators therapy when endometrial thickness <15mm endometrial hyperplasia is less likely to occur [33].
TVU with a "power" angio-Doppler technique can be a valuable diagnostic method in hyperplasia and cancer of the endometrium, and especially useful in the early stages of these pathologies. Szpurek et al. 2000 report irregular vascularity of the endometrium, in 12.2% and 81.2% in patients with hyperplasia and EC respectively [34]. However, Angio Doppler technique as a prognostic factor in EC has not been implemented in the daily practice for young women.
Magnetic Resonance Imaging (MRI): Contrast-enhanced MRI (fused T2- and diffusion-weighted) is the preferred modality in ruling out invasive cancer and myometrial involvement and has an accuracy of 88% [35,36]. Bathen et al., 2000 reported an accuracy of 83% in differentiating between cancer and normal samples by analysing lipid metabolic profiles using nuclear magnetic resonance [37]. Recent studies report that MRI has a sensitivity of 90% and specificity of 98% for the staging of early IB1 tumors. The addition of DWI and DCE imaging enables the detection of tumors smaller than 1cm [38,39]. Bourgioti et al. [40] described a highly accurate tumor origin prediction MRI scoring system that discriminates between EC and cervical cancer, with sensitivity up to 96.6% and specificity up to 100%. However, for the detection of metastatic lymph nodes, moderate sensitivity (43%) and specificity (73%) has been demonstrated [41].
Computed Tomography (CT): CT is the preferable modality in assessing the extrauterine encroachment of EC although CT sensitivity in the detection of adnexal involvement of EC has been reported to be only 60% [42, 43]. Hence, it has been argued that diagnostic laparoscopy is probably essential to be performed to rule out the presence of extrauterine disease before initiating fertility-sparing treatments [44].
Positron Emission Tomography-CT is mainly used for detecting the enlargement of retroperitoneal nodes suspicious for metastatic disease with 100% sensitivity and 94% specificity at assessing nodal disease [45]. Eighty-seven percent of tumor recurrence occurs within 3 years after surgery and it has a 46% chance of it recurring at regional lymph nodes [46].
Hysteroscopic Findings
Hysteroscopy has a determinant role in providing FSS for cases with ES-EC. Primarily hysteroscopy can diagnose the anatomical location, extension and depth of the carcinomatous lesion and consequently can provide together with sonography and MRI the appropriate management options and follow-up plan. Timing and frequency of these examinations is of pivotal importance and will determine the time interval for uterine preservation to achieve a pregnancy. The current hysteroscopy procedure as an outpatient setting provides extra comfort and safety to frequent endometrial examinations even every 3-4 months until the patient gets pregnant. The examination performed by an experienced hysteroscopist should include a structured surveillance and should be meticulous and informative. History and previous hysteroscopies text, images records and video recordings should be reviewed prior to every new hysteroscopic examination. The intrauterine pressure used at the initial stage of hysteroscopy should be kept low as possible because this a) reduces the risk of malignant cell spreading, b) decreases the risk of pain and discomfort of the patient, c) overdistention deforms the normal appearance of the endometrium since in physiological condition the anterior wall is lying over the posterior wall. In addition, the vaginoscopic approach using no speculum, tenaculum or sound provides painless hysteroscopy in an office setting.
The option to use a double flow hysteroscope with a working channel with biopsy forceps seems to be the most acceptable approach allowing multiple direct biopsies. Once a lesion is identified a wide excision biopsy should be followed, trying to include the whole lesion in surface and depth. In case atypical cells are reported by histopathology a revision of the anatomical location should be performed in the following hysteroscopy.
Currently cervical Dilatation and Curettage (D&C) usually follows hysteroscopy, providing an extra safety and reassurance of a healthy endometrium. However, the D&C is done mainly for medical legal reasons, and it is common knowledge that blind curettage as well as Pipelle-blind endometrial sampling have low sensitivity and are insufficient in ruling out EC in high-risk women [47]. Tissue Removal Systems (TRS) can provide an excellent view and fast endometrial curettage by adjusting the depth of endometrial sampling. Hence, TRSs might be proposed and encouraged for high-risk patients with EC after FSS. Lastly, directed wide excision biopsies provide additional safety as any suspected lesion can be accompanied by an adjacent biopsy to verify the clear margins of a cancerous sample [47].
In case of uncertain biopsy results and the need to confirm histopathological results, an additional hysteroscopy and lesion wide excision might be advised to reassure the depth, extend and grade of the endometrial carcinoma. It should also be noted that the staging of EC is by surgical approach and the standard treatment is total hysterectomy, bilateral salpingo-oophorectomy, pelvic washing, and/or lymphadenectomy as portrayed in Figure 1 [48]. Hence, the effort to demarcate and classify the ES-EC cannot replace the standard of surgical staging care but to support the temporal FSS to conception and delivery.
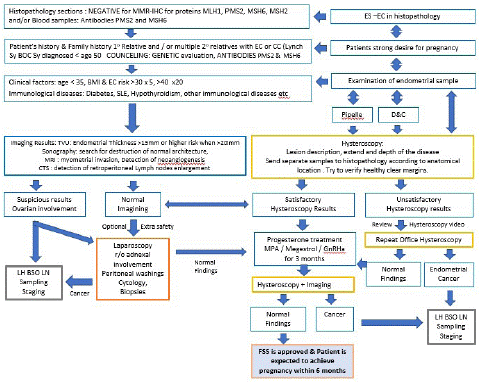
Figure 1: Early-Stage Endometrial Cancer Prognostic Factors influencing the management steps leading to Fertility Sparing Surgery.
