
Research Aricle
Austin J Nucl Med Radiother. 2023; 8(1): 1032.
Metabolic Changes in Medial Temporal Lobe Epilepsy Compared to Healthy Controls Using Advanced [18F] FDG PET SPM Analysis Techniques
Al Sadi R¹*; Bouhali O²; Djekidel M³
1Department of Science, Texas A & M University at Qatar, Education City, Al Rayyan, Doha, Qatar
2Qatar Computing Research Institute, Hamad Bin Khalifa University, Doha, Qatar
3Senior Author, Northwell, New York, USA
*Corresponding author: Rahaf Al Sadi Department of Science, Texas A&M University at Qatar, Education City, Al Rayyan, Doha, Qatar. Email: rahaf.alsadi@qatar.tamu.edu
Received: March 13, 2023 Accepted: April 17, 2023 Published: April 24, 2023
Abstract
Objectives: [18F] FDG PET imaging has emerged as an established modality in the evaluation of medically refractory temporal lobe epilepsy. Advanced techniques improve the sensitivity and detection of abnormalities. We sought to measure changes in metabolic activity using [18F] FDG PET in Medial Temporal Lobe Epilepsy (MTLE) patients compared to healthy controls and evaluate changes in the Seizure Onset Zone (SOZ) and remote areas.
Methods: We evaluated a cohort of 14 MTLE patients. Following a standard brain [18F] FDG PET acquisition, images were co-registered to a healthy control database using the Neuro-MIM software. Z scores were generated for different temporal and extra-temporal lobe structures for significant p values <0.05. Seizure laterality was determined by experienced epileptologists (>10 years) using intracranial or surface depth electrodes.
Results: The mean age of our patient cohort was 33.9 years (range: 9-52 years). We evaluated 7 left MTLE, 1 bilateral MTLE, and 6 right MTLE patients. Significant hypometabolic changes were seen in the amygdala, the hippocampus, and overall, the medial temporal lobe region (Z score of -2.2, -2.3, and -2.1 respectively). There was a smaller decrease in metabolic activity observed in the parahippocampal gyrus, fusiform gyrus, and the lateral temporal lobe (Z score of -1.4, -1.2, and -1.1 respectively). There were no significant differences in the basal ganglia, thalamus, prefrontal cortex, or cingulate gyrus.
Conclusions: Measurable significant differences in FDG brain metabolic activity exist in medial temporal lobe epilepsy patients compared to healthy controls. Our study shows the most significant changes are hypometabolism in the amygdala, hippocampus, and overall, the medial temporal lobe region compared to the lateral temporal lobe.
Keywords: MTLE; Epilepsy; SOZ; FDG PET
Introduction
Epilepsy is a chronic debilitating condition. Its comorbid impact can be as significant as that of severe diabetes. It has a significant socioeconomic impact on the individual patient as well as a prominent health care cost burden [1-3]. In North America, epilepsy incidence is approximately 50/100,000 per year and prevalence is 5-10/1000 [4]. The CDC estimates that about 2.0 million people in the United States have epilepsy and nearly 140,000 Americans develop the condition each year with an increase in patients with symptomatic epilepsy [4]. The lives of epilepsy patients are negatively impacted at multiple levels, with decreased independent living, and limited financial independence, due to their reduced income and less-likeliness to have full-time employment; they have driving restrictions, neuropsychological impairment, and suffer from persistent stigma in developing as well as developed countries [2-6]. The best studies found an overall standardized mortality ratio of 2.3 for epilepsy relative to the general population [4]. There is a need for more research into ways to improve epilepsy management and global awareness of the disproportionate resources allocated to other diseases and lack of funding into epilepsy progress.
Medical treatment has not been shown to impact clinical outcomes or cost-effectiveness [7-9]. While useful, Antiepileptic Drugs (AEDs) exhibit a substantial long-term health care cost and are associated with debilitating side effects [10,11]. Many epilepsy patients and young females of childbearing age, in whom a pregnancy is contemplated, may be affected directly or indirectly by their epilepsy condition itself or by the adverse effects and teratogenicity related to AEDs [12]. In the case of the one third of epilepsy patients who do not respond to AEDs, respective surgical alternatives or minimally invasive novel intracranial devices may be beneficial.
