
Special Article: Human Nutrition
Int J Nutr Sci. 2024; 9(2): 1091.
Prevalence of Vitamin A and D Deficiencies in Children Under Five in South Asia and Africa: A Narrative Review
Nimmathota Arlappa1; Deokare Swateja Ashok2; Billakanti Sai Dheeraja3; Abdul Jaleel4*
1Scientist-G, Department of Public Health Nutrition, ICMR-National Institute of Nutrition, Hyderabad, India
2Intern, Department of Public Health Nutrition, ICMRNational Institute of Nutrition, Hyderabad, India
3PhD Scholar, Department of Public Health Nutrition, ICMR-National Institute of Nutrition, Hyderabad, India
4Scientist-B, Department of Public Health Nutrition, ICMR-National Institute of Nutrition, Hyderabad, India
*Corresponding author: Abdul Jaleel, ICMR-National Institute of Nutrition, Beside Tarnaka Metro Station, Jamai-Osmania PO, Hyderabad-500 007, India. Tel: + 91 9769187487 Email: jaleel.cp@icmr.gov.in
Received: December 03, 2024; Accepted: December 24, 2024; Published: December 31, 2024
Abstract
Background: The prevalence of vitamin A and D deficiencies among children aged 6–59 months remains alarmingly high in African and South Asian countries. This review evaluates the most recent prevalence estimates of these essential micronutrient deficiencies in children under five in these regions.
Methods: This article adopted a narrative review approach. Electronic searches were conducted across multiple databases, focusing on articles and reports published between 2000 and 2024.
Results: South Asia exhibits the highest burden of Vitamin A Deficiency (VAD). Notably, recent prevalence rates among children are alarmingly high in Afghanistan (49.3%), Maldives (43.8%), and Pakistan (37%). In India, while the national prevalence among preschool children is 17.5%, many states continue to face severe public health challenges. In Africa, VAD prevalence remains significant, with Mozambique (71.2%), Zambia (56%), and Morocco (40.9%) reporting the highest rates. Vitamin D deficiency (VDD) is widespread across South Asia, particularly among neonates, with prevalence rates reaching 85%. Preschool children in Sri Lanka (93%), Nepal (91%), Afghanistan (81%), Pakistan (62.7%), and India (35%–76%) are notably affected, while Bangladesh (30%–50%) and Bhutan (43%) also report concerning rates.
Conclusion: African and South Asian countries continue to face a substantial burden of vitamin A and D deficiencies among children under five. Strengthening vitamin-A supplementation programs and ensuring robust social safety net initiatives are critical to mitigating hidden hunger and reducing its impact on the disease burden among the most vulnerable populations.
Keywords: Vitamin A; Vitamin D; Under five children; India; South Asia; Africa
Introduction
Vitamins A and D are critical micronutrients with significant roles in supporting immune function, cellular growth, and overall health outcomes [1]. Vitamin A is vital for a wide range of physiological processes, including vision, cellular communication, epithelial integrity, red blood cell production, immune enhancement, and reproductive health [2-5]. It exists in two primary dietary forms: retinyl esters from animal sources and provitamin A carotenoids, such as β-carotene, found in green and yellow vegetables and fruits. The body converts these carotenoids into retinol, the active form of Vitamin A [6]. Deficiency in Vitamin A often arises from inadequate dietary intake and is exacerbated by recurrent infections, particularly diarrhea and measles [7] Vitamin A deficiency (VAD) has serious implications for maternal and child health. In pregnant women, it increases the risk of complications during childbirth, adverse pregnancy outcomes, and neonatal mortality, and can result in the transmission of deficiency to infants [8]. It is crucial for fetal development, particularly in skeletal and organ formation [9-11]. In children under five, VAD is a significant contributor to mortality, accounting for about 2% of deaths globally in this age group [7].
Similarly, Vitamin D plays a pivotal role in calcium and phosphate absorption, bone mineralization, and the prevention of conditions like rickets and osteomalacia [12]. It also contributes to immune regulation, inflammation reduction, and glucose metabolism [13-21]. Vitamin D is synthesized in the skin through Ultraviolet (UV) exposure, with only limited amounts obtained through natural food sources [22,23]. Once produced, Vitamin D3 (cholecalciferol) is converted in the liver to calcidiol and subsequently in the kidneys to calcitriol, its active form [23,24]. The deficiency of Vitamin D has been identified as a major public health issue worldwide, affecting all age groups. In children, Vitamin D Deficiency (VDD) is the primary cause of rickets, leading to skeletal abnormalities and delayed development [25,26]. Beyond musculoskeletal health, VDD is associated with an increased risk of various extraskeletal conditions, including cardiovascular diseases, cancer, autoimmune disorders, and metabolic syndromes [27,28]. Deficiencies in these essential nutrients are highly prevalent in South Asia and Africa. The prevalence of VAD among children aged 6–59 months in Low-and Middle-Income Countries (LMICs) is alarmingly high, estimated at 29%, with sub-Saharan Africa (48%) and South Asia (44%) being the most affected regions. Together, these areas account for 95% of global VAD-related deaths [7,29,30]. Additionally, it is estimated that over one billion people globally have insufficient Vitamin D levels, with particularly high prevalence rates in South Asia (exceeding 70% in some countries) [31-33]. Addressing these deficiencies remains a critical priority for improving global health outcomes. In this context, we conducted this narrative review of literature to examine the prevalence of VAD and VDD in South Asian and African countries, and its trend over a period of time. This compilation of prevalence data aims to support these regions in assessing the current situation and formulating effective responses to address these pressing public health challenges.