INDEX: ES-EC Early-Stage Endometrial Cancer; EC: Endometrial Cancer; CC: Colorectal Cancer; BOC Sy: Breast Ovarian Cancer syndrome; SLE: Systematic Lupus Erythematosus; r/o: Ruling Out; LH: Laparoscopic Hysterectomy; BSO: Bilateral Salpingoophorectomy; LN: Lymph Nodes; TVU: Trans Vaginal Ultrasound; CTS: Computerized Tomography Scan
Laparoscopy
The occurrence of synchronous or metachronous endometrioid ovarian cancer in Stage I EC limited to the endometrium in up to 25% of cases [49,50].
In a retrospective study by Walsh et al. the incidence of synchronous ovarian cancer in women under 40 years old was reported to be 4.5% (21/471), which is much lower than what is reported elsewhere [51]. Additionally, Song et al. 2013 demonstrated that ES-EC with no additional risks (i.e., Grade 1EC found only on endometrium, TVU showing normal ovaries as well as normal CA-125); ovarian cancer was not detected, proposing that laparoscopy in these cases could be avoided [52,53].
Treatment Response and Risk Assessment prior to decision for Temporal Fertility Sparing Surgery
Patients who received hysteroscopic resection followed by progestin therapy seem to achieve the highest complete remission rate when compared with other fertility-preserving treatments [54-56].
The recommended treatments are 400–600mg oral Medroxyprogesterone Acetate (MPA) daily or 160–320mg Megestrol Acetate (MA) daily. In a prospective study, complete remission was achieved in 55% of women with EC who took 600mg of MPA and low-dose aspirin orally [57-63]. A retrospective study reported complete remission with oral MPA or MA in 115/148 patients (77.7%) MPA was associated with a significantly lower risk of recurrence than MA (p=0.021). No patients showed clinical progression at the time of recurrence and therefore, concluded that FSS treatment is safe [64]. A meta-analysis of 32 studies reported that FSS for EC was associated with a regression rate of 76.2% and a relapse rate of 40.6% [65].
Intrauterine progestin therapy such as levonorgestrel-releasing intrauterine system combined with gonadotropin-release hormone receptor agonist have a satisfactory pregnancy rate and low recurrence rate. In a prospective trial by daily administration of oral MPA 500mg with LNG-IUS, reported a complete remission rate of 87.5% and an average time to complete remission of 9.8 months (±8.9) [66]. Patients received oral progestin only, had higher risk of cancer recurrency and more systemic adverse effects.
Evaluation of the treatment response is crucial and of pivotal importance to identify the patients of low risk and candidates to be allowed to proceed to conception. The general rule without any scientific evidence is progesterone treatment for 3 months followed by re-evaluation of the endometrial cells [44,67,68].
The complete response to progesterone treatment for patients with stage IA (without superficial myometrial invasion) G2, G3 disease was 76.5%, for patients with stage IA (with superficial myometrial invasion) G1 disease was 73.9%, and for patients with stage IA (with superficial myometrial invasion) Grade 2–3 disease, respectively was 87.5%, [69]. Pregnancy outcomes after higher-grade and fertility-sparing treatments associated with pregnancy failure [70].
The recommendation for FSS in ES-EC Grade 1 endometrioid adenocarcinoma confined to the endometrium (or with only superficial myometrial invasion) has been supported by the British Gynecological Cancer Society, European Society of Gynecological Oncology, Society of Gynecologic Oncology, Japan Society of Gynecologic Oncology, and Korean Society of Gynecologic Oncology [69].
Discussion
ES-EC has an excellent prognosis after treatment with hysterectomy and bilateral salpingo-oophorectomy. Hormonal therapy although less effective compared to definitive surgery, still has a response rate of 80% which is comparable to the majority of reviews published in the last 7 years with average response rate of 75.4% [71,72].
However, oncological safety evidence and chance of successful pregnancy after FSS in gynecological malignancy patients are low. Mainly because the results are based on retrospective case series with small cohorts. Moreover, follow-up is often short and incidence of pregnancy and pregnancy outcome are inadequately reported. In most studies, patients with complex hyperplasia are included besides endometrial cancer, which will give a more optimistic response rate and therefore, differ from the current analysis. Although hormonal therapy can be an acceptable alternative in terms of initial response, recurrence rate is as high as 35%. Therefore, it is important that patients pursue pregnancy soon after remission and that hysterectomy is performed after completion of family planning [73].
The fertility-sparing alternative treatment includes hysteroscopic resection and/or curettage in combination with hormonal therapy with progestin with complete remission rates reported to be 50 to 75%, demonstrating a clear concession and high effectiveness [44,64,74]. Nevertheless, strict follow-up with hysteroscopic evaluation and endometrial sampling is advised.
Treatment Strategy
There is no consensus regarding optimal treatment strategy, because different types of progestin, dosage, and administration are used. The chance of complete remission with LNG-IUD is not significantly different compared to oral agents. However, the risk of recurrence is higher after oral administration (45.7% versus 9.5% after LNG-IUD).