Since the late 1800’s, surgery for epilepsy has been shown to be successful in seizure control [13-15]. Since then major advances have been made [16-21]. Quality-of-life outcomes are high, and morbidity/mortality post-surgery are low [11,22-37] especially in temporal lobe epilepsy, but are dependent on the preoperative localization of the Seizure-Onset Zone (SOZ) [22,38]. Seizure freedom is usually high at 2 years [39,40]. A prospective, randomized study demonstrated the superiority of surgical versus medical treatment in temporal lobe epilepsy [39]. Surgical success rates can be improved by better noninvasive techniques to define the SOZ, such as what we are proposing here.
The SOZ, while challenging to identify, is traditionally identified by expert epileptologists using several measurement/techniques, each evaluating a certain aspect of the clinical, structural, functional, or electric-magnetic activity of the brain, in order to determine the boundaries of the SOZ. These methods vary widely in their temporal and spatial resolution. Given that there is no single perfect technique that assesses the SOZ reliably with both high temporal and spatial resolution, combining different approaches is currently the mainstay for modern pre-surgical evaluation. This involves inpatient 24-hour video monitoring of seizures while the patient is undergoing additional scalp or Intracranial Electrocorticography (IEC) or both. These data are analyzed in conjunction with MRI, MEG, and functional imaging studies including PET and SPECT, and used to guide final definition of the SOZ with or without intracranial electrode mapping. This process is challenging even in expert hands. It is guided by clinically heterogeneous and imperfect data and patients.
While MRI is used with great success, it is not always informative in cases where no lesion is found, or multiple lesions are seen. Defining a SOZ in the absence of a lesion evident on an MRI scan is challenging and can adversely impact outcomes [26,41-55]. This is where functional PET imaging with [18F] FDG, [11C] Flumazenil, [11C] AMT and [18F] MPPF has proven superior to MRI in identifying the SOZ necessary to be resected [56-81]. Traditionally, PET evaluation is done through visual review and interpretation of the scan. However, semi-quantitative or quantitative brain PET evaluations have proven more useful with numerous radiotracers including FDG. Hence, we aim here to measure and present the changes in metabolic activity using [18F] FDG PET in MTLE patients compared to healthy controls and to evaluate changes in the SOZ (i.e., medial temporal lobe) and remote areas.
Materials and Methods
Fourteen patients (with an age range of 9-52 years) with Medial Temporal Lobe Epilepsy (MTLE) were evaluated. Presurgical evaluation was carried out, whereby the patients were required to have MRI, Stereo-Electro-Encephalography (SEEG), and inter-ictal FDG PET scans performed.
Each patient underwent a PET scan with [18F] FDG 0.1mCi (3.7MBq)/kg. [18F] FDG was administered 35-45 minutes prior to imaging. A 10-minute static scan was acquired using a PET/CT scanner. PET images were reconstructed using an Ordered-Subsets Expectation Maximization (OSEM) algorithm. The appropriate corrections such as detector dead time scatter and random events, and radioactive decay were applied, in addition to attenuation correction using CT scans. The images were then co-registered to the patient’s MRI and a healthy control database using the MIMneuro (MIM Software, USA).
Consequently Z-scores were generated for different temporal and extra-temporal lobe structures for significant p values <0.05. Scans were processed and reviewed by an experienced nuclear medicine physician.
Results
A total of fourteen patients were evaluated with a mean age of 33.9 Years (range: 9-52 years). There were 7 left MTLE, 1 bilateral MTLE, and 6 right MTLE patients. The Z scores from the scans were evaluated.
As shown in (Figures 1 and 2), significant hypometabolic changes were seen in the amygdala, the hippocampus, and overall the medial temporal lobe region (Z score of -2.2, -2.3 and -2.1 respectively).
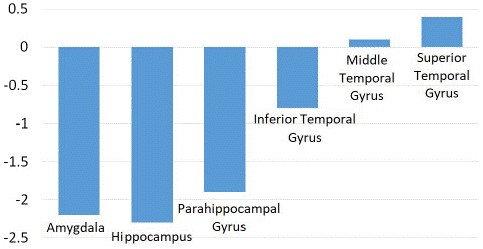
Figure 1: Z score comparison of medial temporal lobe epilepsy patients compared to healthy control patients in thalamus and basal ganglia.