Materials and Methods
Search Strategy
To identify the most relevant literature (both published and gray) on Vitamin A Deficiency (VAD) and Vitamin D Deficiency (VDD) among children under five years of age, we conducted a comprehensive search using keywords across relevant electronic databases. This electronic search was meticulously conducted across multiple reputable databases, including PubMed, Google Scholar, ResearchGate, government reports, and reports by international agencies. A comprehensive set of keywords, encompassing "Vitamin A Deficiency (VAD)," "Vitamin D Deficiency," "vitamin A," "vitamin D," and "micronutrient assessment" was employed to ensure the thorough exploration of relevant literature. Only articles and reports published between 2000 and 2024 were considered for this review. Furthermore, the search was refined through manual examination, whereby pertinent papers were identified and included in the review. This search strategy was deliberately tailored to encompass articles, reports, and documents that addressed the prevalence of vitamin A and D deficiencies in countries in South Asia and Africa.
Prevalence of VDD (%)
Children
Algeria – 2014
8.05
Egypt – 2014
30.00
Egypt – 2016
15.19
Nigeria – 2018
0.00
Nigeria – 2014
7.00
South Africa – 2015
5.00
Tunisia – 2016
40.89
Random effect meta-analysis
10.55
Newborn babies
South Africa – 2019
33.10
Tunisia – 2016
89.66
Random effect meta-analysis
63.72
Table 1: Prevalence of VDD among children in African countries.
Figure 1: Search strategies adopted for identification of studies.
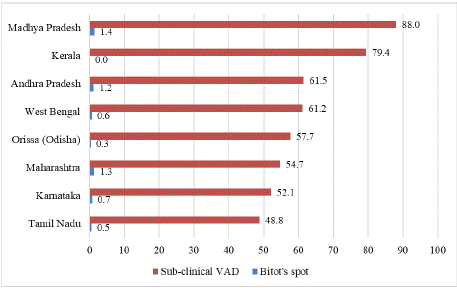
Figure 2: Prevalence of Bitot’s spot and sub-clinical VAD among under-five
children (NNMB-Rural survey 2002-2003) in various states of India.
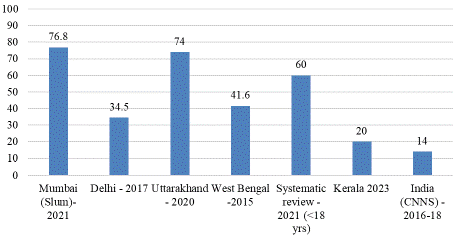
Figure 3: Prevalence of VDD among under-five children in various states
in India.
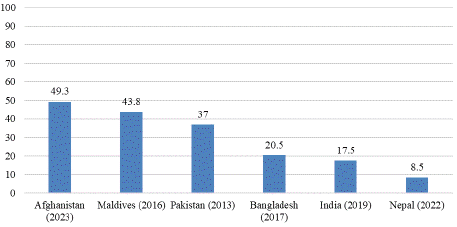
Figure 4: Prevalence of VAD among under-five children in South Asian
countries.
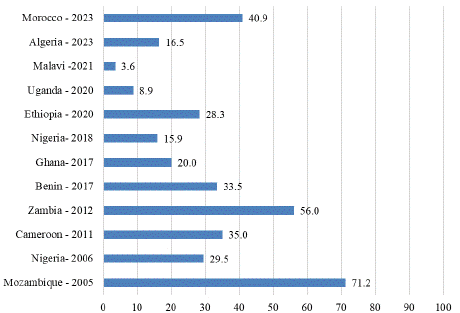
Figure 5: Prevalence of VAD among under five children in African countries.
Based on the relevant papers and reports, we conducted a descriptive and interpretive analysis of the prevalence of VAD and VDD among children under five in South Asia and Africa. The findings are then contextualized and discussed within the broader malnutrition situation of the respective regions.
This review is organized into six sections to provide a comprehensive analysis of Vitamin A and D deficiency in children under five years of age. The first two sections focus on the prevalence and status of Vitamin A and D deficiencies in India. The third and fourth sections expand the scope to examine the prevalence of these deficiencies across other South Asian countries. The final two sections present an overview of the Vitamin A and D deficiency situation in various African countries. Following the results, the review includes a brief discussion to contextualize the findings and provide insights into the implications of these deficiencies. The article concludes with key takeaways and recommendations to address these critical public health challenges.
Results
Vitamin A Deficiency among Under-five Children in India
This section provides an overview of the prevalence of VAD, both clinical (e.g., Bitot's spots) and subclinical, among children under five years of age across various states in India. Cross-sectional studies conducted in rural areas of Maharashtra, West Bengal, and Madhya Pradesh reported the prevalence of Bitot's spots as 1.3%, 0.6%, and 1.4%, respectively. Subclinical VAD prevalence in these states was reported to be 55%, 61%, and 88%, respectively [34-36]. A community-based cross-sectional study across eight Indian states, involving 71,591 preschool children, reported a Bitot's spot prevalence of 0.8%. This study further found that children from Scheduled Caste (SC) and Scheduled Tribe (ST) communities had a 2.4-fold higher risk of developing Bitot's spots compared to children from other social groups [37].
The National Nutrition Monitoring Bureau (NNMB) rural survey (2002–2003), conducted across eight states—Madhya Pradesh, West Bengal, Odisha, Maharashtra, Andhra Pradesh, Karnataka, Tamil Nadu, and Kerala—highlighted VAD as a significant public health concern. Bitot's spot prevalence was at or above the threshold of 0.5% in all surveyed states except Kerala and Odisha [38]. However, subclinical VAD was a severe public health problem (prevalence exceeding 20%) in all the surveyed states.
In Mizoram, the prevalence of clinical VAD among preschool children aged 12–59 months was reported at 4.1%, while subclinical VAD was alarmingly high at 81.2% [39]. In Punjab, a study reported a 5.4% prevalence of Bitot's spots among children aged 6 months to 6 years, indicating a very high prevalence of clinical VAD [40]. In Assam, clinical VAD prevalence among children under five years of age was reported at 6.4% [41], whereas Karnataka reported a prevalence of 2.5% [42]. Similarly, in Meghalaya, 59% of children under five were found to have subclinical VAD, reflecting a severe public health issue [43].