A possible explanation for this difference is that in two out of three studies investigating LNG-IUD, a hysteroscopic resection was also performed, which was not standard in patients who started with oral treatment. In previous studies, this procedure increased the response rate and reduced the chance of recurrence [75]. To the best of our knowledge, most studies are single arm studies, lacking control groups, hence, the combination of LNG-IUD and oral agents improved outcomes are questionable. Metformin has demonstrated to benefit women with obesity, PCOS, and insulin resistance when added to the conservative treatment of endometrial cancer as well as decrease the risk of recurrence and increase both recurrence free survival and overall survival [76,77]. However, groups are heterogeneous (often also including hyperplasia) and the only available randomized control trial does not show a clear benefit from the addition of metformin [78].
The treatment of ES-EC is changing as molecular markers can predict disease recurrency and patients’ prognosis. In the selection process for FSS, these markers are not officially evaluated yet, although they may predict response to conservative treatment. Patients with MMRd tumors may be less responsive than those with POLE mutation (exonuclease domain of the polymerase epsilon gene; presented in 7–12% of EC and 1-2% of colorectal cancers) but the truth is that numbers are too small to draw any conclusions [79]. A relatively high number of fetal losses (31.3%) is reported after FSS for EC, but the limited number of studies describing obstetric outcome can influence this number. Currently, the live birth rate of 72% has been reported by several studies [80].
Progestin Treatment
There are four different combinations of progestin treatment for ES-EC, a) Progesterone (oral) treatment for 3 months, b) Progesterone (oral) + GnRH analogues, c) Progesterone (oral) + Levonorgestrel (IUD), d) Levonorgestrel (IUD) only.
Tock et.al. 2018 evaluated the efficacy and safety of gonadotropin-releasing hormone (GnRH) agonist when combined with laparoscopy to exclude concomitant ovarian tumor and/or other extra-uterine disease for 3 months after endometrial resection; in 18 women suffering ES-EC (G1 EC) and/or Endometrial Intra-epithelial Neoplasia (EIN). Out of 18 patients, six underwent hysterectomy as final treatment. Four of these because of FSS failure. One was diagnosed with recurrency after 10 months, two had a residual lesion and partial response respectively and one appeared to have a residual lesion 3 months after a second FSS for recurrent disease. Two patients had hysterectomy after their family planning was completed [81].
In a 3 months interval follow-up of endometrial sampling by hysteroscopy, Kim et al. report the recurrence rate after FSS to be 50%, 38.9%, 5.5%, 5.5% in EIN, G1 EC, combined histology and G2 EC respectively. After a median follow-up of 40.7 months, 12 patients (66.7%) preserved their uterus and 8 patients (53.3%) were pregnant with a total of 14 pregnancies among those who tried to become pregnant. 33.3% of patients had a stable disease and 66.7% had complete response rate of which 25% relapsed. To conclude GnRH agonist after surgery demonstrated to be an effective fertility-sparing strategy for women with EIN and/or G1 EC. [82]
Subsequent to that study, a multicenter prospective investigation was conducted in order to evaluate the efficacy of combined oral MPA and LNG-IUD treatments. The clearance rate at 6 months was only 37.1% (13/35 patients) attributed to the short treatment and follow-up periods [82].
A meta-analysis revealed that metformin was associated with improved overall survival in EC patients [83] and in combination with MPA, it elicited longer relapse-free survival [84]. Lastly, successful treatment of EC with a GnRH agonist along with an aromatase inhibitor or LNG-IUS and photodynamic therapy was established [85-88].
Genetic Testing
When familial history is highly suspicious of HNPCC, genetic counseling is recommended independent of the MMR status [22]. Furthermore, in the absence of hypermethylation, referral to genetic counseling is recommended to evaluate the presence of a germline mutation.
Testing for MMR status/MSI in EC, patients at higher risk for HNPCC can be identified [89]. Women carriers of MLH1 and MSH2 mismatch genes are in high risk to develop EC and colon cancer.
Ryan et al. suggest gynecological surveillance to be appropriate from age 30 years for those with MSH2 mutations, from age 35 years for those with non-truncating MLH1 mutations, and from age 40 years for those with MSH6 and truncating MLH1 mutations [89]. Women with heterozygous PMS2 mutations do not warrant gynecological surveillance because their absolute risk of gynecological cancer is very low.
Testing for MMRd by IHC or MSI by PCR-based methods does not allow direct identification of patients with HNPCC since MMRd/MSI is frequently due to sporadic events such as bi-allelic somatic mutations or hypermethylation.
The future of ES-EC treatment, as with multiple other cancers, seems to be in the form of molecularly targeted therapies. Pembrolizumab which is a PD-1 inhibitor has already been approved for use in patients with microsatellite instability tumors, however, no other molecular targeted therapies for EC have been approved to date [90,91].
