
Review Article
Austin J Nutr Metab. 2025; 12(1): 1138.
Zinc as a Micronutrient in Cellular Function and Nutritional Balance
Kiran Alluri*
College of Dentistry, University of Nebraska Medical Center, Omaha, Nebraska, 68198, USA
*Corresponding author: Kiran Alluri. Zinc as a Micronutrient in Cellular Function and Nutritional Balance. Austin J Nutr Metab. 2025; 12(1): 1138.
Received: April 26, 2025 Accepted: May 08, 2025 Published: May 13, 2025
Abstract
Zinc performs many physiological effects like cell viability, proliferation, differentiation, bone calcification, insulin packaging, brain development, activation of the immune system, and acts as a cofactor for many enzymes and hormones. Dietary zinc deficiency leads to stunting/growth retardation in humans. In circulation, zinc levels are maintained through its distribution from various tissues. In this regard, macrophages and muscle and bone play an important role.
Cellular and consequently the whole body zinc homeostasis is maintained by coordinated regulation of two major families of zinc metal transporters, ZnTs, and ZIPs with opposite functional activities and an intracellular zinc storage protein metallothionein. ZnTs includes 10 (ZnT1-10) transporters, which decrease cytoplasmic zinc concentrations, and the ZIPs includes 14 ZIPs (1- 14) transporters and increases cytoplasmic zinc concentrations. Fluctuations in the intracellular zinc levels are tightly regulated by these transporter families depending on their localization and in a tissue, cell, and organelle-specific manners. The expression of zinc transporters determines the zinc levels in tissues or cells, which in turn regulate specific gene expression and physiological functions.
It has been proposed that bone may serve as a passive reserve for zinc and may become available during the normal turnover of osseous tissue. This may be due to the differential role of zinc transporters in tissues that contribute to maintaining zinc homeostasis. The overall balance between ZnTs, ZIPs, and MTs might be the reason for zinc assimilation in a specific tissue. Therefore, the present review was carried out to understand the regulation of zinc in maintaining the homeostasis.
Keywords: Dietary zinc; Zinc depletion; Metallothionein; Zinc transporters
Introduction
Zinc is the second most abundant transition metal in the human body after iron. The essentiality of zinc as a trace nutrient was first discovered in the fungus Aspergillus niger in 1869. However, the significance of zinc as a mineral essential for human life was studied half a century ago by Dr. Ananada Siva Prasad [1]. In 1961, he hypothesized that zinc deficiency could account for human growth retardation, and later he established that subjects from Iran and Egypt suffered zinc deficiency, which could be corrected upon zinc supplementation. Severe zinc deficiency in humans leads to alopecia in children and mild to moderate forms of zinc deficiency has been shown to affect physical growth, development, neurological deficits, and impaired immune system and increased susceptibility to diarrhea in children. Zinc supplementation studies have been shown to have health benefits. Zinc deficiency is a serious global public health problem, affecting ~ 2 billion people because of inadequate zinc in the diet [2]. Zinc is involved in linear growth and is been implicated in stunting in children which are a serious nutritional problem in India. According to the National Family Health Survey, 38.4 % of children under the age of 5 years are stunted in the country [3]. Various studies from India have also shown that zinc deficiency exists in the general population [4].
Zinc participates in several metabolic activities and has three major roles in viz., a) catalytic activity b) structural and c) regulatory mechanisms. Zinc transport proteins at the cellular levels are indispensable for the physiology of zinc and are of two types 1) ZnTs and 2) ZIPs. Zinc is the only metal that is part of all enzyme classes. Zinc also functions as an antioxidant metal and therefore has an important role in the inflammatory process. Animal and in vitro studies using human cell lines have given an enormous understanding of the role of cellular zinc transporters along with the storage protein metallothionein (MT) in maintaining zinc homeostasis. Furthermore, genetically inherited diseases such as Ehlers- Danlos, Acrodermatitis, TNZD (Trans neonatal zinc deficiency) diseases or infection, and inflammation conditions have also revealed the importance of zinc transporters in zinc homeostasis. However, there is a wide lacuna existing in our understanding of tissue and cell specific regulation of zinc under conditions prevailing in humans such as zinc depletion (deficiency), repletion (supplementation) and under conditions of inflammation which is essential to develop strategies to prevent and control zinc deficiency. Therefore, the overall aim of the work is to study changes in various exports and import transporters of zinc under conditions of deficiency, supplementation, and inflammation in three human cell lines that have contrasting functions of zinc storage and non-storage in humans. Considering the contrasting functions of these three tissues or cells, this study may provide a lead in identifying an early biomarker that can reflect zinc deficiency much before plasma zinc changes.
The following write up is intended to review the literature on various aspects of zinc metabolism in humans, which will identify knowledge gaps about various zinc transporters. There exists certain uncertainty in the distribution of zinc in various tissues and their role in mobilizing zinc under deficiency and inflammation in establishing homeostasis.
Review of Literature
Metabolism of Zinc
Dietary Sources: Intake of zinc through the diet among different age and gender groups in India ranges from 5.2 to 16.2 mg/day [5]. However, zinc content in the total diet depends on the source of the food items and also food processing methods. It is evident from the table below that red meat is a rich source of zinc, while fruits, roots, and tubers contain fewer quantities of zinc (Table 1). Drinking water or other beverages may contain high levels of zinc if they are stored in metal containers. Dietary Zinc Deficiency: Reduced intake and impaired absorption of zinc due to autosomal recessive inheritable disease acrodermatitis enteropathica, leads to zinc deficiency in humans. As per the International Zinc Nutrition Consultative Group (IZiNCG) guidelines, if >20% of the population has the plasma levels of zinc lower than cut off values <70 μg/dL, the whole population should be considered at risk of zinc deficiency [6]. In India, various studies carried out in 6 states have revealed the prevalence of zinc deficiency ranging from 36.2-73.3% in children, pregnant and nonpregnant women [7-10]. The prevalence of zinc deficiency in the world based on the childhood growth stunting and absorbable zinc in the food is given in (Figure 1).
Type of food
Zinc
Type of food
Zinc
Fruits
0.09-0.4
Egg and egg products
1-1.2
Roots and Tubers
0.1-0.3
Poultry
1.4
Condiments and spices
0.1-0.3
Cereals and millets
2-Jan
Marine fish
0.3
Beef
3
Leafy vegetables
0.5
Meat
4
Fresh water fish
0.6
Nuts and oil seeds
5-Mar
Milk and milk products
0.3 – 2.3
Table 1: Zinc content (mg/100g) in Indian foods.
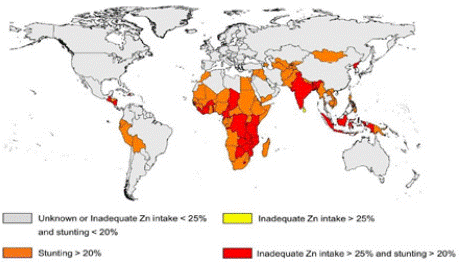
Figure 1: Global prevalence of zinc deficiency based on growth stunting
among children and absorbable zinc in the food
(Source: Wessells et al 2012).
Recommended Dietary Allowances (RDA) for Zinc in Humans: Zinc requirement varies according to age and gender. The requirement is more in growing children, pregnant and lactating women. According to the Institute of Medicine (IOM) the RDA for zinc is 11 mg per day for men and women [12]. The Indian RDA is comparable to the above recommendations (Table 2). These recommendations have been computed based on dietary intake and absorption of zinc in various target groups [5].
Recommended dietary allowance
ICMR-2010
IOM-2001
Man
12
11
Woman
10
8
Pregnant woman
12
8
Boys (13-15 years)
11
11
Girls (13-15 years)
11
9
Table 2: Recommended dietary allowance of zinc (mg/day).
Consequences of Zinc Deficiency
Growth in Children: Growth retardation is the first sign of zinc deficiency condition (Wessells et al 2012). The meta-analysis of studies in developing countries on zinc intervention trails revealed stunted growth in children is due to zinc deficiency [13]. Low levels of maternal zinc decrease the foetal growth, results in low birth weight in new-borns [14]. It was found that growth-promoting response of zinc is due to the coordinate cell signalling mechanism of zinc on the growth-regulating hormone, insulin-like growth factor and insulinlike growth factor-binding protein 3 [15,16].
Development in Children: Zinc levels in mother have propounding impacts on foetus neural development; low levels affect the motor function. Studies of zinc supplementation in school children have improved cognitive behaviour [17]. Further on zinc supplementation has a beneficiary effect on short term memory in children 5-15 years [18].
Pregnancy: In pregnancy, the adverse side effect of zinc deficiency affects both mother and foetus such as placental abruption, prolonged labor, haemorrhage [19]. Zinc supplementation during pregnancy has been shown to reduce about 14%, premature deliveries in lowincome women [20]. The most common pre-eclampsia reported in pregnancy is associated with low levels of zinc [21,22].
Diarrhoea: During zinc deficiency susceptible to infection and diarrhoea increases [23]. Zinc as oral rehydration therapy was recommended by the WHO and UNICEF in diarrheal diseases [24]. Besides, the combination of ORS and zinc in acute and persistent diarrhoea lowered the frequency of hospitalization and also the severity of diarrheal stool output [25, 26]. It has been observed that zinc deficiency might potentiate E. coli to produce toxins for causing diarrhoea [27]. However, the mode of action of zinc in diarrhoea to reduce its severity is not yet understood clearly. It is predicted that Na+ absorption is elevated, whereas Cl- ions secretion outside is inhibited by zinc [28].