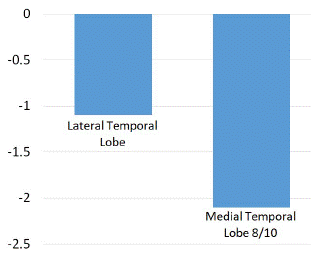
Figure 2: Z score comparison in medial and lateral temporal lobe structures.
There was a smaller decrease in metabolic activity observed in the parahippocampal gyrus, fusiform gyrus, and the lateral temporal lobe (Z score of -1.4, -1.2 and -1.1 respectively). A small decrease was also found in the temporal lobe and pole, as well as the globus pallidus, shown in (Figure 3).
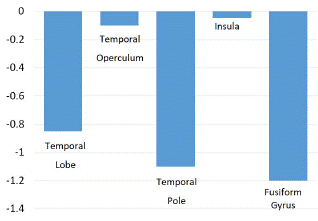
Figure 3: Z score comparison in the temporal lobe, operculum, pole and insula and fusiform gyrus.
There were no significant differences in the basal ganglia, thalamus, prefrontal cortex, cingulate gyrus, or paracentral lobule as shown in (Figures 4, 5 and 6).
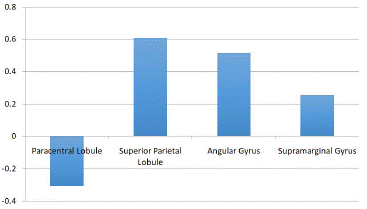
Figure 4: Z score comparison in paracentral and superior parietal lobules and angular and supramarginal gyrus.
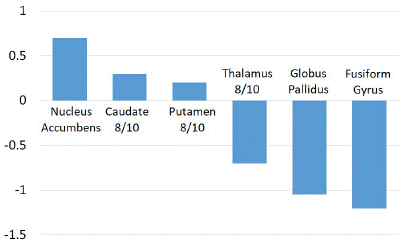
Figure 5: Z score comparison in thalamus and basal ganglia.
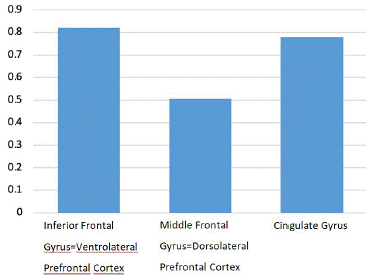
Figure 6: Z score comparison in prefrontal cortex and cingulate gyrus.
Discussion
While there are several tools used in the pre-surgical evaluation of medically refractory epilepsy patients to define cortical zones of epileptic abnormality [82], currently MRI is the most reliable and accurate in providing information with regards to the epileptogenic zone. The inconsistent use of FDG PET clinically is due to the limited sensitivity and specificity of visual qualitative reads. Interpretation is challenging in non-experts’ hands. Even nuclear medicine specialists with extensive expertise and experience can have a difficult time with accurate visual/qualitative interpretation. Even more so in the medial temporal lobe owing to baseline mild physiologic hypometabolism. The latter is due to patient variation in medial temporal lobe metabolism as well as the fact that deep brain structures may display less radiotracer uptake due to technical parameters. Hence, it makes sense to use fully quantitative or semiquantitative techniques with advanced tools such as subtraction and SPM analysis.
The main finding of this study was that the Z scores comparing MTLE patients to healthy controls provided measurable significant differences in [18F]-FDG brain metabolic activity. This technique is accurate, reliable, and reproducible. It provides high inter-reader agreement. It offers increased sensitivity and specificity compared to traditional visual/qualitative interpretations, as shown in (Figure 7). We show that in our cohort using advanced statistical mapping can differentiate lateral temporal lobe from MTLE with lower non-significant z scores in the lateral temporal lobes. Changes in other temporal lobe structures outside the medial temporal lobe were not significant.