Ovarian Involvement Concerns
Adnexal involvement in EC will upgrade the FIGO staging and can have an impact on the overall survival rate. Currently, patients with both endometrial and ovarian involvement in low-grade EC have a favorable outcome [22]. It even seems that these tumors present as synchronal primary tumors rather than metastatic progression [92,93]. Between low-grade EC and ovarian carcinomas there is a clonal relationship. This suggests that cancer secondarily extends to the ovary and primarily arises in the endometrium [94,95]. Consequently, the world health organization reports that treatment should not include any adjuvant modalities for patients with clonally related low-grade EC and treatment should be focused on 2 independent primary carcinomas. However, for this they will need to fulfil the following: (a) low-grade endometrioid morphology, (b) restricted to superficial invasion of the myometrium, (c) nonappearance of lympho-vascular involvement, and (d) nonappearance of additional metastases [22,96]
Evaluation of Post-Treatment Response
To date, there are no available randomized controlled trials comparing different methods of conservative treatment in women with atypical hyperplasia/endometrioid intraepithelial neoplasia or presumed Stage IA Grade 1 EC. According to the literature Dilation and Curettage (D&C), endometrial aspiration biopsy, or hysteroscopic biopsy are all equally accepted as modalities for follow up [97]. However, given the fact the D&C sensitivity in detecting EC is around 60-66%, we would like to express our hesitation regarding this modality [98]. Office hysteroscopy can be performed without any anaesthesia, allows for endometrial cavity review in detail and simultaneously it enables directed biopsies, providing precise diagnosis. Especially in the cases of ES-EC and FSS, hysteroscopy we propose to be selected as the most appropriate procedure for confirming remission or diagnosing relapse of the disease. EC staging and treatment should be made by a multi-disciplinary team of experts based on a comprehensive knowledge of prognostic factors for morbidity, mortality and quality of life and the strong wish of the patient for temporal preservation of the uterus.
ES-EC Patients and Fertility Potential will Determine FSS Decision
Delayed first pregnancy and aging of women in combination with high-definition sonography and cancer awareness is expected to increase the cases of ES-EC during reproductive age as well as the incidence of women with ES-EC interested in FSS. Age is a very important determinator regarding FSS in ES-EC, since fertility potential is compromised especially after the age of 37 due to oocytes carrying the risk of DNA being fragmented [99].
Patients with ES-EC at reproductive age with infertility problem either due to tubal factor, adenomyosis, male with oligo/terato/astheno spermia need artificial reproductive techniques and ovarian stimulation. However, once ovarian hormonal stimulation is needed patients’ safety is compromised. In cases that spontaneous cycle and natural ovulation is considered as the treatment option, FSS might be proposed. Lastly, for parous patients that already had one child and they are trying for another one after ES-EC, with favorable obstetrical history, the age limit might be extended up to 40-years-old for a trial of 6-12 months [100].
Conclusion
The management of Endometrial Cancer requires a stepwise and holistic approach. The increasing number of older premenopausal patients with early-stage endometrial cancer, requiring fertility sparring treatment, calls for a more targeted, personalized, and structured approach in treatment protocols. Since prognostic factors play a vital role to decide for FSS, there is a need for a multicenter, prospective study clarifying further the selection of patients and the guidelines to follow.
References
- World Health Organization. GLOBOCAN 2018: estimated cancer incidence, mortality and prevalence worldwide in 2018. 2018.
- World Health Organization, International Agency for Research on Cancer (IARC), Global Cancer Observatory (GCO). 2020.
- The Netherlands cancer registry, the Netherlands comprehensive cancer organisation (IKNL). 2020.
- Schuurman T, Zilver S, Samuels S, Schats W, Amant F, et al. Fertility-sparing surgery in gynecologic cancer: A systematic review. Cancers (Basel). 2021; 13: 1008.
- Kalogera E, Dowdy SC, Bakkum-Gamez JN. Preserving fertility in young patients with endometrial cancer: current perspectives. Int J Womens Health. 2014; 6: 691-701.
- Bharati R, Jenkins MA, Lindor NM, Le Marchand L, Gallinger S, et al. Does risk of endometrial cancer for women without a germline mutation in a DNA mismatch repair gene depend on family history of endometrial cancer or colorectal cancer? Gynecol Oncol. 2014; 133: 287-92.
- Ryan NAJ, Glaire MA, Blake D, Cabrera-Dandy M, Evans DG, et al. The proportion of endometrial cancers associated with Lynch syndrome: a systematic review of the literature and meta-analysis. Genet Med. 2019; 21: 2167-80.
- Ryan NAJ, Morris J, Green K, Lalloo F, Woodward ER, et al. Association of mismatch repair mutation with age at cancer onset in Lynch syndrome: implications for stratified surveillance strategies. JAMA Oncol. 2017; 3: 1702-6.
- Lachiewicz MP, Kravochuck SE, O’Malley MM, Heald B, Church JM, et al. Prevalence of occult gynecologic malignancy at the time of risk reducing and nonprophylactic surgery in patients with Lynch syndrome. Gynecol Oncol. 2014; 132: 434-7.
- Maxwell GL, Risinger JI, Gumbs C, Shaw H, Bentley RC, et al. Mutation of the PTEN tumor suppressor gene in endometrial hyperplasias. Cancer Res. 1998; 58: 2500-3.
- Zhao P, Li L, Jiang X, Li Q. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J Hematol Oncol. 2019; 12: 54.
- Cho KR, Cooper K, Croce S, Djordevic B, Herrington S, et al. International Society of Gynecological Pathologists (ISGyP) Endometrial Cancer Project: Guidelines From the Special Techniques and Ancillary Studies Group. Int J Gynecol Pathol. 2019; 38: S114-22.