HIV/AIDS: Zinc status of an individual has a major impact on HIV/AIDS patients, increased mortality rate, and progression of the disease was noticed with low serum zinc levels in HIV patients [29]. The importance of zinc further delineated with 45mg/day of zinc to AIDS patients for one month decreased the opportunistic infections [30]. On the other hand, increased zinc intake progressed the disease, and mortality rate was increased. Thus, there exists ambiguity of zinc dose and the stage at which zinc has to be supplemented in AIDS patients [31].
Assessment of Zinc Status
Although, there are no sensitive and reliable biochemical markers for identifying the zinc status; currently, plasma zinc, metallothionein (MT), and zinc-dependent enzymes have been used for assessing the zinc status. Given the above knowledge gap, it is necessary to develop an early biomarker for zinc.
Plasma/serum Zinc: Plasma zinc can be measured using Atomic Absorption spectrophotometry or inductively coupled plasma mass spectrometry (ICPMS). Plasma zinc levels below the cut off value 70μg/dL are used for assessing the inadequacy of zinc [6]. However, zinc is a type-II nutrient during zinc starvation condition other tissues such as bone release zinc into the blood and maintain plasma zinc concentration [32]. Moreover, plasma zinc levels vary based on the inflammation, infection, and plasma protein levels (albumin). These confounders make plasma zinc levels an unreliable biomarker for assessing zinc status.
Metallothionein: Metallothionein (MT) is an intracellular zinc storage protein which is ubiquitously expressed in tissues. Studies reported that metallothionein (MT) levels reflect the zinc status, during zinc depletion and repletion in human. RBC metallothionein levels have been shown to correlate with the zinc changes [33]. Similarly, in monocytes also a direct relation of changes to zinc fluctuations in monocytes MT mRNA was reported [34]. The specificity of MT levels is questionable since MT is affected in inflammation and by heavy metals Co, Ag, Cu, and Cd.
Zinc Dependent Enzymes: Many enzymes contain zinc as an essential component, such as carboxy peptidase, alkaline phosphatase, thymidine kinase and have been used as a marker for assessing zinc status [35]. However, none of these enzymes has correlated clearly with zinc levels [36].
Other Markers: Notably, attempts were made to relate hair and skin zinc contents as a possible biomarker. However, all these attempts were failed due to large variations in the distribution of zinc in these organs. Moreover, these tissues are also affected by many other parameters apart from zinc. It has been reviewed that hair zinc concentration has limited application in population surveys [37].
Digestion and Absorption
Dietary zinc in food is present in the bound form complexed with amino acids and nucleic acids. Hydrochloric acid in the stomach acts on zinc- biomolecule complexes and liberate zinc as free ions. Intestinal enzymes, particularly proteases and nucleases, act on proteins and nucleic acid and liberate zinc.
Zinc is absorbed in the lumen of the small intestine and is transported through specific proteins across the cell membrane into the portal circulation through specific transporters. The type of transport and their role in the absorption of zinc in the enterocyte is described elsewhere (Section 1.9.2)
Absorption of free zinc ions liberated from the food in the stomach is affected by dietary ligands that can promote or inhibit zinc absorption. Cysteine, histidine, phytates reduce the absorption of zinc and ligands like tannic acid promote zinc absorption [38]. The relative proportion of these ligands in the lumen will determine the bioavailability of zinc from a habitual diet. Using stable isotopes of zinc, bioavailability (fractional absorption of zinc) of zinc among Indian adolescent boys and girls have been reported to be about 30% from a rice-based diet [39].
The kinetics of zinc uptake in enterocyte and its resolution into individual components can provide insights into the transport process. Studies have shown that zinc uptake is more complex, as it involves three components [40]. Resolution of this triphasic zinc uptake into individual components shows substantial uptake at concentrations below Km (1) paracellular uptake of about ~30%, (2) mediated uptake and (3) in maintaining steady- state concentrations of cellular zinc by efflux of excess zinc from the cell in human enterocyte Caco-2 cell line.
Among the polyphenols, tannic acid, and components of red grape juice have been shown to increase the zinc uptake in Caco-2 cells [41]. It is not known how the zinc-polyphenols complex enters into enterocytes and suggested non- involvement of specific zinc transporters similar to amino acid zinc complexes [42]. Studies have shown that tannic acid and quercetin significantly enhance zinc uptake and induce metallothionein synthesis, whereas phytates, reduce the zinc absorption by forming indigestible complexes with zinc [41,43].
Circulation, Distribution and Excretion of Zinc
Circulation: The hepatic portal system transfers the absorbed zinc into the liver and distributed to different organs. Zinc in the circulation (plasma zinc ~ 0.1 %) is delivered to tissues in the bound form to albumin (80%) and lesser extent to a2-macroglobulin (20%) [44,45]. Furthermore, zinc (70-80%) in RBCs is bound to the carbonic anhydrase and acts as a source for zinc [46].
Zinc binding site in albumin is located at the domain interface between domains I and II, as shown in (Figure 2). These domains are two amino acids His67 (H), and Asn99 (N) in domain I, and His247 (H) and Asp249 (D) in domain II and a water molecule is included as a fifth ligand [47].
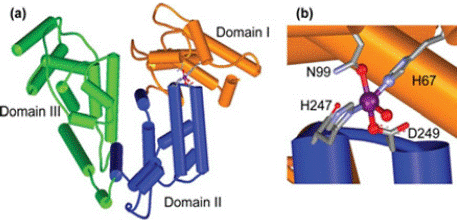
Figure 2: Depiction of zinc binding site on albumin. A) Zinc binding site at the
interface of domains I (orange) and II (blue). B) zinc (purple) site composed
of (H67 and D 249) in domain I, (H247 and D249) in domain II and a fifth
exogenous (water) ligand (Source: Lu et al 2008).
Distribution: Zinc is absorbed and transported from small intestine to the liver by a hepatic portal vein from here it is redistributed to the other tissues. Tissues such as muscle (~60%) and bone (~30%) are the major reservoirs of zinc while skin and liver (~5%) hold relatively fewer concentrations of zinc (Figure 3). Although pancreatic Β- cells contain high levels of zinc compared to other soft tissues, they are not the prominent reservoirs of zinc as muscle and bone [48].
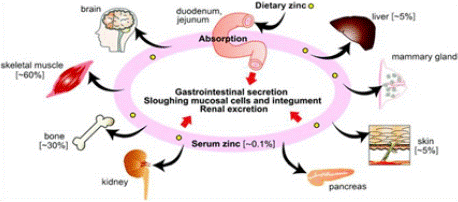
Figure 3: Tissue distribution of zinc in the human body
(Source: Golan et al 2017).
Excretion: Zinc is excreted primarily through the gut; unabsorbed zinc is eliminated through faeces. Besides, bile, pancreatic, and intestinal secretions in the lumen along with mucosal sloughing of the gut also contribute to excrete zinc [49]. The renal system regulates zinc loss through urine as per the dietary zinc intakes [50]. The total daily excretion of zinc is estimated to be around 0.63 mg/day [51].
Biochemical Functions of Zinc
The three important biochemical functions of zinc are (1) catalytic (2) structural and (3) regulatory are presented in (Table 1.3). Zinc has certain special physic-chemical characteristics that enable it to function uniquely. It belongs to the ‘’d’’ block of the periodic table with electrons completely occupying d and s shells. The natural redox state for zinc is Zn2+ and it does not easily undergo oxidation or reduction processes. The chemical features that allow zinc to participate in structural and catalytic roles are as follows:
Role
Biological Process
Function of Zinc
Reference
Catalytic and Structural
DNA and protein synthesis
Zinc is a cofactor for many enzymes including DNA polymerase, RNA polymerase, and reverse transcriptase
[56]
Regulatory
Gene Expression
MTF-1 a zinc responsive transcription factor regulates the number of genes
[57]
Inflammation
IL-6 upregulates expression of zinc importer, ZIP14 in hepatocytes which leads to the accumulation of zinc bound to metallothionein in the liver
[58]
Immunity
Zinc deficiency leads to reprogramming of the immune system
[59]
Catalytic and structural
Enzymes
Zinc acts as a cofactor for all the six classes of enzyme
[60]
Signalling
Zinc regulates the release of neurotransmitter
[61]
Table 3: Examples of biochemical functions of zinc.
• Lewis acid which can accept an electron.
• Chemical tendency to form multiple geometries. Zinc can bind with 2 to 8 ligands.
Structural Role: Many enzymes require zinc as a structural component. The enzyme copper-zinc dismutase (Cu, Zn SOD) is the most prominent zinc-containing enzyme. In this enzyme, zinc stabilizes the protein while copper provides catalytic activity [53]. The detailed structure analysis revealed that the zinc is bound to the protein through the amino acids His-61, His-69, His-78, and Asp-81. Whereas copper binds to the four histidine residues His-44, His-46, His- 61 and His-118, and a water molecule. The imidazole of the His-61 bridges the two metal ions and are separated by 6.3 A° away from each other in (Figure 4). Zinc has an important role in the fast protonation of nitrogen of His-61, which disconnects cuprous ion upon reduction [54] as shown in (Figure 5).
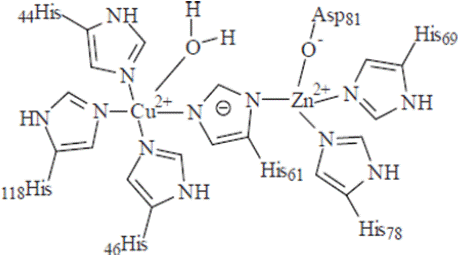
Figure 4: Active site configuration of Cu, Zn-SOD active configuration
(Source: Labadi et al 2009).
