
Research Article
Austin J Nucl Med Radiother. 2025; 9(1): 1033
Verification of an Economic Affordable Substitute for the Radio Chromatography Technique for 68Ga -Labelled Radiopharmaceuticals
Sarepaka V Ramana Murthy*
Homibhabha Cancer Hospital & Research Centre, Aganampudi, Visakhapatnam, Andhrapradesh-530046, India
*Corresponding author: Sarepaka V Ramana Murthy, Homibhabha Cancer Hospital & Research Centre, Aganampudi, Visakhapatnam, Andhrapradesh-530046, India Email: sarepakas2012@gmail.com
Received: April 03, 2025 Accepted: April 17, 2025 Published: April 22, 2025
Abstract
Introduction: Gallium-68 (68Ga) labelled radiopharmaceuticals, like PSMA (Prostate specific membrane antigen), DOTA-TOC (1,4,7,10-tetra azacyclododecane1,4,7,10 tetra acetic acid) and FAPI (Fibroblast activation protein inhibitor) are widely utilized in positron emission computed tomography (PET-CT) imaging. Quality control of these radiopharmaceuticals requires radio chromatography techniques to determine radiochemical purity. However conventional methods such as high-performance liquid chromatography (HPLC) and thin layer chromatography (TLC) can be expensive.
This study explores an economic and affordable method using with a survey meter. The accuracy, precision, linearity and robustness of survey meter technique were assessed and compare to standard TLC method results. The proposed technique could offer a cost-effective and affordable routine chromatographic technique for resource -limited settings without compromising quality standards.
Materials & Methods: PSMA, DOTA-TOC, FAPi peptides were synthesized at HBCH&RC with about (15mCi-27mCi) of eluted 68GaCl3 from automated Ge68-Ga68 generator module. Samples of labelled product were spotted on 1 × 7.5cm TLC strips (silica gel 60 F254) and developed in appropriate mobile phases. Each strip was first analysed on the TLC scanner used at HBCH&RC (standard method), and subsequently, the strip was cut in two pieces and radioactivity from each portion was counted with a small survey meter as well as dose calibrator.
Results and Discussion: The proposed method proved to be accurate (CV of mean RCP <5),
precise (RSD<5%), linear slope to 1, r2 = 0.9) within the RCP range of 90% to 99.8% and Robust (P> 0.05).
Conclusion: The proposed method compared well with the standard method (TLC method) and is suitable as economically affordable.
Keywords: 68Ga-radiopharmaceuticals; Radio chromatography; Survey meter technique
Introduction
Gallium is a P-block element present ingroup13 of the periodic table. The oxidation state of Gallium is +3. 68Ga is a positron emitting isotope of gallium with half-life of 68 minutes. The emission of positron from 68Ga is 88.88% with maximum positron energy of 1.9MeV and the remaining 11.11% of Ga68 is disintegrated by electron capture [1]. 68Ga is generally eluted from the 68Ge-68Ga generator in the hospital-based radio-pharmacy .68Ga can also be obtained from cyclotron via 68Zn (p, n) 68Ga reaction using liquid target. In case of larger scale requirement of 68Ga, cyclotron is preferable otherwise 68Ge/68Ga generator is the better choice for in house labelled radiopharmaceuticals [2]. A 68Ge-68Ga generator is a device which provides 68Ga3+ metal ion. The half life of the parent isotope 68Ge is 271 days and it decays into 68Ga having a half life of 68 minutes.in 1960 Gleason introduced the first 68Ge/68Ga radionuclide generator, and described it as “a positron cow” [3].
The use of 68Ga labelled radiopharmaceuticals such as PSMA, DOTA-TOC, and FAPI has increased in PET-CT (Positron Emission computed Tomography) in clinical practice. 68Ga is having suitable physical, chemical and radioactive properties and also is produced basically from 68Ge-68Ga generator [4].
The radiochemical purity (RCP) of Gallium labelled radiopharmaceuticals (RP) is important to ensure PET-CT image quality. In low-income settings, it may not be possible to use compendial analytical methods or expensive equipment like TLC and HPLC scanner for radiochemical purity analysis. To ensure the efficacy of RP prepared at Homi bhabha cancer hospital& research centre, Visakhapatnam, this study investigates a cost-effective and affordable routine chromatographic method using a simple survey meter technique for verifying the radiochemical purity of Ga68- labelled radiopharmaceuticals. The study compares this approach with conventional methods, focusing on accuracy, precision, linearity and robustness.
Materials and Methods
This study is conducted by using a 1.1GBq (30mCi) of 68Ge- 68Ga generator fully with automated module (40mm lead-lined cabinet). Peptides like 0.5mg PSMA,1.0mg DOTA-TOC, 1.0 FAPI, precursors, cassette set and reagent kits are obtained from ABx, Gmbh company, Germany [5]. Ga68-labeled PSMA, DOTA-TOC, FAPI were synthesized at the radio-pharmacy laboratory of the Department of Nuclear medicine at HBCHRC, Visakhapatnam. The three radiopharmaceuticals were synthesizing by fully automatic methods. The survey meter cum contamination monitor (ROTEM S/N 12819-110) was also check periodically for its accuracy by measuring the exposure rate at 2meters from the known activity of I-131(T1/2= 8.04Days) [6]. TLC scanning was performed by using a Lab logic scanner (ITG, Germany). The same calibrated survey cum contamination monitor used at for the comparative experiments.