- Kheirelseid EA, Miller N, Chang KH, Curran C, Hennessey E, et al. Mismatch repair protein expression in colorectal cancer. J Gastrointest Oncol. 2013; 4: 397-408.
- Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010; 138: 2073-2087.e3.
- Kato M, Takano M, Miyamoto M, Sasaki N, Goto T, et al. DNA mismatch repair-related protein loss as a prognostic factor in endometrial cancers. J Gynecol Oncol. 2015; 26: 40-5.
- Huang M, Djordjevic B, Yates MS, Urbauer D, Sun C, et al. Molecular pathogenesis of endometrial cancers in patients with Lynch syndrome. Cancer. 2013; 119: 3027-33.
- Concin N, Matias-Guiu X, Vergote I, Cibula D, Mirza MR, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer. 2021; 31: 12-39.
- Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983; 15: 10-7.
- Potischman N, Hoover RN, Brinton LA, Siiteri P, Dorgan JF, et al. Case-control study of endogenous steroid hormones and endometrial cancer. J Natl Cancer Inst. 1996; 88: 1127-35.
- Sherman ME, Bur ME, Kurman RJ. p53 in endometrial cancer and its putative precursors: evidence for diverse pathways of tumorigenesis. Hum Pathol. 1995; 26: 1268-74.
- Okuda T, Sekizawa A, Purwosunu Y, Nagatsuka M, Morioka M, et al. Genetics of endometrial cancers. Obstet Gynecol Int. 2010; 2010: 984013.
- Parc YR, Halling KC, Burgart LJ, McDonnell SK, Schaid DJ, et al. Microsatellite instability and hMLH1/hMSH2 expression in young endometrial carcinoma patients: associations with family history and histopathology. Int J Cancer. 2000; 86: 60-6.
- Crosbie EJ, Ryan NAJ, Arends MJ, Bosse T, Burn J, et al. The Manchester International Consensus Group recommendations for the management of gynecological cancers in Lynch syndrome. Genet Med. 2019; 21: 2390-400.
- Saed L, Varse F, Baradaran HR, Moradi Y, Khateri S, et al. The effect of diabetes on the risk of endometrial Cancer: an updated a systematic review and meta-analysis BMC Cancer. 2019; 19: 527.
- Xiao Y, Jianliu W. The role of metabolic syndrome in endometrial cancer: a review. Front Oncol. 2019; 9: 744.
- Aune D, Sen A, Vatten LJ. Hypertension and the risk of endometrial cancer: a systematic review and meta-analysis of case-control and cohort studies. Sci Rep. 2017; 7: 44808.
- Aune D, Sen A, Vatten LJ. Hypertension and the risk of endometrial cancer: a systematic review and meta-analysis of case-control and cohort studies. Sci Rep. 2017; 7: 44808.
- Ding D-C, Chen Weishan, Wang J-H, Lin S-Z. Association between polycystic ovarian syndromeand endometrial, ovarian, and breast cancer: A population-based cohort study in Taiwan Medicine. 2018; 97: 39.
- Shafiee MN, Seedhouse C, Mongan N, Chapman C, Deen S, et al. Up-regulation of genes involved in the insulin signalling pathway (IGF1, PTEN and IGFBP1) in the endometrium may link polycystic ovarian syndrome and endometrial cancer. Mol Cell Endocrinol. 2016; 424: 94-101.
- Nair S, Nguyen H, Salama S, Al-Hendy A. Obesity and the endometrium: adipocyte-secreted proinflammatory TNF a cytokine enhances the proliferation of human endometrial glandular cells. Obstet Gynecol Int. 2013; 2013: 368543.
- Shafiee MN, Ortori CA, Barrett DA, Mongan NP, Abu J, et al. Lipidomic biomarkers in polycystic ovary syndrome and endometrial cancer. Int J Mol Sci. 2020; 21: 4753.
- Tofiloska V, Velik-Stefanovska V, Dimitrov G. The connection between the endometrial thickness and the risk of endometrial malignancy in postmenopausal women. Open Access Maced J Med Sci. 2019; 7: 2263-6.
- Zhang G, Yu X, Sun Z, Zhu L, Lang J. Value of endometrial thickness in diagnosis of endometrial hyperplasia during selective estrogen receptor modulator therapy in premenopausal breast cancer patients. J Gynecol Obstet Hum Reprod. 2021; 50: 101929.
- Szpurek D, Sajdak S, Moszynski R, Roszak A. Estimation of neovascularisation in hyperplasia and carcinoma of endometrium using a ”power” angio-Doppler technique. Eur J Gynaecol Oncol. 2000; 21: 405-7.
- Kinkel K, Kaji Y, Yu KK, Segal MR, Lu Y, et al. Radiologic staging in patients with endometrial cancer: a meta-analysis. Radiology. 1999; 212: 711-8.
- Faria SC, Devine CE, Rao B, Sagebiel T, Bhosale P. Imaging and staging of endometrial cancer. Semin Ultrasound CT MR. 2019; 40: 287-94.
- Bathen TF, Krane J, Engan T, Bjerve KS, Axelson D. Quantification of plasma lipids and apolipoproteins by use of proton NMR spectroscopy, multivariate and neural network analysis. NMR Biomed. 2000; 13: 271-88.