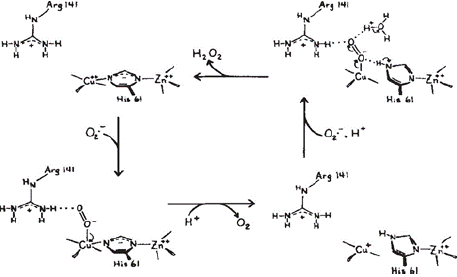
Figure 5: Catalytic mechanism of Cu, Zn SOD (Source: Tainer et al 1983).
Catalytic Role: Carbonic anhydrase, alkaline phosphatase, Ca- ATPase, 5’nucleotidase, and carboxypeptidase enzymes depend on zinc for their catalytic activity. During catalysis of these enzymes, amino acids such as histidine, glutamic acid, and threonine present at the active site form tetra dentate co-ordination with zinc and function as a Lewis acid. One of the best examples of such a process is the hydration of CO2 to H2CO3 by carbonic anhydrase, as shown in (Figure 6). The detailed structure analysis revealed that the HCAII (human carbonic anhydrase) enzyme is a functional 29-kDa monomer consisting of a 10-stranded, twisted Β-sheet. The active site is located at the bottom of a 15-Å cone-shaped cavity that leads to the center of the protein. The active site includes a zinc ion coordinated tetrahedrally by 3 histidine residues (His-94, His-96, and His-119) and a water molecule/hydroxide ion (Wat-263) as a fourth ligand [55]. Regulatory Role: Zinc regulates the gene expression at the transcriptional level of various cellular activities such as DNA synthesis, mitosis, cell division and protein synthesis mediated via zinc finger proteins or zinc finger motifs [56, 62]. MTF-1 (metal transcription factor) is an important zinc finger protein involved during heavy metal stress and activates the transcription of metallothionein, which is a zinc-responsive protein [63]. MTF-1 has a critical role in regulating genes; any abnormal function in MTF-1 leads to catastrophic effects. MTF-1 malfunction in null mutation leads to a lethal effect on foetal development [64]. However, the total number of genes that are under the regulation of MTF-1 is not yet deciphered.
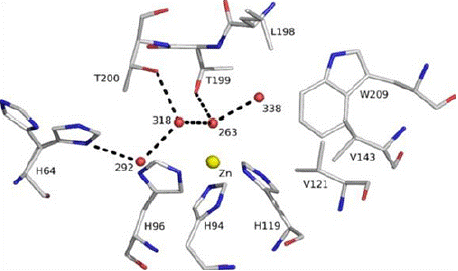
Figure 6: The active site of human carbonic anhydrase, The zinc ion is
tetrahedrally coordinated by His-94, His-96, and His-119 and catalytic water
(Wat-263). The deep water (Wat-338) sits in a hydrophobic pocket lined by
Leu-198, Trp-209, Val-143, and Val-121 at the bottom of the active site. Wat-
318 is in a hydrophilic environment toward the mouth of the active site. The
proton shuttle His-64, shown in both “in” and “out” positions, is linked via
Wat-292 and Wat-318 to the catalytic water. Hydrogen bonds are depicted as
dotted lines, and waters are labelled with numbers only. (Source: Sjöblomet
al 2009).
Zinc Finger Motifs: Zinc in several proteins co-ordinate and chelate through cysteine, histidine residues. This stabilizes the protein structure by the formation of zinc finger-like structures known as zinc finger motifs [65]. Several different structural families are known, and they typically function as interaction modules that bind to DNA, RNA, proteins, or small molecules which are involved in the cell growth and differentiation (Figure 7).
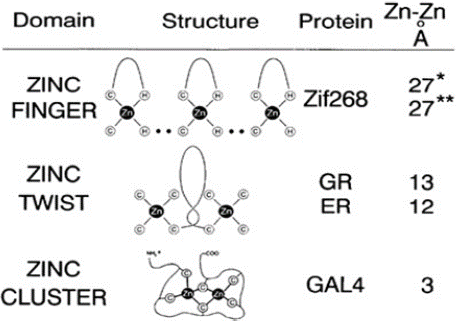
Figure 7: Zinc binding sites in gene regulatory proteins. In the zinc finger
type (MTF-1), zinc is coordinated to 2 Cys and 2 His residues (Structure has
been defined using a 3-finger construct Zif268). In zinc twist type (estrogen
or steroid hormone receptors) zinc is co- ordinated to 4 cysteine residues.
(Source: Vallee et al 1993).al 2009).
Transcription factor-IIIA (TF-IIIA) is the first transcription factor containing zinc and identified as zinc finger type regulates the gene expression by binding to DNA. In steroid hormone receptors such as estrogen and glucocorticoid receptors has a zinc twist type of zinc finger motif have been described while in testosterone, and vitamin D zinc has a structural role [46, 66]. In protein (insulin) also zinc coordinates with histidine and or cysteine residues for proper hexameric conformation of the protein [67]. Similar, kind of structural motifs exist in viruses also the best notable example is the nucleocapsid protein of HIV [66].
Cell Signalling: Zinc plays an important role in cell signalling mechanisms, in neurons zinc act as signalling ion and reduces the activity of N-methyl-D-aspartate (NMDA) receptors while increasing the activity of gamma-aminobutyric acid (GABA) receptors [68,69]. Zinc can act as an insulin-mimetic signalling molecule and elevates the glucose uptake [70,71]. Similarly, earlier, it was reported that treatment of 3T3-L1 adipocytes with Zn elevated glucose uptake and lipogenesis, by stimulating the tyrosine phosphorylation of insulin receptor [72]. In diabetic mice, zinc deficiency enhances the hepatic injury by the degradation of NrF2, while zinc maintains NrF2 levels, via the Akt activation which prevents the Fyn transportation of NrF2 into the cytoplasm for degradation [73]. Zinc inhibits protein tyrosine phosphatase 1B (PTP1B), a cytoplasmic phosphatase that interacts with the insulin receptor and catalyzes its dephosphorylation resulting in the attenuation of insulin signalling [74]. Thus, zinc supplementation to diabetes can be considered for further studies to determine the possibilities of generating a very rational therapy.
Cellular and Sub Cellular Distribution of Zinc
The cellular and subcellular zinc homeostasis is maintained through zinc uptake, distribution, storage, and efflux (Figure 8). Normally zinc levels in the cells vary from picomolar to micromolar depending on the physiological needs of the tissue. In neurons and HT-29 cells, zinc concentration is 396μM and 264μM, respectively [75,76] and in mammalian oocytes at the time of maturation zinc levels increases to zillions of atoms [77].
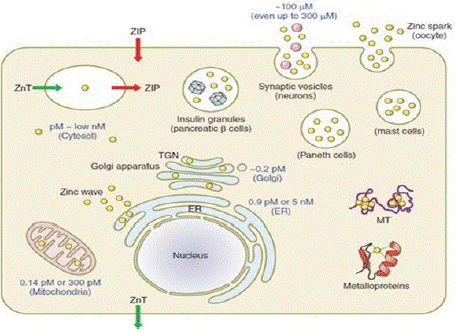
Figure 8: Schematic representation of cellular and sub cellular zinc
distribution and levels (Source: Kambe et al 2015).
At subcellular level mitochondria contains a low amount of zinc (picomolar range), endoplasmic reticulum contains a maximum of 5 nM while the golgi complex contains the lowest zinc concentration (~0.2pM). All these processes of zinc trafficking across the cells are achieved by the zinc-specific transporters along with co-ordinated regulation of MT, a zinc-binding protein. Collectively, these proteins function together and regulate the intracellular zinc levels between the cytoplasm and cellular organelles known as “buffering” and “muffling” of zinc [78,79].
Cellular Regulation of Zinc Metabolism
Metallothionein: Mammalian metallothionein (MT) is an intracellular zinc-binding protein, and it is a small ~7 kDa cellular protein with a high (~30%) content of cysteine residues and are present in four major isoforms (MT1–MT4) which are tissue-specific in expression (Table 4) [79].
Isoform
Tissue
MT-I and MT-II
Ubiquitously expressed majorly on
liver and kidneyMT-III
Neurons
MT-IV
Skin and G.I.T
Table 4: Isoforms of MT expression.
Metallothionein (MT), regulates the zinc absorption and excretion to zinc supplementation and in inflammation [81]. In addition, dietary zinc restriction results in diminished MT synthesis [82]. Structural studies of unsaturated MT have proven to be a challenge, mainly due to the unfolded and highly dynamic structure.
To date, only one X-ray structure of metallothionein (Figure 1.9 A) has been solved: hepatic rat MT-II. Metallothionein folds into two separate domains with two Zn–S clusters, one with 3 Zn(II) and 9 Cys (Β-cluster) and the other with 4 Zn(II) and 11 Cys (a-cluster). In both domains, metal ions are present in tetrahedral geometry and tetrathiolate environments, where sulfur donors have bound to one or two (bridging donors) metal ions. Here, the X-ray structure is based on Cd(II)-induced in vivo protein, which has one Cd(II) ion in the Β- domain at position IV and four ions in all positions (I, V, VI, and VII) in the a-domain (Figure 9B). MT reconstituted in vitro also binds seven Zn (II) or seven Cd(II) ions. Most of the current knowledge regarding the structural, biophysical and even biochemical properties of MTs is based on cadmium MTs, which are more convenient to study due to their better spectroscopic properties, less dynamic nature or higher protein affinity, in comparison with a zinc counterpart [83].