A cohort study involving 216 children aged 6–59 months attending Anganwadis in Chandigarh revealed a high prevalence (35.1%) of subclinical VAD (serum retinol < 0.7 µmol/L), which qualifies as a severe public health problem. The study further revealed that children with suboptimal complementary feeding practices had a 2.23 times higher risk of VAD [44]. In Maharashtra, a study of 1,341 children aged 3–6 years across 36 Anganwadis in Pune reported ocular morbidities in 6% of the children [45]. In Kerala, a study among tribal children under five years of age found a 12% prevalence of subclinical VAD [46].
The Comprehensive National Nutrition Survey (CNNS) reported an overall prevalence of VAD at 17.5% among preschool children in India, classifying it as a severe public health problem (=20% prevalence) in 10 states, including Andhra Pradesh, Telangana, Madhya Pradesh, Chhattisgarh, Bihar, Jharkhand, Haryana, Assam, Tripura, and Mizoram. Additionally, nine states reported moderate VAD prevalence (10–19.9%), with the lowest prevalence in Goa (2%) and the highest in Jharkhand (43%) [47].
Recent findings from Karnataka, Meghalaya, Chandigarh, and Maharashtra, combined with the CNNS data, underscore that both clinical and subclinical VAD remain significant public health challenges across many parts of India, particularly among the socially and economically vulnerable communities.
Vitamin D Deficiency among Under-five Children in India
This section outlines the prevalence of Vitamin D Deficiency (VDD) in children under five years of age across various states in India. Overall, there is a limited number of studies focusing specifically on VDD among this age group. However, existing evidence highlights the widespread and severe nature of the problem.
A recent systematic review on micronutrient deficiencies in India identified VDD as the most prevalent micronutrient deficiency, affecting 61% of the population [48]. The deficiency is notably high among children in the northern, western, and southern regions of the country. For instance, an analysis of serum 25-hydroxycholecalciferol levels among 310 preschool children attending a paediatric hospital in Kolkata found that 41.6% had VDD, with 16.2% experiencing severe deficiency [49].
Data from the Comprehensive National Nutrition Survey (CNNS, 2016–2018) revealed that 14% of preschool children in India suffer from VDD. However, the prevalence varies significantly by state, ranging from 25% to 50% in states such as Punjab, Uttarakhand, Manipur, Delhi, Haryana, Rajasthan, Gujarat, and Jammu & Kashmir [47,50].
Certain subpopulations exhibit alarmingly high prevalence rates. For example, a study among children aged 1–5 years living in urban slums in Mumbai reported a VDD prevalence of 76.8% [51]. Similarly, a Randomized Controlled Trial (RCT) conducted among 960 children aged 6–30 months in Delhi found that 34.5% were affected by VDD [52]. In Uttarakhand, a study of 200 infants at a tertiary care center revealed that 74% had VDD, with nearly half suffering from severe deficiency. Logistic regression analysis demonstrated a significant positive correlation between maternal and infant VDD in this population [53]. In Kerala, a recent study among 398 tribal children reported a VDD prevalence of 20% [46]. These findings highlight the widespread prevalence of VDD among children in India, with significant regional variations.
Vitamin A Deficiency among Under-five Children in South Asian Countries
South Asia remains the region with the highest burden of VAD globally, affecting an estimated 44–50% of preschool children [29]. Among South Asian countries, India reports the highest prevalence of both clinical and subclinical VAD, historically affecting 62% of preschool children [29]. However, more recent data indicate significant progress, with the prevalence of VAD among preschool children declining to 17.5% [54]. Despite this improvement, VAD remains a severe public health issue (=20% prevalence) in at least 10 states, indicating the need for sustained public health interventions [47].
In Pakistan, no comprehensive studies have been conducted to quantify the national burden of VAD among preschool children [29]. However, clinical cases of VAD in children under six years of age have been widely reported across major provinces [55]. The prevalence of VAD among preschool children in Pakistan is notably high at 37% [29]. In Sri Lanka, VAD remains a significant public health concern, despite the implementation of supplementation programs for children and pregnant and lactating women. Subclinical VAD persists as a challenge, suggesting gaps in program effectiveness [29]. Similarly, in Bangladesh, the prevalence of subclinical VAD among preschool children stands at 20.5% [56].
Afghanistan has the highest reported prevalence of VAD among South Asian countries, with 49.3% of children aged 0–5 years affected, representing a critical public health issue [57]. In contrast, Nepal reports a lower prevalence of 8.5% among children under five [58]. In the Maldives, the burden of VAD is significant, with clinical VAD affecting 16.8% and subclinical VAD affecting 43.8% of children aged six months to six years [59]. These findings underscore the persistent and varied burden of VAD across South Asia, with majority of the countries facing severe challenges.
Vitamin D Deficiency among Under-five Children in South Asian Countries
Vitamin D Deficiency (VDD) has become a widespread endemic condition across South Asia, posing a significant public health challenge [60]. A recent systematic review of VDD among children and adolescents in South Asia revealed alarmingly high prevalence rates, with the highest reported in Afghanistan (96.2%), followed by Pakistan (94%), India (64%), Bangladesh (35.5%), Nepal (35%), and Sri Lanka (25%) [61]. The review also highlighted that VDD prevalence is most severe among neonates (85%), followed by schoolage children (57%) and preschool children (55%).
In Afghanistan, the 2013 National Nutrition Survey reported that 81% of children aged 6–59 months were affected by VDD, with 16.8% experiencing severe deficiency [62]. In Pakistan, children and adolescents aged 6–18 years are among the most affected populations [63]. According to the National Nutrition Survey, VDD affects 62.7% of children under five, with severe deficiency reported in 13.2% [64]. A study among preschool children in Karachi found that approximately 60% had VDD, while 15% had insufficient Vitamin D levels [65]. Another recent study reported that 47% of children under five had VDD, and 24% had insufficient levels, leaving only 29% with sufficient Vitamin D levels [63].