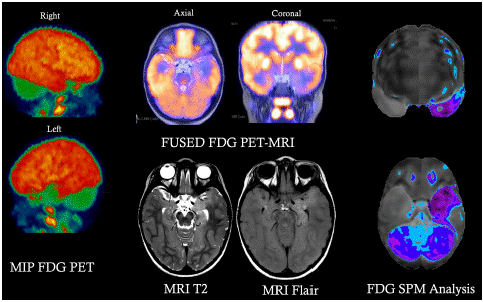
Figure 7: An FDG PET/MRI co-registered scan for an epilepsy patient showing a well-defined area of focal hypometabolism.
This increases the confidence in proceeding with surgery in these MTLE patients. Lack of changes outside the medial temporal lobe structures is something that cannot be ascertained visually/qualitatively with certainty. Although if present these changes would have negative prognostic determinants. Changes along the epilepsy network (basal ganglia, thalami and extratemporal cortical regions) were not significant in our cohort.
In essence, measurable significant differences in [18F] FDG brain metabolic activity exist in medial temporal lobe epilepsy patients compared to healthy controls and advanced techniques offer a superior diagnostic performance.
Conclusions
Measurable significant differences in FDG brain metabolic activity exist in medial temporal lobe epilepsy patients compared to healthy controls. Our study shows the most significant changes are hypometabolism in the amygdala, hippocampus, and overall, the medial temporal lobe structures compared to the lateral temporal lobe. SPM analysis allows for an accurate and highly sensitive evaluation of medically refractory temporal lobe epilepsy patients and should be used as a standard in surgical epilepsy centers.
References
- Leonardi M, Ustun TB. The global burden of epilepsy. Epilepsia. 2002; 43: 21-25.
- Burneo JG, Jette N, Theodore W, Begley C, Parko K, et al. Disparities in epilepsy: report of a systematic review by the North American Commission of the International League Against Epilepsy. Epilepsia. 2009; 50:2285-2295.
- Angalakuditi M, Angalakuditi N. A comprehensive review of the literature on epilepsy in selected countries in emerging markets. Neuropsychiatric disease and treatment. 2011; 7: 585-597.
- Theodore WH, Spencer SS, Wiebe S, Langfitt JT, Ali A, et al. Epilepsy in North America: a report prepared under the auspices of the global campaign against epilepsy, the International Bureau for Epilepsy, the International League Against Epilepsy, and the World Health Organization. Epilepsia. 2006; 47: 1700-1722.
- Dua T, de Boer HM, Prilipko LL, Saxena S. Epilepsy Care in the World: results of an ILAE/IBE/WHO Global Campaign Against Epilepsy survey. Epilepsia. 2006; 47: 1225-1231.
- Meinardi H, Scott RA, Reis R, Sander JW. The treatment gap in epilepsy: the current situation and ways forward. Epilepsia. 2001; 42: 136-149.
- Connock M, Frew E, Evans BW, Bryan S, Cummins C, et al. The clinical effectiveness and cost-effectiveness of newer drugs for children with epilepsy. A systematic review. Health Technol Assess. 2006; 10: ix-118.
- Wilby J, Kainth A, Hawkins N, Epstein D, Mclntosh H, et al. Clinical effectiveness, tolerability and cost-effectiveness of newer drugs for epilepsy in adults: a systematic review and economic evaluation. Health Technol Assess. 2005; 9: 1-157.
- Schmidt D. Efficacy of new antiepileptic drugs. Epilepsy currents/American Epilepsy Society. 2011; 11: 9-11.
- Glauser T, Ben-Menachem E, Bourgeois B, Cnaan A, Chadwick D, et al. ILAE treatment guidelines: evidence-based analysis of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2006; 47: 1094-1120.
- Tolman JA, Faulkner MA. Treatment options for refractory and difficult to treat seizures: focus on vigabatrin. Therapeutics and clinical risk management. 2011; 7: 367-375.
- Morrow J, Russell A, Guthrie E, Parsons L, Robertson I, et al. Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. Journal of neurology, neurosurgery, and psychiatry. 2006; 77: 193-198.
- Taylor DC. One hundred years of epilepsy surgery: Sir Victor Horsley's contribution. Journal of neurology, neurosurgery, and psychiatry. 1986; 49: 485-488.