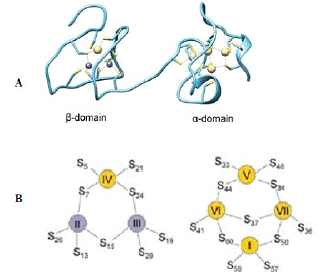
Figure 9: Crystal and cluster organization of hepatic rat MT2. (A) Crystal
structure of Zn2Cd5MTisolated from rat liver (B) Schematic representation
of β and a metallothionein clusters in MT2. Yellow and grey refer to Cd and
zinc found in the crystal structure. Metal ion numbering corresponds to the
order of Cd-NMR chemical shifts of Cd7MT2. (Source: Drozd et al 2018).
Cellular and Sub Cellular Zinc Transporters
Two major families of zinc transporters at cellular and sub-cellular levels belong to the cation diffusion facilitators (CDF), also known as the SLC30 family. These include 10 ZnTs (1-10) involved in exporting zinc and 14 Zrt Irt-like Proteins ZIPs (1-14), known as the SLC39 family involved in importing zinc. Both of these proteins are tissuespecific in expression and have a distinct response to zinc intakes and also for hormones and cytokines [85]. A review of zinc efflux transporters and their tissue specificity subcellular localization and methodology based for their identification is given in (Table 5). Here, ZnTs are the effluxers which decrease the cellular zinc levels based on their localization. Among ZnTs, ZnT-1 has ubiquitously expressed protein which has been localized in the basolateral membrane in enterocytes and renal tubular cells; zinc intake elevated ZnT-1 protein in rats [86]. Hence these observations on zinc intakes might lead to a current consensus that ZnT-1 functions mainly as a zinc exporter in zinc homeostasis under conditions of excess of zinc.
Protein
Cellular location
Subcellular localization
Technique used for identification
ZnT-1
Ubiquitous
Plasma membrane
[102]
ZnT-2
Mammary gland, pancreas, prostate, retina, intestine, kidney
Endosome, lysosome, secretory vesicle, plasma membrane
[103]
ZnT-3
Brain, pancreas testis
Synaptic vesicles
[104]
ZnT-4
Mammary gland, placenta, prostate, kidney, brain
Endosome, secretory vesicle, plasma membrane
[105]
ZnT-5
Heart, prostate, ovary, testis, bone
Golgi, vesicles, plasma membrane
[106]
ZnT-6
Brain, lung, intestine
Golgi vesicles
[107]
ZnT-7
Intestine, stomach, pancreas, muscle
Golgi vesicles
[108]
ZnT-8
Pancreas, adrenal gland, thyroid
Secretory granules
[109]
ZnT-9
Brain, muscle, kidney
Endoplasmic reticulum
[110]
ZnT-10
Brain, retina, liver
Golgi, plasma membrane
[111]
Table 5: Tissue and sub cellular expression and localization of zinc efflux transporters.
Similarly, another set of zinc transporters ZIPs are the zinc influxers increase the zinc levels depending on their site of localization. A review of zinc efflux transporters their tissue specificity subcellular localization and methodology based for their identification is given in (Table 6). Importantly, ZIP-4 was well studied of all the ZIPs, major transporter involved in the absorption of zinc at the intestinal region to dietary zinc status. Consequently, mutations in ZIP-4 levels leads to low absorption of zinc at the intestine [87]. ZIP-4 is the main transporter involved in the absorption of zinc at the intestine [88] whereas ZIP-5 located at the basolateral membrane of enterocyte involved in the regulation of zinc excretion [89]. An isoform of ZnT- 5, ZnT-5 B regulates the zinc absorption and excretion at the apical membrane of enterocyte in either way (exit and entry) of zinc [90]. A schematic representation of zinc absorption at the enterocyte is shown in (Figure 10).
Protein
Cell location
Subcellular localization
Technique used for identification
ZIP-1
Prostate, small intestine, kidney, liver, pancreatic a cells
Plasma membrane
[112]
ZIP-2
Epithelial cells, ovary, liver, skin, uterine, prostate
Plasma membrane
[113]
ZIP-3
Testes, Pancreatic cells
Plasma membrane
[114]
ZIP-4
Small intestine, stomach, colon, cecum, kidney, pancreatic β cells
Plasma membrane
[115]
ZIP-5
Liver, kidney, spleen, colon, pancreas
Plasma membrane
[116]
ZIP-6
Testis, pancreatic β cells
Plasma membrane
[117]
ZIP-7
Brain, liver, pancreatic β cells
Golgi, ER
[118]
ZIP-8
Pancreas, placenta, lung, liver, testis, thymus, RBC.
Plasma membrane endosomes, Lysosome
[119]
ZIP-9
Prostate
Golgi
[120]
ZIP-10
Testis, kidney, breast, pancreatic a cells, RBCs.
Plasma membrane
[121]
ZIP-11
Mammary gland, testis, stomach, ileum, and cecum
Golgi
[122]
ZIP-12
Neurons, endothelial, smooth muscle, and interstitial cells
Unknown
[93]
ZIP-13
Retinal pigment, epithelial cell line, osteoblasts
Endoplasmic reticulum
[123]
ZIP-14
Liver, heart, placenta, lung, brain, pancreatic a cells.
Plasma membrane, mitochondria, Lysosome
[124]
Table 6: Tissue and sub cellular expression and localization of zinc influx transporters.
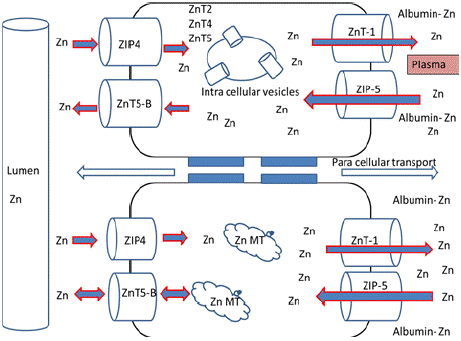
Figure 10: Zinc absorption at the enterocyte; Absorbed zinc is effluxed into
circulation by ZnT-1 at the basolateral side while ZIP-5 eliminates the excess
zinc in the circulation effluxing into lumen via ZIP-5 and ZnT-5B axis.
Studies using human enterocyte Caco-2 cell line have shown that ZIP-4 transporter is most responsive to cellular zinc status, while efflux could be through the ZnTs (SLC30) family of efflux transporters, most plausibly ZnT-1 and also zinc may be transported through paracellular route [40]. Thus, absorbed zinc functions either bound to MT or exist as labile zinc. Since zinc is present in all these diverse forms, which makes it difficult to assess accurately the intracellular distribution status of zinc. Earlier studies on human lung adenocarcinoma cells, reported the compartmentalization of total intracellular zinc in the cytoplasm and nucleus [91]. Fluctuations in intracellular zinc levels regulated by these transporter families or any changes in zinc transporter localization and function results in zinc dyshomeostasis, which affects signalling pathways involved in cell proliferation, differentiation, and death.
Zinc transporters deregulation leads to catastrophic effects on B cell, T cells maturation, and activation via cytokines IL-2, IL-6, IL- 10 [59]. Cytokines such as IL-6, IL-10, IL-12, IL-15, GM-CSF, and interferons (IFNs), activate Janus kinases (JAKs) and signal transducer and activator of transcription (STATs) factors to exert their effects [93]. However, zinc inhibits the IL-6 activation of STATs. Further on, ZIP-12 involvement in cell signalling mechanism was observed in the central nervous system (CNS) via activation of CREB cAMP response element binding protein [93].
Similarly, ZIP-7 has a key role in glucose metabolism in skeletal muscle, and ZnT-7 knockout mouse have shown suppression of insulin signalling pathway in myocytes [94,95]. In addition, ZnT-3 null mice have decreased insulin gene expression and insulin secretion that resulted in hyperglycemia [96]. Moreover, ZnT-8 plays a critical role in the synthesis and secretion of insulin and therefore represents a pharmacological target for treating disorders of insulin secretion, including diabetes [97]. Thus, zinc transporters completely regulate the cellular and total body zinc levels for maintaining physiological functions. It was found that infection and inflammation also affect the ZIPs expression, of interest ZIP-14 levels, were upregulated in inflammation condition leading to high levels of zinc [98].
Similarly, studies on lipopolysaccharide (LPS) mediated inflammation has shown that zinc transporter ZIP-8 modulate the zinc levels [99,100]. A schematic representation of zinc transporters localization is represented in (Figure 11) 3.10. Functional Role of Zinc in Monocytes, Muscle and Bone
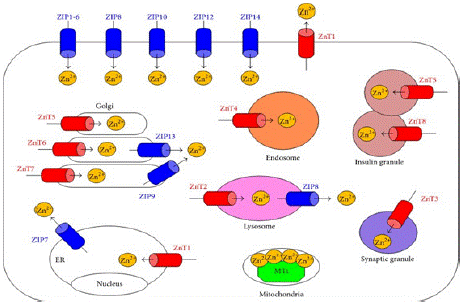
Figure 11: Generalized schematic representation of localization of zinc
transporters in cellular and sub cellular organells (source: Hojyo et al 2016).
Monocytes: Monocytes differentiate into macrophage clears the pathogens or foreign substances from the body by phagocytosis. In addition, these cells activate other immune cells by secreting cytokines. Lipopolysaccharide (LPS) is a gram-negative bacterial cell wall component, binds to the TLR-4 receptor leads to activation of signalling pathway thereby secretion of proinflammatory cytokines IL-1, IL-6 and tumour necrosis factor [125]. Zinc deficiency in monocytes hampers the production of TNF-a and IL-6 cytokines [126]. However, zinc acts as an anti-inflammatory reduce the cytokines levels. It has been shown that zinc influences more than 1000 genes in THP-1 monocytes, which are involved in signal transduction and cytokine production [127].