In India, VDD is prevalent across various regions and demographics. For example, 41.6% of preschoolers in Kolkata were found to have VDD [49], while nationally, 14% of preschool children were deficient, with rates exceeding 20% in 10 states [47,50]. Urban slums in Mumbai reported a very high prevalence of 76.8% among children aged 1–5 years [51]. Additionally, 34.5% of children aged 6–30 months in Delhi were reported to have VDD [52]. In Uttarakhand, 74% of infants at a tertiary care center were affected, many with severe deficiency [53]. Among tribal children in Kerala, the prevalence of VDD was 20% [46].
In Bangladesh, VDD and vitamin D insufficiency rates are also significant. Among children aged 0–1 year, 31.9% had VDD, and 52.2% had insufficient levels. For children aged 2–5 years, 38.1% were deficient, and 50% had insufficient levels [66]. A randomized controlled trial of children aged >2–59 months with severe pneumonia attending Hospital found that 50% had VDD [67]. Another study reported VDD in 30.1% of infants under one year and 35% of children aged 2–5 years [68]. Additionally, VDD was reported in 47% of the children 12–24 months [69].
In Nepal, VDD was reported in 91.1% of apparently healthy children aged 12–60 months [70]. A more recent study found a VDD prevalence of 77% among children aged 1–5 years [71]. Similarly, in Sri Lanka, high rates of VDD have been documented among infants and children. Among 30 exclusively breastfed infants under six months of age, 93.3% were found to have VDD [72]. Another study of 100 apparently healthy children revealed that 78% were Vitamin D deficient [73]. Furthermore, a cross-sectional survey of children aged 1–5 years in the Bope-Poddala and Galle municipalities reported a VDD prevalence of 28%, with an additional 7% of children identified as vitamin D insufficient [74].
In Bhutan, a cross-sectional study found that 43.2% of children under five years of age were vitamin D deficient [75]. However, no studies examining VDD prevalence among children under five in the Maldives were identified. These findings underscore the widespread and variable prevalence of VDD across South Asian countries, with certain populations—such as neonates, and children in urban slums— experiencing particularly high rates.
Vitamin A Deficiency among Under-five Children in African Countries
The prevalence of VAD among African preschool children was estimated at 32%, affecting approximately 33 million children [76]. Additionally, 1.5% of children in Africa are reported to suffer from xerophthalmia, representing approximately 1.5 million cases [77]. In Ethiopia, a community-based cross-sectional study found the prevalence of Bitot’s spots at 1.46% and night blindness at 1.22%, both exceeding the World Health Organization (WHO) thresholds for defining a public health problem [78]. A systematic review of VAD in Ethiopia noted a decline in subclinical VAD prevalence from 55.7% in 1990 to 28.3% in 2019. However, these rates remain alarmingly high, indicating that VAD persists as a severe public health issue in the country [79].
In Nigeria, the national prevalence of VAD among children under five was 29.5%, with significant variation across agroecological zones: 31.3% in the dry savanna, 24.0% in the moist savanna, and 29.9% in the humid forest [80]. More recent data reported a reduced prevalence of 15.9% among preschool children, suggesting some progress in addressing the issue [81]. In Algeria, a cross-sectional study involving 133 preschool children found a VAD prevalence of 16.5% [82]. Similarly, Benin reported a VAD prevalence of 33.5% among children under five [83]. In Ghana, VAD affects approximately 20% of children, with a higher prevalence in the northern belt (31%) compared to lower rates among children from wealthier households (9%) [84].
Mozambique has one of the highest recorded VAD prevalence rates among children under five, at 71.2%. An estimated 2.3 million children in Mozambique are vitamin A deficient, with VAD being the attributable cause of over 30,000 deaths annually. This represents 34.8% of all-cause mortality among children under five [85]. In Cameroon, 35% of children aged 12–59 months were reported to have VAD [86]. In rural Zambia, 56% of preschool children were found to have VAD based on plasma retinol levels [87]. In Uganda, VAD prevalence among children aged 6–59 months was reported at 8.9% [88]. A recent study in Malawi found a much lower VAD prevalence of 3.6% among preschool children [89]. Conversely, in Morocco, VAD prevalence remains high, affecting 40.9% of preschool children [90].
Vitamin D Deficiency among Under five Children in African Countries
Vitamin D Deficiency (VDD) remains a significant public health issue among children under five in Africa, contributing to conditions such as rickets and increased susceptibility to infectious diseases [91]. A multi-country study involving children aged 0–8 years in Kenya, Uganda, Burkina Faso, The Gambia, and South Africa reported VDD prevalence rates of 6.3%, 5.5%, 6.1%, 10.2%, and 15.4%, respectively [91]. A study among children with sickle cell anemia in southwest Nigeria revealed a VDD prevalence of 12.6% [92]. However, VDD prevalence varies widely across regions and populations in Africa, as shown in a systematic review [93]. A meta-analysis included in this review estimated the random-effect pooled prevalence of VDD among children to be 10.55%. Among newborns, the pooled prevalence was even higher, at 63.72%, with 33.1% prevalence reported in South Africa (2019) and 89.66% in Tunisia (2016). The review reported the prevalence of VDD among children in specific countries as follows:
Another systematic review analyzing seven studies involving 2,736 healthy children reported a pooled VDD prevalence of 50.06%, with the lowest prevalence in Botswana (17%) and the highest in Ethiopia (90%) [94]. The same review assessed 684 sick children and found a pooled VDD prevalence of 39.36%, with the lowest prevalence in Botswana (21%) and the highest in Tanzania (70.2%) [94]. In South Africa, a recent study reported a VDD prevalence of 20.3% among children under five [95].
Discussion
This narrative review presents the latest prevalence data on vitamin A and vitamin D deficiencies in countries across South Asia and Africa. This review found that South Asia continues to bear the highest burden of VAD, with India reporting the highest prevalence of both clinical and subclinical VAD in the region. Other South Asian countries, such as Afghanistan, Maldives, Pakistan, and Bangladesh, also exhibit a high prevalence of VAD.