PSMA, DOTA-TOC, FAPI peptides were synthesized at HBCH&RC with about (15mCi-27mCi) of eluted 68GaCl3 from automated Ge68-Ga68 generator module. Samples of labelled product were spotted on 1 × 7.5cm TLC strips (silica gel 60 F254) and developed in appropriate mobile phases (0.1M sodium citrate). Each strip was first analysed on the TLC scanner used at HBCH&RC (standard method), and subsequently, the strip was cut in two pieces and radioactivity from each portion was counted with a small survey meter as well as dose calibrator. The percentage RCP for each TLC strip was calculated using both counting methods. Internationally accepted validation parameters were applied as well as statistical analysis (USP 37<1225>2008) done. Additionally, linearity of the survey meter was determined with Ga68. Obtained readings with the survey meter were also plotted against the TLC scanner results.
Verification of Quantitative Analytical Techniques
Sample Preparation
15mCi-27mCi of 68GaCl3 was used to prepare Ga68-labelled radiopharmaceuticals like Ga68-PSMA, Ga68-DOTA-TOC and Ga68-FAPi. All the radiochemical purity was checked with TLC scanner between approximately 90% and 100%.
Chromatography for Labelled Radiopharmaceuticals
3μl of 68Galabelled product samples were spotted on 7.5 cm iTLC-SG strips. For PSMA, DOTA-TOC, FAPI the strips were then developed in 0.1M sodium citrate as mobile phase, under these conditions 68Ga-DOTA-TOC, PSMA and FAPi remained at the origin and the impurity (Ga-cl3) migrated with the solvent front. After developing, each strip was first scanned on the TLC scanner (Model: SCAN-RAM PET/ SPECT, Radio TLC SCANNER, make: Lab logic system ltd.) using with wide range of gamma energies (60- 1500KeV). The scan time was 100 s and background counts were subtracted. Within 5-10 min thereafter the strip was cut in 2 pieces at 0.5 cm from the origin. The radioactivity from each portion of the strip was counted for 2 min with the survey meter cum contamination monitor (BAK-1880 S/N 12819-110) which is having gamma energy range from 17 Kev to 3 MeV (measurement range from 0-7200 μSv/Hr or 0 to 500kcps). Each strip was placed flat in the bottom of a 15 cm deep container and the counter placed at the top of the container to ensure counting geometry was the same for all samples. Each count rate reading was recorded with background count rate subtracted. From these values, the percentage of radiochemical purity of each strip ([% RCP = Activity in desired form / Total activity]) x 100 was calculated.
Results and Discussion
The Significance of Method Validation
Standard analytical methods are provided in the package of radiopharmaceutical kits and described in pharmacopeia monographs [7]. However, implementing these methods can be challenging in nuclear medicine radio pharmacy setup with limited financial resources due to insufficient equipment and a shortage of consumables. Additionally, these techniques require significant time to complete, which can delay in the administration of short half radiopharmaceuticals to patients. Given these limitations, there is a strong need for analytical methods that are more practical, simpler, faster and cost-effective.
Accuracy
RCP values from three replicates of each sample were determined along with the standard deviation and coefficient of variation (CV). The accuracy criteria for the radiopharmaceutical component were considered met if the mean of the standard method differed by not more than 5% from the mean value of the proposed method (Table 1, Figure 1).
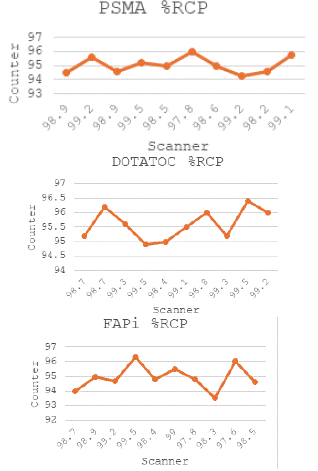
Figure 1: Counter response compared with RCP values determined with
TLC.
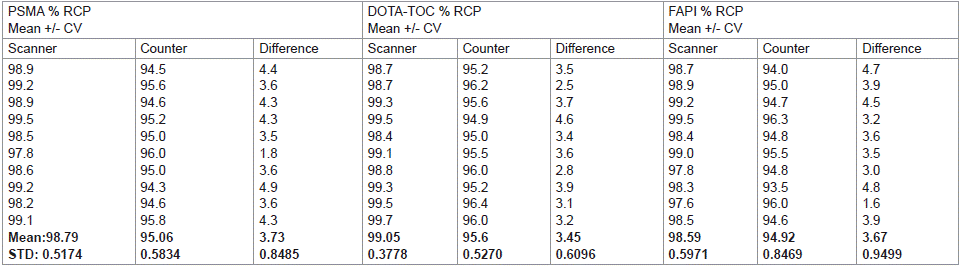
Table 1: Accuracy of the proposed method for the 3 compounds.