Muscle: Zinc regulates skeletal muscle contraction and relaxation. It was found that zinc potentiates the twitch tension in sartorius muscle in frogs [128]. In addition, zinc in the small concentrations of 0.001 mM improved the contractility of the glycerinated fiber, while in a high concentration such as 0.25 mM reduced the contractility [129-131]. Thus, zinc levels affect the functional properties of muscle. Zinc has propounding impacts on athletes. Usually, their diet mostly consists of carbohydrate-rich and less of proteins and fats, which leads to a decrease in dietary zinc levels [132].
Bone: Bone remodeling, i.e. formation and absorption of bone, is under precise regulation; osteoblasts deposit calcified bone matrix, and osteoclasts absorb it. Zinc is a potent inhibitor of osteoclastogenesis and tightly regulates the osteoclast function [133]. Any change or abnormal activities of osteoclasts leads to bone deformities, one of the best examples is osteoporosis, in which bone resorption takes place majorly. While bone is the prominent reservoir of zinc, its significant role in zinc homeostasis has been described in many studies [134,135].
Tissue Response During Zinc Depletion and Repletion
Zinc, the most abundant intracellular element, is found in all body tissues, with 85% of the whole body zinc in muscle and bone (Table 7) and remaining minor quantities in the skin and liver, pancreas. Prolonged intake of low dietary zinc changes the homeostatic mechanism of zinc, which no longer maintains the lost zinc, and a negative zinc balance occurs. Earlier observations [136] on low zinc diet, i.e. 0.06 μmol/g leads to a decrease in the whole body zinc content compared to control animals. During this period of zinc depletion, the loss of zinc is not uniform in hair, skin, heart and skeletal muscle zinc concentrations remained constant, whereas plasma, liver, bone and testes zinc concentrations dropped significantly.
Tissue (μmol g-1dry wt)
Normal
Depleted
Repleted
Liver
1.73 ± 0.05
1.0±0.07
1.63±0.05
Bone
3.54±0.23
1.28±0.16
2.14±0.10
Testes
2.32±0.32
1.09±0.14
2.64±0.24
Hair
1.63±0.16
1.59±0.27
1.61±0.32
Skin
0.69±0.08
0.84±0.03
0.73±0.07
Heart
0.77±0.02
0.73±0.03
0.79±0.04
Skeletal muscle soleus
4.55±0.61
4.14±0.37
3.84±0.19
EDL (Extensor Digitorum longus)
0.73±0.05
0.79±0.04
0.77±0.04
AT (Anterior tibialis)
0.64±0.10
0.56±0.12
0.57±0.13
Table 7: Effect of zinc repletion and depletion on tissue concentration of zinc in rats.
Studies showed that bone releases zinc which composes 10–20% primarily from a rapidly turning over pool whereas the second major pool turns over more slowly and does not release zinc in required conditions [137]. Furthermore, bone accumulates zinc in zinc excess situations and releases zinc in depletion conditions [135]. Therefore, when dietary intakes of zinc are low bone seems to function, as a reserve of zinc by releasing zinc.
Tissue Response of Zinc Under Inflammation: Zinc levels in serum also change during infection and inflammation, redistribution of kidney zinc levels in association with increased MT expression was reported in coxsackievirus Type B infection [138]. During inflammation, the liver sequesters zinc, perhaps as an adaptive response to limit zinc bioavailability to pathogenic microbes [139, 140]. Zinc levels in inflammation were regulated by cytokines (IL-1a) to tissue- specific zinc distribution into the liver, bone marrow, and bone, skin, intestine [141].
Rationale and Key Research Questions
Zinc is a typical type-II nutrient as there is no onset of clinical symptoms of deficiency, but growth retardation can be observed [142]. A transient mild zinc deficiency may not alter plasma zinc concentration (12-16μM), whereas persistent deficiency results in decreased plasma Zn levels [143]. In addition, bone and liver also contribute to plasma zinc during zinc depletion [32]. Moreover, plasma levels of zinc vary in pathological conditions [144]. Some of the important studies in these directions are highlighted below.
Zinc supplementation and deficiency have been shown to influence the metabolic functions in monocytes a non-storage tissue, bone, and muscle storage tissues [127,145,146]. Contrasting effects of changes in tissue concentration of zinc have been shown during zinc repletion-depletion in rats. It is interesting to note that bone depletes zinc while muscle retains it during depletion. This contrasting observation in the two major storage tissues of zinc appears to play an important role in zinc homeostasis, especially in maintaining plasma zinc levels during deficiency. A schematic model of depletion of zinc from these two zinc storage organ and plasma concentration is given in (Figure 12). Thus, the contribution of monocytes, muscle, and bone in maintaining zinc homeostasis is still not yet established, and there is scope for studying the role of these tissues in isolation or in tandem in maintaining zinc homeostasis.
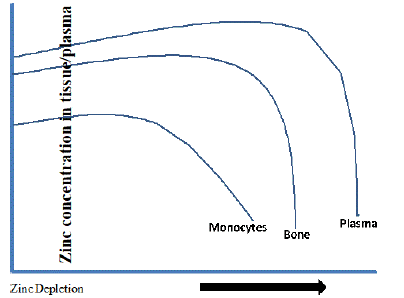
Figure 12: Directional change in plasma/tissue zinc levels to zinc depletion.
During zinc depletion, the loss of zinc is not uniform across all tissues. Zinc levels in skeletal muscle, skin, and heart are maintained to normal levels, while in bone, monocytes, liver, testes, and plasma zinc levels are declined [136]. Bone can apparently accumulate zinc in times of excess and release zinc during depletion [135]. Hence, it has been proposed that bone may serve as a passive reservoir for zinc and may become available during the normal turnover of osseous tissue. This may be due to the differential role of zinc transporters in tissues that contribute to maintaining zinc homeostasis. However, zinc released from bone comes primarily from a rapidly turning over pool that composes 10–20% of the total bone zinc [147]. In vitro studies on mouse pre- osteoblasts cells showed the importance of zinc transporters such as ZnT-2 on bone mineralization [148]. Notably, ZnT-5 knock out studies reported crucial role in osteopenia [149]. Previous reports on PBMCs, osteoblasts, cancerous tissues, and THP- 1 monocyte cell lines have widened our knowledge on whole body zinc regulation. However, there is a wide lacuna in our understanding of the mechanism of zinc homeostasis. Zinc being a type-II nutrient, during zinc deficiency clinical symptoms appear only with severe zinc depletion indicating that there are no conventional zinc reservoirs as like type-I nutrient iron [150]. Unlike iron metabolism, where hepcidin plays a critical role in systemic iron homeostasis [151], biological regulators of zinc homeostasis have not been identified. A low-molecular-weight (2 kDa) zinc-regulated humoral factor, which is induced in zinc deficiency, associated with immune functions and development in smooth muscle cells is reported [152].
The present review was aimed to understand the contribution of zinc storage and non-storage contrasting tissues during zinc sufficiency, depletion, and in inflammation. This will be addressed using suitable human cell lines representing these tissues and experimental system to mimic conditions of zinc repletion, deficiency and in inflammatory conditions in humans. Here, known zinc export and import transporters, ZIPs and ZnTs, were quantified using qRTPCR. The results of these experiments may lead to the identification of a possible biomarker for zinc.
Future Research Questions
1. Can there be a difference in the expression levels of zinc transporters in zinc storage (bone, muscle) and non-storage (monocyte) cell lines?
2. Can there be a difference in the expression levels of zinc transporters when cells are subjected to depletion and repletion in storage and non-storage cell lines?
3. Can there be a difference in the expression levels of zinc transporters in zinc storage and non-storage cell lines to inflammation?
Funding
The author acknowledges Council Scientific Industrial Research (CSIR)-New Delhi, India for the financial assistance to Mr. Kiran Alluri as JRF and SRF (09/484/(0050)/2012-EMR-1).
Orcid id: 0009-0007-1567-3434.
References
- Prasad AS, Halsted JA, Nadimi M. Syndrome of iron deficiency anemia, hepatosplenomegaly, hypogonadism, dwarfism and geophagia. The American journal of medicine. 1961; 31: 532-546.
- Bafaro E, Liu Y, Xu Y, Dempski RE. The emerging role of zinc transporters in cellular homeostasis and cancer. Signal transduction and targeted therapy. 2017; 2: 17029.
- NFHS-4-National Family Health Survey 2015-16: International Institute for Population Sciences, India, 2017.
- Menon KC, Skeaff SA, Thomson CD, Gray AR, Ferguson EL, Zodpey S, et al. Concurrent micronutrient deficiencies are prevalent in nonpregnant rural and tribal women from central India. Nutrition. 2011; 27: 496-502.
- ICMR-Nutrient Requirements & Recommended Dietary Allowances for Indians A Report of the Expert Working Group of the: National Institute of Nutrition; 2010.
- Wessells KR, Brown KH. Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PloS one. 2012; 7: e50568.
- Kapil U, Pathak P, Singh P, Singh C. Zinc and magnesium nutrition amongst pregnant mothers of urban slum communities in Delhi: a pilot study. Indian pediatrics. 2002; 39: 365-368.
- Pathak P, Kapil U, Kapoor SK, Dwivedi SN, Singh R. Magnitude of zinc deficiency among nulliparous nonpregnant women in a rural community of Haryana State, India. Food and Nutrition Bulletein. 2003: 24: 368-371.
- Dhingra U, Hiremath G, Menon VP, Dhingra P, Sarkar A, Sazawal S. Zinc deficiency: descriptive epidemiology and morbidity among preschool children in peri-urban population in Delhi, India. Journal of health, population, and nutrition. 2009; 27: 632.
- Kapil U, Jain K. Magnitude of zinc deficiency amongst under five children in India. The Indian Journal of Pediatrics. 2011; 78: 1069-1072.