- Duguid C. Macewen of Glasgow; a recollection of the chief. Edinburgh. Oliver and Boyd. 1957.
- Schramm J, Clusmann H. The surgery of epilepsy. Neurosurgery. 2008; 62: 463-481.
- Wilson SJ, Engel J Jr. Diverse perspectives on developments in epilepsy surgery. Seizure: the journal of the British Epilepsy Association. 2010; 19: 659-668.
- Jobst BC, Darcey TM, Thadani VM, Roberts DW. Brain stimulation for the treatment of epilepsy. Epilepsia. 2010; 51: 88-92.
- Saillet S, Langlois M, Feddersen B, Minotti L, Vercueil L, et al. Manipulating the epileptic brain using stimulation: a review of experimental and clinical studies. Epileptic disorders: international epilepsy journal with videotape. 2009; 11: 100-112.
- Fisher R, Salanova V, Witt T, Worth R, Henry T, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010; 51: 899-908.
- Morrell M. Brain stimulation for epilepsy: can scheduled or responsive neurostimulation stop seizures? Current opinion in neurology. 2006; 19: 164-168.
- Theodore WH, Fisher R. Brain stimulation for epilepsy. Acta neurochirurgica. Supplement. 2007; 97: 261-272.
- McClelland S 3rd, Guo H, Okuyemi KS. Population-based analysis of morbidity and mortality following surgery for intractable temporal lobe epilepsy in the United States. Archives of neurology. 2011; 68: 725-729.
- Locharernkul C, Kanchanatawan B, Bunyaratavej K, Srikijvilaikul T, Deesudchit T, et al. Quality of life after successful epilepsy surgery: evaluation by occupational achievement and income acquisition. Journal of the Medical Association of Thailand. 2005; 88: S207-213.
- Tellez-Zenteno JF, Dhar R, Hernandez-Ronquillo L, Wiebe S. Long-term outcomes in epilepsy surgery: antiepileptic drugs, mortality, cognitive and psychosocial aspects. Brain: a journal of neurology. 2007; 130: 334-345.
- Tellez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain: a journal of neurology. 2005; 128: 1188-1198.
- Tellez-Zenteno JF, Hernandez Ronquillo L, Moien-Afshari F, Wiebe S. Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy research. 2010; 89: 310-318.
- Hamiwka L, Macrodimitris S, Tellez-Zenteno JF, Metcalfe A, Wiebe S, et al. Social outcomes after temporal or extratemporal epilepsy surgery: a systematic review. Epilepsia. 2011; 52: 870-879.
- Benifla M, Rutka JT, Otsubo H, Lamberti-Pasculli M, Elliott I, et al. Long-term seizure and social outcomes following temporal lobe surgery for intractable epilepsy during childhood. Epilepsy research. 2008; 82: 133-138.
- Hynes CA, Mar RA. A case study of long-term cognitive and social functioning following a right temporal lobectomy in infancy. Neurocase. 2008; 15: 37-46.
- Langfitt JT, Wood BL, Brand KL, Brand J, Erba G. Family interactions as targets for intervention to improve social adjustment after epilepsy surgery. Epilepsia. 1999; 40: 735-744.
- Derry PA, Rose KJ, McLachlan RS. Moderators of the effect of preoperative emotional adjustment on postoperative depression after surgery for temporal lobe epilepsy. Epilepsia. 2000; 41: 177-185.
- Velasco AL, Boleaga B, Brito F, Jimenez F, Gordillo JL, et al. Absolute and relative predictor values of some non-invasive and invasive studies for the outcome of anterior temporal lobectomy. Archives of medical research. 2000; 31: 62-74.
- Gonnaud PM. Specific medico-social supports for drug-resistant partial epilepsies]. Revue neurologique. 2004; 160: 5S301-307.
- Wilson SJ, Bladin PF, Saling MM, Pattison PE. Characterizing psychosocial outcome trajectories following seizure surgery. Epilepsy & behavior: E&B. 2005; 6: 570-580.