Recent studies from India, including the Comprehensive National Nutrition Survey (CNNS), confirm that subclinical VAD remains a significant public health challenge in many parts of the country. To combat this issue, India has been implementing the National Prophylaxis Program, which administers an oral dose of 200,000 IU of vitamin A concentrate to preschool children every six months. According to the National Family Health Survey-5 (NFHS- 5), Vitamin A Supplementation (VAS) coverage among children aged 9–35 months was 71.2% [96]. It is important to note that the high prevalences of VAD persists despite the implementation of the programs. Although the Vitamin A Supplementation (VAS) program has successfully reached many of the most vulnerable districts, significant gaps remain, as not all at-risk and socially disadvantaged children benefit from the intervention [97].
In addition to supplementation, India has prioritized preventive measures, such as promoting early initiation of breastfeeding, exclusive breastfeeding, and the fortification of hydrogenated fats with vitamin A. Furthermore, recent efforts have included integrating fortified rice into social assistance programs. These programs aim to replace unfortified rice with rice enriched with essential micronutrients, including iron, zinc, folic acid, vitamin A, thiamine, riboflavin, niacin, and vitamins B6 and B12, thereby leveraging existing systems that serve nutritionally vulnerable populations [98].
This review also highlights that VDD is a widespread endemic condition across South Asia, with certain regions and populations— such as neonates and children in urban slums—experiencing particularly high prevalence rates. In India, the prevalence of VDD among children under five ranges from 35% to 77%. Pakistan has a VDD prevalence of 63%. Similarly, Bangladesh reports a prevalence ranging from 30% to 50%, while children in Bhutan have a VDD prevalence of 43%.
This review also found that VAD prevalence among preschool children remains high in parts of Africa. Countries such as Mozambique, Zambia, Morocco, Cameroon, and Ethiopia also report significant rates of VAD, underscoring the global nature of this public health issue. Similarly, VDD is highly prevalent in countries like Tunisia, particularly among newborns. Egypt also has a significant VDD burden among its child population.
Conclusion
Hidden hunger, characterized by micronutrient deficiencies, remains a critical public health challenge in South Asia and Africa, two regions with persistently poor health outcomes. Children in these regions are particularly affected by deficiencies in Vitamin A and Vitamin D, with the prevalence of Vitamin D Deficiency (VDD) in South Asia reaching concerning levels. It is imperative to enhance Vitamin A and D supplementation programs, prioritizing the delivery of these interventions to the most at-risk populations.
Author Statements
Acknowledgements
The authors would like to thank the Global Alliance for Improved Nutrition (GAIN) in India for its support.
Conflict of Interest
The authors declare that they have no competing interests.
Authors Contribution
NA conceptualized the topic and design of the review; DSA and BSD conducted searching of articles and acquisition of final papers/ reports; AJ, DSA, and BSD contributed to the analysis of the data; NA, AJ, DSA, and BSD contributed to the interpretation of the data; DSA and BSD contributed to writing the first draft of the manuscript. AJ and NA contributed to critically revising the manuscript. All authors are in agreement with the manuscript and declare that the content has not been published elsewhere.
References
- Džopalic T, Božic-Nedeljkovic B, Jurišic V. The role of vitamin A and vitamin D in modulation of the immune response with a focus on innate lymphoid cells. Central-European journal of immunology. 2021; 46: 264-9.
- Brouzes CMC, Darcel N, Tomé D, Dao MC, Bourdet-Sicard R, Holmes BA, et al. Urban Egyptian Women Aged 19-30 Years Display Nutrition Transition- Like Dietary Patterns, with High Energy and Sodium Intakes, and Insufficient Iron, Vitamin D, and Folate Intakes. Current developments in nutrition. 2020; 4: nzz143.
- Elrayah EE, Rogers L, Doggui R, Al-Jawaldeh A. Vitamin D Insufficiency and Deficiency in the Eastern Mediterranean Region (EMR)-Misconceptions in Public Health Practice: A Scoping Review 2019-2020. Journal of nutritional science and vitaminology. 2020; 66: 389-95.
- Wirth JP, Petry N, Tanumihardjo SA, Rogers LM, McLean E, Greig A, et al. Vitamin A Supplementation Programs and Country-Level Evidence of Vitamin A Deficiency. Nutrients. 2017; 9: 190.
- Arlappa N. Vitamin A supplementation policy: A shift from universal to geographical targeted approach in India considered detrimental to health and nutritional status of under 5 years children. European journal of clinical nutrition. 2023; 77: 1-6.
- Carazo A, Macáková K, Matoušová K, Krcmová LK, Protti M, Mladenka P. Vitamin A Update: Forms, Sources, Kinetics, Detection, Function, Deficiency, Therapeutic Use and Toxicity. Nutrients. 2021; 13.
- Stevens GA, Bennett JE, Hennocq Q, Lu Y, De-Regil LM, Rogers L, et al. Trends and mortality effects of vitamin A deficiency in children in 138 lowincome and middle-income countries between 1991 and 2013: a pooled analysis of population-based surveys. The Lancet Global health. 2015; 3: e528-36.
- Georgieff MK. Iron deficiency in pregnancy. American journal of obstetrics and gynecology. 2020; 223: 516-24.
- Cabezuelo MT, Zaragozá R, Barber T, Viña JR. Role of Vitamin A in Mammary Gland Development and Lactation. 2020; 12: 80.
- Gannon BM, Jones C, Mehta S. Vitamin A Requirements in Pregnancy and Lactation. Current developments in nutrition. 2020; 4: nzaa142.
- Maia SB, ASR, Caminha MFC, da Silva SL, Callou Cruz RSB, Carvalho Dos Santos C, et al. Vitamin A and Pregnancy: A Narrative Review. Nutrients. 2019; 11.
- Laird E, Ward M, McSorley E, Strain JJ, Wallace J. Vitamin D and bone health: potential mechanisms. Nutrients. 2010; 2: 693-724.
- DeLuca HF. The metabolism and functions of vitamin D. Advances in experimental medicine and biology. 1986; 196: 361-75.
- Dominguez LJ, Farruggia M, Veronese N, Barbagallo M. Vitamin D Sources, Metabolism, and Deficiency: Available Compounds and Guidelines for Its Treatment. 2021; 11: 255.