Robustness
Factors such as mobile phase composition, and ambient temperature can influence the stationary and mobile phase flow. In this work, we assessed the reliability of a counting system, which is independent of chemical separation, improper positioning of the survey meter could cause to differences in counting geometry between the two parts of chromatography strips. Considering the possibility of slight variations in counting, the robustness of the counting system was checked by comparing counts with a properly aligned counter to a slightly titled (angle up to 10 degree) counter. No significant differences in results were found (Table 2, Figure 2).
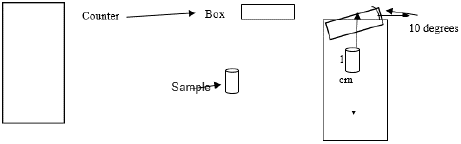
Figure 2: Illustration of the robustness test (Reading with counter at normal
position and 10-degree angle).
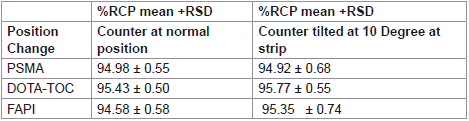
Table 2: Robustness of the counter.
Repeatability
The repeatability precision was assessed with three replicates of a spiked sample and the relative standard deviation (RSD) was calculated (Table 3).
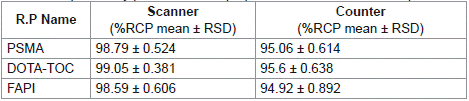
Table 3: Repeatability precision of the proposed method for the 3 compounds.
Precision
The precision was assessed by chromatography of 3 replicates of spiked samples performed by 2 different operators, the distribution of activity on the strips was quantified by using the two different counting systems. Mean RCP values and RSD were calculated. The statistical evaluation using ANOVA was performed of the complete data set where results are grouped by each operator and instrument were analysed with acceptance criteria stating no significant difference at 95% CI (P = 0.05) (Table 4).
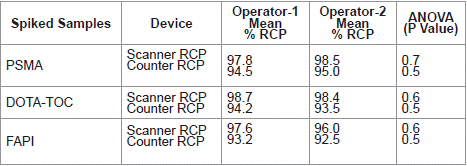
Table 4: Intermediate Precision results with two different operators (n=5,
acceptance criteria (P = 0.05).
Linearity
To evaluate linearity, the survey meter’s response to Ga68-cl3 was evaluated.
A 6 MBq point source of Ga68-cl3 was measured repeatedly over a period of 150 min. The measured count rate was then plotted against the calculated activity of the point source. The recorded count rate was then plotted against the calculated activity of source. The linearity results include the equation from the linear regression obtained from a plot of % RCP determined with the counter (proposed method) against the RCP values determined with the scanner (the validated control method of the GMP compliant radio pharmacy), key parameters such as slope, correlation coefficient and Y intercept were analysed. According to acceptance criteria, the slope should be close to 1, and the coefficient of determination r2 of the graph is equal to 0.979 (Figure 3).
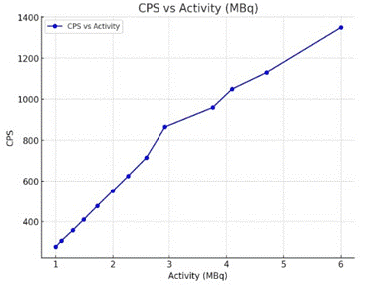
Figure 3: Counter response vs 68Ga -cl3 activity.
Specificity
The effectiveness of chromatographic techniques in distinguishing various chemical components such as the active pharmaceutical ingredient and any impurities. In our assessment of the method for quantifying radioactive distribution, we utilized TLC for chemical separation and used the same strip for both counting techniques. As a result, specificity was not considered in our validation. For the same reason, we did not assess impurities other than free gallium.
Conclusion
In compared to the standard method, our alternative affordable method demonstrated linear, accurate, specificity in the range of product concentrations. It was also precise and robust. It has increased awareness among our staff about the importance of validation and cost effective.
References
- Romero E, Martinez A, Oteo M, et al. Development and long term evaluation of a new Ge68/Ga68 generator based on nano-SnO2 for PET imaging. Sci Rep. 2020; 10: 12756.
- Rodnick Melissa E, Sollert carina. stark cyclotron-based production of 68Ga,[68Ga] GaCl3, and [68Ga] Ga-PSMA -11 from liquid target.
- Note on Ge-68 production methods in 1996. 2010.
- Manual vs. automated 68Ga-radiolabeling - a comparison of optimized processes1Michael Meisenheimer1,2, Stefan Kürpig1, Markus Essler1, Elisabeth Eppard2. 2020.
- Breeman WAP, Jong M, Blois E, Bernard BF, Konijnenberg M, Krenning EP. Radiolabelling DOTA-peptides with 68Ga. Eur J Nucl Med Mol Imaging. 2005; 32: 478-485.
- Estimation of Whole-Body radiation exposure to nuclear medicine professional during synthesis of Lu-177Lutetium –labelled radiopharmaceuticals. Indian J Nucl Med. 2017; 2: 89-92.
- A better method of quality control for 99mTc-tetrofosmin. Journal of nuclear medicine technology. 2004; 32: 72-78.