- Longvah T, Anantan I, Bhaskarachary K, Venkaiah K. Indian food composition tables. Hyderabad: National Institute of Nutrition, Indian Council of Medical Research; 2017.
- IOM - Institute of medicine, Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc.
- Hambidge M. Human zinc deficiency. The Journal of nutrition. 2000; 130: 1344S-1349S.
- Wang H, Hu YF, Hao JH, Chen YH, Su PY, Wang Y, et al. Maternal zinc deficiency during pregnancy elevates the risks of fetal growth restriction: a population-based birth cohort study. Scientific reports. 2015; 5: 11262.
- MacDonald RS. The role of zinc in growth and cell proliferation. The Journal of nutrition. 2000; 130: 1500S-1508S.
- Alves CX, Vale SH, Dantas MM, Maia AA, Franca MC, Marchini JS, et al. Positive effects of zinc supplementation on growth, GH, IGF1, and IGFBP3 in eutrophic children. Journal of Pediatric Endocrinology and Metabolism. 2012; 25: 881-887.
- De Moura JE, de Moura EN, Alves CX, de Lima Vale SH, Dantas MM, de Araújo Silva A, et al. Oral zinc supplementation may improve cognitive function in schoolchildren. Biological trace element research. 2013; 155: 23- 28.
- Khor GL, Misra S. Micronutrient interventions on cognitive performance of children aged 5-15 years in developing countries. Asia Pacific journal of clinical nutrition. 2012; 21: 476-486.
- Shah D, Sachdev HP. Zinc deficiency in pregnancy and fetal outcome. Nutrition reviews. 2006; 64: 15-30.
- Mahomed K, Bhutta ZA, Middleton P. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database of Systematic Reviews. 2007.
- Akhtar S, Begum S, Ferdousi S. Calcium and zinc deficiency in preeclamptic women. Journal of Bangladesh Society of Physiologist. 2011; 6: 94-99.
- Ma Y, Shen X, Zhang D. The relationship between serum zinc level and preeclampsia: a meta-analysis. Nutrients. 2015; 7: 7806-7820.
- Penny ME, Marin RM, Duran A, Peerson JM, Lanata CF, Lönnerdal B, et al. Randomized controlled trial of the effect of daily supplementation with zinc or multiple micronutrients on the morbidity, growth, and micronutrient status of young Peruvian children. The American journal of clinical nutrition. 2004; 79: 457-465.
- WHO/UNICEF JOINT STATEMENT, Clinical management of acute diarrhoea. 2004.
- Aggarwal R, Sentz J, Miller MA. Role of zinc administration in prevention of childhood diarrhea and respiratory illnesses: a meta-analysis. Pediatrics. 2007; 119: 1120-1130.
- Scrimgeour AG, Lukaski HC. Zinc and diarrheal disease: current status and future perspectives. Current Opinion in Clinical Nutrition & Metabolic Care. 2008; 11: 711-717.
- Wapnir RA. Zinc deficiency, malnutrition and the gastrointestinal tract. The Journal of nutrition. 2000; 130: 1388S-1392S.
- Hoque KM, Sarker R, Guggino SE, Tse CM. A new insight into pathophysiological mechanisms of zinc in diarrhea. Annals of the New York Academy of Sciences. 2009; 1165: 279.
- Lai H, Lai S, Shor-Posner G, Ma F, Trapido E, Baum MK. Plasma zinc, copper, copper: zinc ratio, and survival in a cohort of HIV-1-infected homosexual men. Journal of acquired immune deficiency syndromes. J Acquir Immune Defic Syndr. 2001; 27: 56-62.
- Mocchegiani E, Muzzioli M. Therapeutic application of zinc in human immunodeficiency virus against opportunistic infections. The Journal of nutrition. 2000; 130: 1424S-1431S.
- Baum MK, Shor-Posner G, Campa A. Zinc status in human immunodeficiency virus infection. The Journal of nutrition. 2000; 130: 1421S-1423S.
- Hennigar SR, Kelley AM, McClung JP. Metallothionein and zinc transporter expression in circulating human blood cells as biomarkers of zinc status: a systematic review. Advances in Nutrition. 2016; 7: 735-746.
- Grider A, Bailey LB, Cousins RJ. Erythrocyte metallothionein as an index of zinc status in humans. Proceedings of the National Academy of Sciences. 1990; 87: 1259-1262.
- Sullivan VK, Burnett FR, Cousins RJ. Metallothionein expression is increased in monocytes and erythrocytes of young men during zinc supplementation. The Journal of nutrition. 1998; 128: 707-713.
- Ruz M, Cavan KR, Bettger WJ, Thompson L, Berry M, Gibson RS. Development of a dietary model for the study of mild zinc deficiency in humans and evaluation of some biochemical and functional indices of zinc status. The American journal of clinical nutrition. 1991; 53: 1295-1303.
- Wood RJ. Assessment of marginal zinc status in humans. The Journal of nutrition. 2000; 130: 1350S-1354S.
- Lowe NM, Fekete K, Decsi T. Methods of assessment of zinc status in humans: a systematic review. The American journal of clinical nutrition. 2009; 89: 2040S-2051S.
- Iyengar V, Pullakhandam R, Nair KM. Dietary ligands as determinants of iron– zinc interactions at the absorptive enterocyte. Journal of food science. 2010; 75: H260-264.
- Nair KM, Brahmam GN, Radhika MS, Dripta RC, Ravinder P, Balakrishna N, et al. Inclusion of guava enhances non-heme iron bioavailability but not fractional zinc absorption from a rice-based meal in adolescents. The Journal of nutrition. 2013; 143: 852-858.
- Iyengar V, Pullakhandam R, Nair KM. Iron-zinc interaction during uptake in human intestinal Caco-2 cell line: kinetic analyses and possible mechanism. Indian journal of biochemistry and biophysics. 2009; 46: 299-306.
- Sreenivasulu K, Raghu P, Ravinder P, Nair KM. Effect of dietary ligands and food matrices on zinc uptake in Caco-2 cells: implications in assessing zinc bioavailability. Journal of agricultural and food chemistry. 2008; 56: 10967- 10972.
- Wapnir RA, Stiel L. Zinc intestinal absorption in rats: specificity of amino acids as ligands. The Journal of nutrition. 1986; 116: 2171-2179.
- Savage JE, Yohe JM, Pickett EE, O’Dell BL. Zinc metabolism in the growing chick. Tissue concentration and effect of phytate on absorption. Poultry Science. 1964; 43: 420-426.
- Reyes JG. Zinc transport in mammalian cells. American Journal of Physiology- Cell Physiology. 1996; 270: C401-410.
- Barnett JP, Blindauer CA, Kassaar O, Khazaipoul S, Martin EM, Sadler PJ, Stewart AJ. Allosteric modulation of zinc speciation by fatty acids. Biochimica et Biophysica Acta (BBA)-General Subjects. 2013; 1830: 5456-5464.
- Vallee BL, Lewis HD, Altschule MD, GIBSON JG. The relationship between carbonic anhydrase activity and zinc content of erythrocytes in normal, in anemic and other pathologic conditions. Blood. 1949; 4: 467-478.
- Lu J, Stewart AJ, Sadler PJ, Pinheiro TJ, Blindauer CA. Albumin as a zinc carrier: properties of its high-affinity zinc-binding site.Biochemical Society Transactions. 2008; 36: 1317-1321.
- Zalewski PD, Millard SH, Forbes IJ, Kapaniris O, Slavotinek A, Betts WH, et al. Video image analysis of labile zinc in viable pancreatic islet cells using a specific fluorescent probe for zinc. Journal of Histochemistry & Cytochemistry. 1994; 42: 877-884.
- Krebs NF. Overview of zinc absorption and excretion in the human gastrointestinal tract. The Journal of nutrition. 2000; 130: 1374S-1377S.
- Johnson PE, Hunt CD, Milne DB, Mullen LK. Homeostatic control of zinc metabolism in men: zinc excretion and balance in men fed diets low in zinc. The American journal of clinical nutrition. 1993; 57: 557-565.
- Gibson RS, King JC, Lowe N. A review of dietary zinc recommendations. Food and nutrition bulletin. 2016; 37: 443-460.
- Golan Y, Kambe T, Assaraf YG. The role of the zinc transporter SLC30A2/ ZnT2 in transient neonatal zinc deficiency. Metallomics. 2017; 9: 1352-1366.
- Labádi I, Benko M, Markó K, Szilágyi I. Mimicking a Superoxide Dismutase (SOD) Enzyme by copper (II) and zinc (II)-complexes. Reaction Kinetics and Catalysis Letters. 2009; 96: 327-333.
- Tainer JA, Getzoff ED, Richardson JS, Richardson DC. Structure and mechanism of copper, zinc superoxide dismutase. Nature. 1983; 306: 284.
- Sjo¨blom B, Polentarutti M, Djinovic-Carugo K. Structural study of X-ray induced activation of carbonic anhydrase. Proceedings of the National Academy of Sciences. 2009; 106: 10609-10613.
- Wu FY, Wu CW. Zinc in DNA replication and transcription. Annual review of nutrition. 1987; 7: 251-272.
- Langmade SJ, Ravindra R, Daniels PJ, Andrews GK. The transcription factor MTF-1 mediates metal regulation of the mouse ZnT1 gene. Journal of Biological Chemistry. 2000; 275: 34803-34809.
- Aydemir TB, Sitren HS, Cousins RJ. The zinc transporter Zip14 influences c-Met phosphorylation and hepatocyte proliferation during liver regeneration in mice. Gastroenterology. 2012; 142: 1536-1546.