- Zhang S, Miller DD, Li W. Non-Musculoskeletal Benefits of Vitamin D beyond the Musculoskeletal System. 2021; 22: 2128.
- Christakos S, Dhawan P, Porta A, Mady LJ, Seth T. Vitamin D and intestinal calcium absorption. Molecular and cellular endocrinology. 2011; 347: 25-9.
- Sanlier N, Guney-Coskun M. Vitamin D, the immune system, and its relationship with diseases. Egyptian Pediatric Association Gazette. 2022; 70: 39.
- Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiological reviews. 2016; 96: 365-408.
- Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocrine reviews. 2008; 29: 726-76.
- Pilz S, Verheyen N, Grübler MR, Tomaschitz A, März W. Vitamin D and cardiovascular disease prevention. Nature reviews Cardiology. 2016; 13: 404-17.
- Rosen CJ, Adams JS, Bikle DD, Black DM, Demay MB, Manson JE, et al. The nonskeletal effects of vitamin D: an Endocrine Society scientific statement. Endocrine reviews. 2012; 33: 456-92.
- Holick MF. Vitamin D: A millenium perspective. Journal of cellular biochemistry. 2003; 88: 296-307.
- IOM. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Washington (DC): Institute of Medicine (US). 2011.
- Norman AW HHVDIEJ, Macdonald IA, Zeisel SH. Present Knowledge in Nutrition. 10 ed. Washington DC: Wiley-Blackwell. 2012.
- Silva MC, Furlanetto TW. Intestinal absorption of vitamin D: a systematic review. Nutrition reviews. 2018; 76: 60-76.
- Holick MF. Vitamin D deficiency. The New England journal of medicine. 2007; 357: 266-81.
- Pilz S, Kienreich K, Tomaschitz A, Lerchbaum E, Meinitzer A, März W, et al. Vitamin D and cardiovascular disease: update and outlook. J Scandinavian Journal of Clinical Laboratory Investigation. 2012; 72: 83-91.
- Anagnostis P, Karras S, Goulis DG. Vitamin D in human reproduction: a narrative review. 2013; 67: 225-35.
- Akhtar S, Ahmed A, Randhawa MA, Atukorala S, Arlappa N, Ismail T, et al. Prevalence of vitamin A deficiency in South Asia: causes, outcomes, and possible remedies. Journal of health, population, and nutrition. 2013; 31: 413-23.
- Zhao T, Liu S, Zhang R, Zhao Z, Yu H, Pu L, et al. Global Burden of Vitamin A Deficiency in 204 Countries and Territories from 1990-2019. Nutrients. 2022; 14.
- Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? The Journal of steroid biochemistry and molecular biology. 2014; 144: 138-45.
- Nimitphong H, Holick MF. Vitamin D status and sun exposure in southeast Asia. Dermato-endocrinology. 2013; 5: 34-7.
- Siddiqee MH, Bhattacharjee B, Siddiqi UR, MeshbahurRahman M. High prevalence of vitamin D deficiency among the South Asian adults: a systematic review and meta-analysis. BMC public health. 2021; 21: 1823.
- Arlappa N, Laxmaiah A, Balakrishna N, Harikumar R, Brahmam GN. Clinical and sub-clinical vitamin A deficiency among rural pre-school children of Maharashtra, India. Annals of human biology. 2008; 35: 606-14.
- Arlappa N, Balakrishna N, Laxmaiah A, Nair KM, Brahmam GNv. Prevalence of clinical and sub-clinical vitamin A deficiency among rural preschool children of West Bengal, India. Indian pediatrics. 2011; 48: 47-9.
- Arlappa N, Balakrishna N, Laxmaiah A, Raghu P, Rao VV, Nair KM, et al. Prevalence of vitamin A deficiency and its determinants among the rural preschool children of Madhya Pradesh, India. Annals of human biology. 2011; 38: 131-6.
- Laxmaiah A, Arlappa N, Balakrishna N, Mallikarjuna Rao K, Galreddy C, Kumar S, et al. Prevalence and determinants of micronutrient deficiencies among rural children of eight states in India. Annals of nutrition & metabolism. 2013; 62: 231-41.
- NIN. Prevalence of Vitamin A deficiency among pre-school children in rural areas. Hyderabad: ICMR-National Institute of Nutrition. 2006.
- Lalmalsawma P. Assessment of vitamin A deficiency among pre-school children in a rural area of Mizoram, North-east India. 2005.
- Shah RA QM, Khan AA. Magnitude of vitamin A deficiency in poor communities of the four selected districts of Punjab using - Rapid Assessment Technique Ann King Edward Med. 2016; 11: 314–8.
- Das R TN, Mondal A. A Prevalence Study of Vitamin A Deficiency Ocular Morbidity among Preschool children in Southern Assam. Int J Community Med Public Heal. 2016; 3: 852–4.
- Godbole M, Kavya NP, Nekar MS, Bant DD. A cross-sectional study to assess prevalence and pattern of ocular morbidity among pre-school children attending anganwadi centres of Hubballi taluk in South India. International Journal Of Community Medicine And Public Health. 2019; 6: 545–9.
- Meshram, II, Neeraja G, Longvah T. Vitamin A Deficiency, Anemia, and Nutritional Status of under 5-Year Children from Northeast India. Indian journal of community medicine: official publication of Indian Association of Preventive & Social Medicine. 2021; 46: 673-9.
- Yadav R, Patial A, Bharti B, Attri SV, Bhatia P. Dietary Vitamin A Intake, Coverage of Vitamin A Megadose Supplementation, and Prevalence of Vitamin A Deficiency among Marginalized Children 6-59 Months in Anganwadis of Chandigarh: A Multistage Cluster Sampling Survey. Indian journal of community medicine: official publication of Indian Association of Preventive & Social Medicine. 2021; 46: 692-6.
- Kalamkar S, Gogate PM, Kaur H, Phadke SP, Shinde A. Prevalence of ocular morbidity in preschool children in Pune, Maharashtra. Journal of Clinical Ophthalmology and Research. 2022; 10.