- Sala E, Rockall AG, Freeman SJ, Mitchell DG, Reinhold C. The added role of MR imaging in treatment stratification of patients with gynecologic malignancies: what the radiologist needs to know. Radiology. 2013; 266: 717-40.
- Park JY, Kim EN, Kim DY, Suh DS, Kim JH, et al. Comparison of the validity of magnetic resonance imaging and positron emission tomography/computed tomography in the preoperative evaluation of patients with uterine corpus cancer. Gynecol Oncol. 2008; 108: 486-92.
- Bourgioti C, Chatoupis K, Panourgias E, Tzavara C, Sarris K, et al. Endometrial vs. cervical cancer: development and pilot testing of a magnetic resonance imaging (MRI) scoring system for predicting tumor origin of uterine carcinomas of indeterminate histology. Abdom Imaging. 2015; 40: 2529-40.
- Bourgioti C, Chatoupis K, Moulopoulos LA. Current imaging strategies for the evaluation of uterine cervical cancer. World J Radiol. 2016; 8: 342-54.
- Lin G, Ng KK, Chang CJ, Wang JJ, Ho KC, et al. Myometrial invasion in endometrial cancer: diagnostic accuracy of diffusion-weighted 3.0-T MR imaging: initial experience. Radiology. 2009; 250: 784-92.
- Zerbe MJ, Bristow R, Grumbine FC, Montz FJ. Inability of preoperative computed tomography scans to accurately predict the extent of myometrial invasion and extracorporal spread in endometrial cancer. Gynecol Oncol. 2000; 78: 67-70.
- Won S, Kim MK, Seong SJ. Fertility-sparing treatment in women with endometrial cancer. Clin Exp Reprod Med. 2020; 47: 237-44.
- Park JY, Kim EN, Kim DY, Suh DS, Kim JH, et al. Comparison of the validity of magnetic resonance imaging and positron emission tomography/computed tomography in the preoperative evaluation of patients with uterine corpus cancer. Gynecol Oncol. 2008; 108: 486-92.
- Sohaib SA, Houghton SL, Meroni R, Rockall AG, Blake P, et al. Recurrent endometrial cancer: patterns of recurrent disease and assessment of prognosis. Clin Radiol. 2007; 62: 28-34.
- Kim MK, Seong SJ, Song T, Kim ML, Yoon BS, et al. Comparison of dilatation & curettage and endometrial aspiration biopsy accuracy in patients treated with high-dose oral progestin plus levonorgestrel intrauterine system for early-stage endometrial cancer. Gynecol Oncol. 2013; 130: 470-3.
- Practice bulletin number 149. Practice Bulletin No. 149: endometrial cancer. Obstet Gynecol. 2015; 125: 1006-26.
- Minig L, Franchi D, Boveri S, Casadio C, Bocciolone L, et al. Progestin intrauterine device and GnRH analogue for uterus-sparing treatment of endometrial precancers and well-differentiated early endometrial carcinoma in young women. Ann Oncol. 2011; 22: 643-9.
- Sideri M, Minig L, Franchi D, Casadio C, Boveri S, et al. Reply to should diagnostic laparoscopy be conducted before hormonal treatment in early-stage endometrial cancer? Ann Oncol. 2011; 22: 749.
- Walsh C, Holschneider C, Hoang Y, Tieu K, Karlan B, et al. Coexisting ovarian malignancy in young women with endometrial cancer. Obstet Gynecol. 2005; 106: 693-9.
- Duska LR, Garrett A, Rueda BR, Haas J, Chang Y, Fuller AF. Endometrial cancer in women 40 years old or younger. Gynecol Oncol. 2001; 83: 388-93.
- Song T, Seong SJ, Bae DS, Suh DH, Kim DY, et al. Synchronous primary cancers of the endometrium and ovary in young women: a Korean Gynecologic Oncology Group Study. Gynecol Oncol. 2013; 131: 624-8.
- Yang B, Xu Y, Zhu Q, Xie L, Shan W, et al. Treatment efficiency of comprehensive hysteroscopic evaluation and lesion resection combined with progestin therapy in young women with endometrial atypical hyperplasia and endometrial cancer. Gynecol Oncol. 2019; 153: 55-62.
- Chae SH, Shim SH, Lee SJ, Lee JY, Kim SN, Kang SB. Pregnancy and oncologic outcomes after fertility-sparing management for early stage endometrioid endometrial cancer. Int J Gynecol Cancer. 2019; 29: 77-85.
- Giampaolino P, Di Spiezio Sardo A, Mollo A, Raffone A, Travaglino A, et al. Hysteroscopic endometrial focal resection followed by levonorgestrel intrauterine device insertion as a fertility-sparing treatment of atypical endometrial hyperplasia and early endometrial cancer: a retrospective study. J Minim Invasive Gynecol. 2019; 26: 648-56.
- Greenwald ZR, Huang LN, Wissing MD, Franco EL, Gotlieb WH. Does hormonal therapy for fertility preservation affect the survival of young women with early-stage endometrial cancer? Cancer. 2017; 123: 1545-54.