- Fraker PJ, King LE. Reprogramming of the immune system during zinc deficiency. Annual Review of Nutrition. 2004; 24: 277-298.
- Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiological reviews. 1993; 73: 79-118.
- McAllister BB, Dyck RH. Sound Processing: A new role for zinc in the brain. eLife. 2017; 6: e31816.
- Shields JM, Christy RJ, Yang VW. Identification and characterization of a gene encoding a gut-enriched Krüppel-like factor expressed during growth arrest. Journal of Biological Chemistry. 1996; 271: 20009-20017.
- Davis SR, Cousins RJ. Metallothionein expression in animals: a physiological perspective on function. The Journal of nutrition. 2000; 130: 1085-1088.
- Günes Ç, Heuchel R, Georgiev O, Müller KH, Lichtlen P, Blüthmann H, et al. Embryonic lethality and liver degeneration in mice lacking the metalresponsive transcriptional activator MTF-1. EMBO J. 1998; 17: 2846-2854.
- Klug A, Schwabe JW. Protein motifs 5. Zinc fingers. The FASEB journal. 1995; 9: 597-604.
- Berg JM, Shi Y. The galvanization of biology: a growing appreciation for the roles of zinc. Science. 1996; 271: 1081-1085.
- Ueda T, Kikuchi A, Ohga N, Yamamoto J, Takai Y, Vogel US, et al. X-ray Structure of an Unusual Ca2+ Site and the Roles of Zn2+ and Ca2+ in the Assembly, Stability, and Storage of the Insulin Hexamer. Biochemistry. 1991; 30: 917-924.
- Westbrook GL, Mayer ML. Micromolar concentrations of Zn 2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature. 1987; 328: 640.
- Sensi SL, Paoletti P, Koh JY, Aizenman E, Bush AI, Hershfinkel M. The neurophysiology and pathology of brain zinc. Journal of neuroscience. 2011; 31: 16076-16085.
- Ilouz R, Kaidanovich O, Gurwitz D, Eldar-Finkelman H. Inhibition of glycogen synthase kinase-3Β by bivalent zinc ions: insight into the insulin-mimetic action of zinc. Biochemical and biophysical research communications. 2002; 295: 102-106.
- Yoshikawa Y, Ueda E, Kojima Y, Sakurai H. The action mechanism of zinc (II) complexes with insulinomimetic activity in rat adipocytes. Life sciences. 2004; 75: 741-751.
- Tang XH, Shay NF. Zinc has an insulin-like effect on glucose transport mediated by phosphoinositol-3-kinase and Akt in 3T3-L1 fibroblasts and adipocytes. The Journal of nutrition. 2001; 131: 1414-1420.
- Zhang C, Lu X, Tan Y, Li B, Miao X, Jin L, et al. Diabetes-induced hepatic pathogenic damage, inflammation, oxidative stress, and insulin resistance was exacerbated in zinc deficient mouse model. PLoS One. 2012; 7: e49257.
- Bellomo E, Massarotti A, Hogstrand C, Maret W. Zinc ions modulate protein tyrosine phosphatase 1B activity. Metallomics. 2014; 6: 1229-1239.
- Krezel A, Maret W. Zinc-buffering capacity of a eukaryotic cell at physiological pZn. Journal of Biological Inorganic Chemistry. 2006; 11: 1049-1062.
- Colvin RA, Bush AI, Volitakis I, Fontaine CP, Thomas D, Kikuchi K, Holmes WR. Insights into Zn2+ homeostasis in neurons from experimental and modeling studies. American Journal of Physiology-Cell Physiology. 2008; 294: C726-742.
- Kim AM, Vogt S, O’halloran TV, Woodruff TK. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nature chemical biology. 2010; 6: 674.
- Colvin RA, Holmes WR, Fontaine CP, Maret W. Cytosolic zinc buffering and muffling: their role in intracellular zinc homeostasis. Metallomics. 2010; 2: 306-317.
- Maret W. Metals on the move: zinc ions in cellular regulation and in the coordination dynamics of zinc proteins. Biometals. 2011; 24: 411-418.
- Thirumoorthy N, Sunder AS, Kumar KM, Ganesh GN, Chatterjee M. A review of metallothionein isoforms and their role in pathophysiology. World journal of surgical oncology. 2011; 9: 54.
- Philcox JC, Sturkenboom M, Coyle P, Rofe AM. Metallothionein in mice reduces intestinal zinc loss during acute endotoxin inflammation, but not during starvation or dietary zinc restriction. The Journal of nutrition. 2000; 130: 1901-1909.
- Szczurek EI, Bjornsson CS, Taylor CG. Dietary zinc deficiency and repletion modulate metallothionein immunolocalization and concentration in small intestine and liver of rats. The Journal of nutrition. 2001; 131: 2132-2138.
- Drozd A, Wojewska D, Peris-Díaz MD, Jakimowicz P, Krezel A. Crosstalk of the structural and zinc buffering properties of mammalian metallothionein-2. Metallomics. 2018; 10: 595-613.
- Kambe T, Tsuji T, Hashimoto A, Itsumura N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiological reviews. 2015; 95: 749-784.
- Liuzzi JP, Cousins RJ. Mammalian zinc transporters. Annual review of nutrition. 2004; 24: 151-172.
- McMahon RJ, Cousins RJ. Mammalian zinc transporters. The Journal of nutrition. 1998; 128: 667-670.
- Andrews GK. Regulation and function of Zip4, the acrodermatitis enteropathica gene. Biochemical Society Transactions. 2008: 1242-1246.
- Cousins RJ. Gastrointestinal factors influencing zinc absorption and homeostasis. International Journal for Vitamin and Nutrition Research. 2010; 80: 243.
- Geiser J, De Lisle RC, Andrews GK. The zinc transporter Zip5 (Slc39a5) regulates intestinal zinc excretion and protects the pancreas against zinc toxicity. PloS one. 2013; 8: e82149.
- Valentine RA, Jackson KA, Christie GR, Mathers JC, Taylor PM, Ford D. ZnT5 variant B is a bidirectional zinc transporter and mediates zinc uptake in human intestinal Caco- 2 cells. Journal of biological chemistry. 2007; 282: 14389-14393.
- Yuan N, Wang YH, Li KJ, Zhao Y, Hu X, Mao L, et al. Effects of exogenous zinc on the cellular zinc distribution and cell cycle of A549 cells. Bioscience, biotechnology, and biochemistry. 2012: 120216.
- Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, et al. A family of cytokine-inducible inhibitors of signalling. Nature. 1997; 387: 917.
- Chowanadisai W, Graham DM, Keen CL, Rucker RB, Messerli MA. Neurulation and neurite extension require the zinc transporter ZIP12 (slc39a12). Proceedings of the National Academy of Sciences. 2013; 110: 9903-9908.
- Huang L, Kirschke CP, Lay YA, Levy LB, Lamirande DE, Zhang PH. Znt7-null mice are more susceptible to diet-induced glucose intolerance and insulin resistance. Journal of Biological Chemistry. 2012; 287: 33883-33896.
- Myers SA, Nield A, Chew GS, Myers MA. The zinc transporter, Slc39a7 (Zip7) is implicated in glycaemic control in skeletal muscle cells. PloS one. 2013; 8: e79316.
- Smidt K, Jessen N, Petersen AB, Larsen A, Magnusson N, Jeppesen JB, et al. SLC30A3 responds to glucose-and zinc variations in Β-cells and Is critical for insulin production and in vivo glucose- metabolism during Β-cell stress. PLoS One. 2009; 4: e5684.
- Chistiakov DA, Voronova NV. Zn2+-transporter-8: A dual role in diabetes. Biofactors. 2009; 35: 356-363.
- Liuzzi JP, Lichten LA, Rivera S, Blanchard RK, Aydemir TB, Knutson MD, et al. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proceedings of the National Academy of Sciences. 2005; 102: 6843-6848.
- Kitamura H, Morikawa H, Kamon H, Iguchi M, Hojyo S, Fukada T, et al. Tolllike receptor–mediated regulation of zinc homeostasis influences dendritic cell function. Nature immunology. 2006; 7: 971.
- Haase H, Ober-Blöbaum JL, Engelhardt G, Hebel S, Heit A, Heine H, Rink L. Zinc signals are essential for lipopolysaccharide-induced signal transduction in monocytes. The Journal of Immunology. 2008; 181: 6491- 6502.
- Hojyo S, Fukada T. Roles of zinc signaling in the immune system. Journal of immunology research. 2016; 6762343.
- Palmiter RD, Findley SD. Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. European Molecular Biology Organization journal. 1995; 14: 639-649.
- Lopez V, Kelleher SL. Zinc transporter-2 (ZnT2) variants are localized to distinct subcellular compartments and functionally transport zinc. Biochemical Journal. 2009; 422: 43-52.
- Wenzel HJ, Cole TB, Born DE, Schwartzkroin PA, Palmiter RD. Ultrastructural localization of zinc transporter-3 (ZnT-3) to synaptic vesicle membranes within mossy fiber boutons in the hippocampus of mouse and monkey. Proceedings of the National Academy of Sciences. 1997; 94: 12676-12681.
- Ranaldi G, Perozzi G, Truong-Tran A, Zalewski P, Murgia C. Intracellular distribution of labile Zn (II) and zinc transporter expression in kidney and MDCK cells. American Journal of Physiology-Renal Physiology. 2002; 283: F1365-1375.
- Jackson KA, Helston RM, McKay JA, O’Neill ED, Mathers JC, Ford D. Splice variants of the human zinc transporter ZnT5 (SLC30A5) are differentially localized and regulated by zinc through transcription and mRNA stability. Journal of Biological Chemistry. 2007; 282: 10423-10431.