- Jaleel A, Arlappa N, Ramakrishna KS, Sunu PV, Jayalakshmi G, Neeraja G, et al. Examining the Triple Burden of Malnutrition: Insights from a Community- Based Comprehensive Nutrition Survey among Indigenous Tribal Children (0-19 Years) in the Western Ghats Hills of India. Nutrients. 2023; 15.
- MoHFW U, Population Council. Comprehensive National Nutrition Survey (CNNS) National Report. New Delhi. 2019.
- Venkatesh U, Sharma A, Ananthan VA, Subbiah P, Durga R. Micronutrient’s deficiency in India: a systematic review and meta-analysis. Journal of nutritional science. 2021; 10: e110.
- Basu S, Gupta R, Mitra M, Ghosh A. Prevalence of vitamin d deficiency in a pediatric hospital of eastern India. Indian journal of clinical biochemistry: IJCB. 2015; 30: 167-73.
- Rana G, Abraham RA, Sachdev HS, Nair KM, Kumar GT, Agarwal PK, et al. Prevalence and Correlates of Vitamin D Deficiency Among Children and Adolescents From a Nationally Representative Survey in India. Indian pediatrics. 2023; 60: 202-6.
- Surve S, Begum S, Chauhan S, Khatkhatay MI, Joshi B. Determinants of Vitamin D Deficiency Among Under-five Children in Urban Slums of Mumbai, India. Indian pediatrics. 2021; 58: 888-9.
- Chowdhury R, Taneja S, Bhandari N, Sinha B, Upadhyay RP, Bhan MK, et al. Vitamin-D deficiency predicts infections in young north Indian children: A secondary data analysis. PloS one. 2017; 12: e0170509.
- Chacham S, Rajput S, Gurnurkar S, Mirza A, Saxena V, Dakshinamurthy S, et al. Prevalence of Vitamin D Deficiency Among Infants in Northern India: A Hospital Based Prospective Study. Cureus. 2020; 12: e11353.
- Kundu S, Rai B, Shukla A. Prevalence and determinants of Vitamin A deficiency among children in India: Findings from a national cross-sectional survey. Clinical Epidemiology and Global Health. 2021; 11: 100768.
- Khan MA, Khan MD. Classification of 154 clinical cases of vitamin A deficiency in children (0-15 years) in a tertiary hospital in North West Frontier Province Pakistan. JPMA The Journal of the Pakistan Medical Association. 2005; 55: 77-8.
- Rahman S, Rahman AS, Alam N, Ahmed AS, Ireen S, Chowdhury IA, et al. Vitamin A deficiency and determinants of vitamin A status in Bangladeshi children and women: findings of a national survey. Public Health Nutr. 2017; 20: 1114-25.
- Fahim O, Shahim S, Shams AN, Muhammadi AF, Djazayery A, Esmaillzadeh A. Double burden of malnutrition in Afghanistan: Secondary analysis of a national survey. PloS one. 2023; 18: e0284952.
- Chitekwe S, Parajuli KR, Paudyal N, Haag KC, Renzaho A, Issaka A, et al. Individual, household and national factors associated with iron, vitamin A and zinc deficiencies among children aged 6–59 months in Nepal. Matern Child Nutr. 2022; 18: e13305.
- Laverack G. Maldives Health Profiles. Male, Republic of Maldives: Minisrty of Health. 2016.
- Salim N, Sattar MA, Adnan A. High prevalence of vitamin D deficiency in Pakistan and miscarriages: A hazard to pregnancies. Annals of medicine and surgery. 2022; 82: 104634.
- Siddiqee MH, Bhattacharjee B, Siddiqi UR, Rahman MM. High burden of hypovitaminosis D among the children and adolescents in South Asia: a systematic review and meta–analysis. Journal of health, population, and nutrition. 2022; 41: 10.
- UNICEF. National Nutrition Survey Afghanistan 2013. Afghanistan: UNICEF. 2013.
- Arshad S, Zaidi SJA. Vitamin D levels among children, adolescents, adults, and elders in Pakistani population: a cross–sectional study. BMC public health. 2022; 22: 2040.
- UNICEF. National Nutrition Survey 2018. Pakistan: UNICE and Nutrition Wing Ministry of National Health Services. 2018.
- Moorani KN, Mustufa MA, Hasan SF, Kubar N. Vitamin D status in under five children in diverse communities of Karachi. Pakistan journal of medical sciences. 2019; 35: 414–9.
- Zaman S, Hawlader M, Biswas A, Hasan M, Jahan Ma, Ahsan G. High Prevalence of Vitamin D Deficiency among Bangladeshi Children: An Emerging Public Health Problem. Health & place. 2017; 9: 12.
- Chowdhury F, Shahid ASMSB, Tabassum M, Parvin I, Ghosh PK, Hossain MI, et al. Vitamin D supplementation among Bangladeshi children underfive years of age hospitalised for severe pneumonia: A randomised placebo controlled trial. PloS one. 2021; 16: e0246460.
- Ahmed SS. Vitamin D Deficiency among the Children: A Silent Epidemic in a Selected Rural Area of Bangladesh. Journal of Enam Medical College. 2021; 10: 93–8.
- Das S, Hasan MM, Mohsin M, Jeorge DH, Rasul MG, Khan A–R, et al. Sunlight, dietary habits, genetic polymorphisms and vitamin D deficiency in urban and rural infants of Bangladesh. Scientific reports. 2022; 12: 3623.
- Avagyan D, Neupane SP, Gundersen TE, Madar AA. Vitamin D status in preschool children in rural Nepal. Public Health Nutr. 2016; 19: 470–6.
- Rauniyar LP, Kafle SP, Ahmad E, Koirala N. Vitamin D Status At Admission In Children Admitted To The Pediatric Intensive Care Unit At A Tertiary Care Center In Eastern Nepal. Int J Acad Med Pharm. 2023; 5: 304–308.
- Jagzape T, Khan S. Vitamin–D levels in exclusively breast–fed infants less than six months of age: Do they need supplementation? Sri Lanka Journal of Child Health. 2014.