- Eftekhar Z, Izadi-Mood N, Yarandi F, Shojaei H, Rezaei Z, et al. Efficacy of megestrol acetate (megace) in the treatment of patients with early endometrial adenocarcinoma: our experiences with 21 patients. Int J Gynecol Cancer. 2009; 19: 249-52.
- Shirali E, Yarandi F, Eftekhar Z, Shojaei H, Khazaeipour Z. Pregnancy outcome in patients with stage 1a endometrial adenocarcinoma, who conservatively treated with megestrol acetate. Arch Gynecol Obstet. 2012; 285: 791-5.
- Mao Y, Wan X, Chen Y, Lv W, Xie X. Outcomes of conservative therapy for young women with early endometrial adenocarcinoma. Fertil Steril. 2010; 93: 283-5.
- Ushijima K, Yahata H, Yoshikawa H, Konishi I, Yasugi T, et al. Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. J Clin Oncol. 2007; 25: 2798-803.
- Wang CJ, Chao A, Yang LY, Hsueh S, Huang YT, et al. Fertility-preserving treatment in young women with endometrial adenocarcinoma: a long-term cohort study. Int J Gynecol Cancer. 2014; 24: 718-28.
- Ohyagi-Hara C, Sawada K, Aki I, Mabuchi S, Kobayashi E, et al. Efficacies and pregnant outcomes of fertility-sparing treatment with medroxyprogesterone acetate for endometrioid adenocarcinoma and complex atypical hyperplasia: our experience and a review of the literature. Arch Gynecol Obstet. 2015; 291: 151-7.
- Park JY, Kim DY, Kim JH, Kim YM, Kim KR, et al. Long-term oncologic outcomes after fertility-sparing management using oral progestin for young women with endometrial cancer (KGOG 2002). Eur J Cancer. 2013; 49: 868-74.
- Kim MK, Seong SJ, Kim YS, Song T, Kim ML, et al. Combined medroxyprogesterone acetate/levonorgestrel-intrauterine system treatment in young women with early-stage endometrial cancer. Am J Obstet Gynecol. 2013; 209: 358.e1-4.
- Niwa K, Tagami K, Lian Z, Onogi K, Mori H, Tamaya T. Outcome of fertility-preserving treatment in young women with endometrial carcinomas. BJOG. 2005; 112: 317-20.
- Cade TJ, Quinn MA, Rome RM, Neesham D. Progestogen treatment options for early endometrial cancer. BJOG. 2010; 117: 879-84.
- Gunderson CC, Fader AN, Carson KA, Bristow RE. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review. Gynecol Oncol. 2012; 125: 477-82.
- Chae SH, Shim SH, Lee SJ, Lee JY, Kim SN, et al. Pregnancy and oncologic outcomes after fertility-sparing management for early stage endometrioid endometrial cancer. Int J Gynecol Cancer. 2019; 29: 77-85.
- Gallos ID, Yap J, Rajkhowa M, Luesley DM, Coomarasamy A, Gupta JK. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: A systematic review and metaanalysis. Am J Obstet Gynecol. 2012; 207: 266.e1-12.
- Gunderson CC, Fader AN, Carson KA, Bristow RE. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: A systematic review. Gynecol Oncol. 2012; 125: 477-82.
- Frumovitz M, Gershenson DM. Fertility-sparing therapy for young women with endometrial cancer. Expert Rev Anticancer Ther. 2006; 6: 27-32.
- Park JY, Lee SH, Seong SJ, Kim DY, Kim TJ, et al. Progestin re-treatment in patients with recurrent endometrial adenocarcinoma after successful fertility-sparing management using progestin. Gynecol Oncol. 2013; 129: 7-11.
- Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up. Ann Oncol Consensus Conference on Endometrial Cancer: Diagnosis, treatment and follow-up. 2016; 27: 16-41.
- Fan Z, Li H, Hu R, Liu Y, Liu X, Gu L. Fertility-preserving treatment in young women with Grade 1 presumed stage IA endometrial adenocarcinoma: A meta-analysis. Int J Gynecol Cancer. 2018; 28: 385-93.
- Hall C, Stone RL, Gehlot A, Zorn KK, Burnett AF. Use of metformin in obese women with Type I endometrial cancer is associated with a reduced incidence of cancer recurrence. Int J Gynecol Cancer. 2016; 26: 313-7.
- Ko EM, Walter P, Jackson A, Clark L, Franasiak J, et al. Metformin is associated with improved survival in endometrial cancer. Gynecol Oncol. 2014; 132: 438-42.
- Yang BY, Gulinazi Y, Du Y, Ning CC, Cheng YL, et al. Metformin plus megestrol acetate compared with megestrol acetate alone as fertility-sparing treatment in patients with atypical endometrial hyperplasia and well-differentiated endometrial cancer: A randomised controlled trial. BJOG. 2020; 127: 848-57.
- Gerstl B, Sullivan E, Vallejo M, Koch J, Johnson M, et al. Reproductive outcomes following treatment for a gynecological cancer diagnosis: A systematic review. J Cancer surviv. 2019; 13: 269-81.
- Tock S, Jadoul P, Squifflet JL, Marbaix E, Baurain JF, et al. Fertility sparing treatment in patients with early stage endometrial cancer, using a combination of surgery and GnRH agonist: a monocentric retrospective study and review of the literature. Front Med (Lausanne). 2018; 5: 240.