- Huang L, Kirschke CP, Gitschier J. Functional characterization of a novel mammalian zinc transporter, ZnT6. Journal of Biological Chemistry. 2002; 277: 26389-26395.
- Kirschke CP, Huang L. ZnT7, a novel mammalian zinc transporter, accumulates zinc in the Golgi apparatus. Journal of Biological Chemistry. 2003; 278: 4096-4102.
- Chimienti F, Devergnas S, Pattou F, Schuit F, Garcia-Cuenca R, Vandewalle B, et al. In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. Journal of cell science. 2006; 119: 4199-4206.
- Perez Y, Shorer Z, Liani-Leibson K, Chabosseau P, Kadir R, Volodarsky M, et al. SLC30A9 mutation affecting intracellular zinc homeostasis causes a novel cerebro-renal syndrome. Brain. 2017; 140: 928-939.
- Bosomworth HJ, Thornton JK, Coneyworth LJ, Ford D, Valentine RA. Efflux function, tissue-specific expression and intracellular trafficking of the Zn transporter ZnT10 indicate roles in adult Zn homeostasis. Metallomics. 2012; 4: 771-779.
- Gaither LA, Eide DJ. The human ZIP1 transporter mediates zinc uptake in human K562 erythroleukemia cells. Journal of Biological Chemistry. 2001; 276: 22258-22264.
- Peters JL, Dufner-Beattie J, Xu W, Geiser J, Lahner B, Salt DE, Andrews GK. Targeting of the mouse Slc39a2 (Zip2) gene reveals highly cell-specific patterns of expression, and unique functions in zinc, iron, and calcium homeostasis. Genesis. 2007; 45: 339-352.
- Dufner-Beattie J, Huang ZL, Geiser J, Xu W, Andrews GK. Generation and characterization of mice lacking the zinc uptake transporter ZIP3. Molecular and cellular biology. 2005; 25: 5607-5615.
- Wang K, Zhou B, Kuo YM, Zemansky J, Gitschier J. A novel member of a zinc transporter family is defective in acrodermatitis enteropathica. The American Journal of Human Genetics. 2002; 71: 66-73.
- Wang F, Kim BE, Petris MJ, Eide DJ. The mammalian Zip5 protein is a zinc transporter that localizes to the basolateral surface of polarized cells. Journal of Biological Chemistry. 2004; 279: 51433-51441.
- Croxford TP, McCormick NH, Kelleher SL. Moderate zinc deficiency reduces testicular Zip6 and Zip10 abundance and impairs spermatogenesis in mice. The Journal of nutrition. 2011; 141: 359-365.
- Hogstrand C, Kille P, Nicholson RI, Taylor KM. Zinc transporters and cancer: a potential role for ZIP7 as a hub for tyrosine kinase activation. Trends in molecular medicine. 2009; 15: 101-111.
- He L, Girijashanker K, Dalton TP, Reed J, Li H, Soleimani M, Nebert DW. ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: characterization of transporter properties. Molecular pharmacology. 2006; 70: 171-180.
- Matsuura W, Yamazaki T, Yamaguchi-Iwai Y, Masuda S, Nagao M, Andrews GK, Kambe T. SLC39A9 (ZIP9) regulates zinc homeostasis in the secretory pathway: characterization of the ZIP subfamily I protein in vertebrate cells. Bioscience, biotechnology, and biochemistry. 2009; 73: 1142-1148.
- Kaler PA, Prasad RA. Molecular cloning and functional characterization of novel zinc transporter rZip10 (Slc39a10) involved in zinc uptake across rat renal brush-border membrane. American Journal of Physiology-Renal Physiology. 2007; 292: F217-229.
- Martin AB, Aydemir TB, Guthrie GJ, Samuelson DA, Chang SM, Cousins RJ. Gastric and colonic zinc transporter ZIP11 (Slc39a11) in mice responds to dietary zinc and exhibits nuclear localization. The Journal of nutrition. 2013; 143: 1882-1888.
- Bin BH, Fukada T, Hosaka T, Yamasaki S, Ohashi W, Hojyo S, et al. Biochemical characterization of human ZIP13 protein a homo- dimerized zinc transporter involved in the spondylocheiro dysplastic ehlers-danlos syndrome. Journal of Biological Chemistry. 2011; 286: 40255-40265.
- Liuzzi JP, Aydemir F, Nam H, Knutson MD, Cousins RJ. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proceedings of the National Academy of Sciences. 2006; 103: 13612-13617.
- Bailly S, Fay M, Ferrua B, Gougerot-Pocidalo MA. Ciprofloxacin treatment in vivo increases the ex vivo capacity of lipopolysaccharide-stimulated human monocytes to produce IL-1, IL-6 and tumour necrosis factor-alpha. Clinical & Experimental Immunology. 1991; 85: 331-334.
- Mayer LS, Uciechowski P, Meyer S, Schwerdtle T, Rink L, Haase H. Differential impact of zinc deficiency on phagocytosis, oxidative burst, and production of pro- inflammatory cytokines by human monocytes. Metallomics. 2014; 6: 1288-1295.
- Cousins RJ, Blanchard RK, Popp MP, Liu L, Cao J, Moore JB, Green CL. A global view of the selectivity of zinc deprivation and excess on genes expressed in human THP-1 mononuclear cells. Proceedings of the National Academy of Sciences. 2003; 100: 6952-6957.
- Isaacson A, Sandow A. Effects of zinc on responses of skeletal muscle. The Journal of general physiology. 1963; 46: 655-677.
- Edman KP. The Effect of Zinc and Certain Other Bivalent Metal Ions on the Isometric Tension Development of Glycerol-extracted Muscle Fibre Bundles. Acta Physiologica Scandinavica. 1958; 43: 275-291.
- Edman KP. The Binding of Zinc to Glycerol-extracted Muscle, and Its Relaxing Effect. Acta Physiologica Scandinavica. 1959; 46: 209-227.
- Edman KP. Zinc-Induced Relaxation of Muscle Fibres. Acta Physiologica Scandinavica. 1960; 49: 330-342.
- Micheletti A, Rossi R, Rufini S. Zinc status in athletes. Sports medicine. 2001; 31: 577-582.
- Khadeer MA, Sahu SN, Bai G, Abdulla S, Gupta A. Expression of the zinc transporter ZIP1 in osteoclasts. Bone. 2005; 37: 296-304.
- Jackson MJ. Physiology of zinc: general aspects. In Zinc in human biology. Springer, London. Editor Colin F. Mills. 1989; 1- 14.
- Emmert JL, Baker DH. Zinc stores in chickens delay the onset of zinc deficiency symptoms. Poultry science. 1995; 74: 1011-1021.
- Jackson MJ, Jones DA, Edwards RH. Tissue zinc levels as an index of body zinc status. Clinical Physiology. 1982; 2: 333-343.
- Zhou JR, Canar MM, Erdman Jr JW. Bone zinc is poorly released in young, growing rats fed marginally zinc-restricted diet. The Journal of nutrition. 1993; 123: 1383-1388.
- Ilbäck NG, Glynn AW, Wikberg L, Netzel E, Lindh U. Metallothionein is induced and trace element balance changed in target organs of a common viral infection. Toxicology. 2004; 199: 241-250.
- Klasing KC. Effect of inflammatory agents and interleukin 1 on iron and zinc metabolism. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1984; 247: R901-904.
- Beisel WR. Metabolic response to infection. Annual review of medicine. 1975; 26: 9-20.
- DiSilvestro RA, Cousins RJ. Glucocorticoid independent mediation of interleukin-1 induced changes in serum zinc and liver metallothionein levels. Life sciences. 1984; 35: 2113-2118.
- Golden MH. Specific deficiencies versus growth failure: type I and type II nutrients. SCN news. 1995: 10-14.
- Prasad AS. Zinc: an overview. Nutrition (Burbank, Los Angeles County, Calif.). 1995; 11: 93-99.
- Cousins RJ, Leinart AS. Tissue-specific regulation of zinc metabolism and metallothionein genes by interleukin 1. The FASEB journal. 1988; 2: 2884- 2890.
- Yamaguchi M, Oishi H, Suketa Y. Zinc stimulation of bone protein synthesis in tissue culture: activation of aminoacyl-tRNA synthetase. Biochemical pharmacology. 1988; 37: 4075-4080.
- Jinno N, Nagata M, Takahashi T. Marginal zinc deficiency negatively affects recovery from muscle injury in mice. Biological trace element research. 2014; 158: 65-72.
- Zhou JR, Canar MM, Erdman Jr JW. Bone zinc is poorly released in young, growing rats fed marginally zinc-restricted diet. The Journal of nutrition. 1993; 123: 1383-1388.
- Nagata M, Lönnerdal B. Role of zinc in cellular zinc trafficking and mineralization in a murine osteoblast-like cell line. The Journal of nutritional biochemistry. 2011; 22: 172-178.
- Inoue K, Matsuda K, Itoh M, Kawaguchi H, Tomoike H, Aoyagi T, et al. Osteopenia and male-specific sudden cardiac death in mice lacking a zinc transporter gene, Znt5. Human molecular genetics. 2002; 11: 1775-1784.
- King JC, Shames DM, Woodhouse LR. Zinc homeostasis in humans. The Journal of nutrition. 2000; 130: 1360S-1366S.
- Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004; 306: 2090-2093.
- Ou O, Allen-Redpath K, Urgast D, Gordon MJ, Campbell G, Feldmann J, et al. Plasma zinc’s alter ego is a low-molecular-weight humoral factor. The FASEB Journal. 2013; 27: 3672-3682.