- Burneo JG, Black L, Martin R, Devinsky O, Pacia S, et al. Race/ethnicity, sex, and socioeconomic status as predictors of outcome after surgery for temporal lobe epilepsy. Archives of neurology. 2006; 63: 1106-1110.
- Dupont S, Tanguy ML, Clemenceau S, Adam C, Hazemann P, Baulac M. Long-term prognosis and psychosocial outcomes after surgery for MTLE. Epilepsia. 2006; 47: 2115-2124.
- von Lehe M, Lutz M, Kral T, Schramm J, Elger CE, et al. Correlation of health-related quality of life after surgery for mesial temporal lobe epilepsy with two seizure outcome scales. Epilepsy & behavior: E&B. 2006; 9: 73-82.
- Bergen DC. Results of epilepsy surgery: still so much to learn. Epilepsy currents/American Epilepsy Society. 2006; 6: 80-82.
- Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. The New England journal of medicine. 2001; 345: 311-318.
- Elsharkawy AE, Alabbasi AH, Pannek H, Schulz R, Hoppe M, et al. Outcome of frontal lobe epilepsy surgery in adults. Epilepsy research. 2008; 81: 97-106.
- Tonini C, Beghi E, Berg AT, Bogliun G, Giordano L, et al. Predictors of epilepsy surgery outcome: a meta-analysis. Epilepsy research. 2004; 62: 75-87.
- Ryvlin P, Bouvard S, Le Bars D, De Lamerie G, Gregoire MC, et al. Clinical utility of flumazenil-PET versus [18F]fluorodeoxyglucose-PET and MRI in refractory partial epilepsy. A prospective study in 100 patients. Brain: a journal of neurology. 1998; 121: 2067-2081.
- Richardson MP, Koepp MJ, Brooks DJ, Duncan JS. 11C-flumazenil PET in neocortical epilepsy. Neurology. 1998; 51: 485-492.
- Bouvard S, Costes N, Bonnefoi F, Lavenne F, Mauguiere F, et al. Seizure-related short-term plasticity of benzodiazepine receptors in partial epilepsy: a [11C]flumazenil-PET study. Brain: a journal of neurology. 2005; 128: 1330-1343.
- Hammers A, Koepp MJ, Labbe C, Brooks DJ, Thom M, et al. Neocortical abnormalities of [11C]-flumazenil PET in mesial temporal lobe epilepsy. Neurology. 2001; 56: 897-906.
- Koepp MJ, Hammers A, Labbe C, Woermann FG, Brooks DJ, et al. 11C-flumazenil PET in patients with refractory temporal lobe epilepsy and normal MRI. Neurology. 2000; 54: 332-339.
- Ryvlin P, Bouvard S, Le Bars D, Mauguiere F. Transient and falsely lateralizing flumazenil-PET asymmetries in temporal lobe epilepsy. Neurology. 1999; 53: 1882-1885.
- Juhasz C, Nagy F, Muzik O, Watson C, Shah J, et al. [11C]Flumazenil PET in patients with epilepsy with dual pathology. Epilepsia. 1999; 40: 566-574.
- Koepp MJ, Labbe C, Richardson MP, Brooks DJ, Van Paesschen W, et al. Regional hippocampal [11C]flumazenil PET in temporal lobe epilepsy with unilateral and bilateral hippocampal sclerosis. Brain: a journal of neurology. 1997; 120: 1865-1876.
- Koepp MJ, Richardson MP, Labbe C, Brooks DJ, Cunningham VJ, et al. 11C-flumazenil PET, volumetric MRI, and quantitative pathology in mesial temporal lobe epilepsy. Neurology. 1997; 49: 764-773.
- Debets RM, Sadzot B, van Isselt JW, Brekelmans GJ, Meiners LC, et al. Is 11C-flumazenil PET superior to 18FDG PET and 123I-iomazenil SPECT in presurgical evaluation of temporal lobe epilepsy? Journal of neurology, neurosurgery, and psychiatry. 1997; 62: 141-150.
- Richardson MP, Koepp MJ, Brooks DJ, Fish DR, Duncan JS. Benzodiazepine receptors in focal epilepsy with cortical dysgenesis: an 11C-flumazenil PET study. Annals of neurology. 1996; 40: 188-198.