- Kim MK, Seong SJ, Kang SB, Bae DS, Kim JW, et al. Six months response rate of combined oral medroxyprogesterone/levonorgestrel-intrauterine system for early-stage endometrial cancer in young women: a Korean Gynecologic-Oncology Group Study. J Gynecol Oncol. 2019; 30: e47.
- Meireles CG, Pereira SA, Valadares LP, Rêgo DF, Simeoni LA, et al. Effects of metformin on endometrial cancer: systematic review and meta-analysis. Gynecol Oncol. 2017; 147: 167-80.
- Mitsuhashi A, Habu Y, Kobayashi T, Kawarai Y, Ishikawa H, et al. Long-term outcomes of progestin plus metformin as a fertility-sparing treatment for atypical endometrial hyperplasia and endometrial cancer patients. J Gynecol Oncol. 2019; 30: e90.
- Zhou H, Cao D, Yang J, Shen K, Lang J. Gonadotropin-releasing hormone agonist combined with a levonorgestrel-releasing intrauterine system or letrozole for fertility-preserving treatment of endometrial carcinoma and complex atypical hyperplasia in young women. Int J Gynecol Cancer. 2017; 27: 1178-82.
- Pashov AI, Tskhay VB, Ionouchene SV. The combined GnRH-agonist and intrauterine levonorgestrel-releasing system treatment of complicated atypical hyperplasia and endometrial cancer: a pilot study. Gynecol Endocrinol. 2012; 28: 559-61.
- Zhang Z, Huang H, Feng F, Wang J, Cheng N. A pilot study of gonadotropin-releasing hormone agonist combined with aromatase inhibitor as fertility-sparing treatment in obese patients with endometrial cancer. J Gynecol Oncol. 2019; 30: e61.
- Choi MC, Jung SG, Park H, Cho YH, Lee C, et al. Fertility preservation via photodynamic therapy in young patients with early-stage uterine endometrial cancer: a long-term follow-up study. Int J Gynecol Cancer. 2013; 23: 698-704.
- Falcone F, Laurelli G, Losito S, Di Napoli M, Granata V, et al. Fertility preserving treatment with hysteroscopic resection followed by progestin therapy in young women with early endometrial cancer. J Gynecol Oncol. 2017; 28: e2.
- Cancer Genome Atlas Research Network, Kandoth C, Schultz N, Cherniack AD, Akbani R, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013; 497: 67-73.
- Lachance JA, Darus CJ, Rice LW. Surgical management and postoperative treatment of endometrial carcinoma. Rev Obstet Gynecol. 2008; 1: 97-105.
- Ott PA, Bang YJ, Berton-Rigaud D, Elez E, Pishvaian MJ, et al. Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: results from the KEYNOTE- 028 Study. J Clin Oncol. 2017; 35: 2535-41.
- Connell PP, Rotmensch J, Waggoner S, Mundt AJ. The significance of adnexal involvement in endometrial carcinoma. Gynecol Oncol. 1999; 74: 74-9.
- Soliman PT, Slomovitz BM, Broaddus RR, Sun CC, Oh JC, et al. Synchronous primary cancers of the endometrium and ovary: a single institution review of 84 cases. Gynecol Oncol. 2004; 94: 456-62.
- Schultheis AM, Ng CKY, De Filippo MR, Piscuoglio S, Macedo GS, et al. Massively parallel sequencing-based clonality analysis of synchronous endometrioid endometrial and ovarian carcinomas. J Natl Cancer Inst. 2016; 108: djv427.
- Anglesio MS, Wang YK, Maassen M, Horlings HM, Bashashati A, et al. Synchronous endometrial and ovarian carcinomas: evidence of clonality. J Natl Cancer Inst. 2016; 108: djv428.
- Turashvili G, Gómez-Hidalgo NR, Flynn J, Gonen M, Leitao MM, et al. Risk-based stratification of carcinomas concurrently involving the endometrium and ovary. Gynecol Oncol. 2019; 152: 38-45.
- Dijkhuizen FP, Mol BW, Brölmann HA, Heintz AP. The accuracy of endometrial sampling in the diagnosis of patients with endometrial carcinoma and hyperplasia: a meta-analysis. Cancer. 2000; 89: 1765-72.
- Stock RJ, Kanbour A. Prehysterectomy curettage. Obstet Gynecol. 1975; 45: 537-41.
- Maria UF, Danilo C, Alberto V, Gemma F, Roberta V, et al. Advanced maternal age in IVF: still a challenge? The present and the future of its treatment. Frontiers in Endocrinology. 2019; 10: 94.
- Bogani G, Dowdy SC, Cliby WA, Ghezzi F, Rossetti D, et al. Management of endometrial cancer: issues and controversies. Eur J Gynaecol Oncol. 2016; 37: 6-12.
- Hegazy AA. Postponing menopause and lengthening fertile age for women’s Good Health: A potential hope. World J Gynecol Womens Health. 2021; 5: 1-2.
- Hegazy AA. Potentiality of postponing menopause through ovarian auto-graft transplantation. J Gynecol. Reprod Med. 2020; 4: 29-31.
- Hegazy AA. Is there any mean to postpone the menopausal ovarian senescence? Int J Fertil Steril. 2020; 13: 346-7.