- Dhillon PK, Narang GS, Arora S, Kukreja S. A hospital based prospective study of vitamin D deficiency in a selected group of apparently healthy children one to five years of age. Sri Lanka Journal of Child Health. 2015.
- Jayawardana P, Liyanage G. Vitamin D level and bone profile among 1– to 5–year–old children in Galle municipality and Bope–Poddala areas in Sri Lanka. SAGE open medicine. 2023; 11: 20503121231195997.
- Dhakal GP, Sharma KP, Bajgai GP, Sharma TR, Bajgai TM, Tenzin J, et al. Vitamin D Status among the Population Visiting Jigme Dorji Wangchuck National Referral Hospital, Bhutan. Indian journal of endocrinology and metabolism. 2023; 27: 436–9.
- Sanjoaquin MA, Molyneux ME. Malaria and vitamin A deficiency in African children: a vicious circle? Malaria journal. 2009; 8: 134.
- West KP Jr. Extent of vitamin A deficiency among preschool children and women of reproductive age. The Journal of nutrition. 2002; 132: 2857s–66s.
- Abrha T, Girma Y, Haile K, Hailu M, Hailemariam M. Prevalence and associated factors of clinical manifestations of vitamin a deficiency among preschool children in asgede–tsimbla rural district, north Ethiopia, a community based cross sectional study. Archives of Public Health. 2016; 74: 4.
- Sahile Z, Yilma D, Tezera R, Bezu T, Haileselassie W, Seifu B, et al. Prevalence of Vitamin A Deficiency among Preschool Children in Ethiopia: A Systematic Review and Meta–Analysis. 2020; 2020: 8032894.
- Maziya–Dixon BB, Akinyele IO, Sanusi RA, Oguntona TE, Nokoe SK, Harris EW. Vitamin A Deficiency Is Prevalent in Children Less Than 5 y of Age in Nigeria12. The Journal of nutrition. 2006; 136: 2255–61.
- Abolurin OO, Adegbola AJ, Oyelami OA, Adegoke SA, Bolaji OO. Vitamin A deficiency among under–five Nigerian children with diarrhoea. African health sciences. 2018; 18: 737–42.
- Amel A. Risk factors for vitamin A deficiency in pre-school children in an eastern Algerian city (Constantine) 2023.
- Alaofè H, Burney J, Naylor R, Taren D. Prevalence of anaemia, deficiencies of iron and vitamin A and their determinants in rural women and young children: a cross-sectional study in Kalalé district of northern Benin. Public Health Nutr. 2017; 20: 1203-13.
- Ghana Uo, GroungWork, Wisconsin-Madison Uo, Trust K-W, UNICEF. Ghana-Micronutrient-Survey-2017. Accra - Ghana: University of Ghana. 2017.
- Aguayo VM, Kahn S, Ismael C, Meershoek S. Vitamin A deficiency and child mortality in Mozambique. Public Health Nutr. 2005; 8: 29-31.
- Engle-Stone R, Haskell MJ, Ndjebayi AO, Nankap M, Erhardt JG, Gimou MM, et al. Plasma retinol-binding protein predicts plasma retinol concentration in both infected and uninfected Cameroonian women and children. The Journal of nutrition. 2011; 141: 2233-41.
- Hotz C, Chileshe J, Siamusantu W, Palaniappan U, Kafwembe E. Vitamin A intake and infection are associated with plasma retinol among pre-school children in rural Zambia. Public Health Nutr. 2012; 15: 1688-96.
- Ssentongo P, Ba DM, Ssentongo AE, Fronterre C, Whalen A, Yang Y, et al. Association of vitamin A deficiency with early childhood stunting in Uganda: A population-based cross-sectional study. PloS one. 2020; 15: e0233615.
- Williams AM, Tanumihardjo SA, Rhodes EC, Mapango C, Kazembe B, Phiri F, et al. Vitamin A deficiency has declined in Malawi, but with evidence of elevated vitamin A in children. The American journal of clinical nutrition. 2021; 113: 854-64.
- WHO. Nutrition country profile: Morocco. 2023.
- Mogire RM, Morovat A, Muriuki JM, Mentzer AJ, Webb EL, Kimita W, et al. Prevalence and predictors of vitamin D deficiency in young African children. BMC medicine. 2021; 19: 115.
- Adegoke SA, Smith OS, Adekile AD, Figueiredo MS. Relationship between serum 25-hydroxyvitamin D and inflammatory cytokines in paediatric sickle cell disease. Cytokine. 2017; 96: 87-93.
- Mogire RM, Mutua A, Kimita W, Kamau A, Bejon P, Pettifor JM, et al. Prevalence of vitamin D deficiency in Africa: a systematic review and metaanalysis. The Lancet Global Health. 2020; 8: e134-e42.
- Shaka MF, kabthymer RH, Meshesha MD, Borde MT. Vitamin D deficiency among apparently healthy children and children with common medical illnesses in Sub-Saharan Africa: A systematic review and meta-analysis. Annals of Medicine and Surgery. 2022; 75: 103403.
- Carboo JA, Malan L, Lombard M, Maleka N, Nienaber A, Dolman-Macleod RC. Predictors of vitamin D status in undernourished and well-nourished children 6–59 months old, in the JB Marks Municipality of South Africa. South African Journal of Clinical Nutrition. 2024; 37: 155-165.
- IIPS I. National Family Health Survey -5. Mumbai: International Institute of Population Sciences. 2021.
- Aguayo VM, Bhattacharjee S, Bhawani L, Badgaiyan N. India’s vitamin A supplementation programme is reaching the most vulnerable districts but not all vulnerable children. New evidence from the seven states with the highest burden of mortality among under-5s. Public Health Nutr. 2015; 18: 42-9.
- WFP. THE PROOF IS IN THE PILOT: 9 INSIGHTS FROM INDIA’S RICE FORTIFICATION PILOT- TO-SCALE APPROACH. Rome, Italy: World Food Programme. 2022.