- Chugani HT, Kumar A, Kupsky W, Asano E, Sood S, et al. Clinical and histopathologic correlates of 11C-alpha-methyl-L-tryptophan (AMT) PET abnormalities in children with intractable epilepsy. Epilepsia. 2011; 52: 1692-1698.
- Phi JH, Paeng JC, Lee HS, Chang Wang K, Kyu Cho B, et al. Evaluation of focal cortical dysplasia and mixed neuronal and glial tumors in pediatric epilepsy patients using 18F-FDG and 11C-methionine pet. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2010; 51: 728-734.
- Maehara T, Nariai T, Arai N, Kawai K, Shimizu H, et al. Usefulness of [11C]methionine PET in the diagnosis of dysembryoplastic neuroepithelial tumor with temporal lobe epilepsy. Epilepsia. 2004; 45: 41-45.
- Didelot A, Mauguiere F, Redoute J, Bouvard S, Lothe A, et al. Voxel-based analysis of asymmetry index maps increases the specificity of 18F-MPPF PET abnormalities for localizing the epileptogenic zone in temporal lobe epilepsies. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2010; 51: 1732-1739.
- Merlet I, Ostrowsky K, Costes N, Ryvlin P, Isnard J, et al. 5-HT1A receptor binding and intracerebral activity in temporal lobe epilepsy: an [18F]MPPF-PET study. Brain: a journal of neurology. 2004; 127: 900-913.
- Assem-Hilger E, Lanzenberger R, Savli M, Wadsak W, Mitterhauser M, et al. Central serotonin 1A receptor binding in temporal lobe epilepsy: a [carbonyl-(11)C]WAY-100635 PET study. Epilepsy & behavior: E&B. 2010; 19: 467-473.
- Meschaks A, Lindstrom P, Halldin C, Farde L, Savic I. Regional reductions in serotonin 1A receptor binding in juvenile myoclonic epilepsy. Archives of neurology. 2005; 62: 946-950.
- Filakovszky J, Gerber K, Bagdy G. A serotonin-1A receptor agonist and an N-methyl-D-aspartate receptor antagonist oppose each others effects in a genetic rat epilepsy model. Neuroscience letters. 1999; 261: 89-92.
- Merlet I, Ryvlin P, Costes N, Dufournel D, Isnard J, et al. Statistical parametric mapping of 5-HT1A receptor binding in temporal lobe epilepsy with hippocampal ictal onset on intracranial EEG. NeuroImage. 2004; 22: 886-896.
- Savic I, Lindstrom P, Gulyas B, Halldin C, Andree B, et al. Limbic reductions of 5-HT1A receptor binding in human temporal lobe epilepsy. Neurology. 2004; 62: 1343-1351.
- Toczek MT, Carson RE, Lang L, Ma Y, Spanaki MV, et al. PET imaging of 5-HT1A receptor binding in patients with temporal lobe epilepsy. Neurology. 2003; 60: 749-756.
- Didelot A, Ryvlin P, Lothe A, Merlet I, Hammers A, et al. PET imaging of brain 5-HT1A receptors in the preoperative evaluation of temporal lobe epilepsy. Brain: a journal of neurology. 2008; 131: 2751-2764.
- Lin TW, de Aburto MA, Dahlbom M, Huang LL, Marvi MM, et al. Predicting seizure-free status for temporal lobe epilepsy patients undergoing surgery: prognostic value of quantifying maximal metabolic asymmetry extending over a specified proportion of the temporal lobe. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2007; 48: 776-782.
- Muzik O, Chugani DC, Shen C, da Silva EA, Shah J, et al. Objective method for localization of cortical asymmetries using positron emission tomography to aid surgical resection of epileptic foci. Computer aided surgery: official journal of the International Society for Computer Aided Surgery. 1998; 3: 74-82.
- Willmann O, Wennberg R, May T, Woermann FG, Pohlmann-Eden B. The contribution of 18F-FDG PET in preoperative epilepsy surgery evaluation for patients with temporal lobe epilepsy A meta-analysis. Seizure: the journal of the British Epilepsy Association. 2007; 16: 509-520.
- Kumar A, Juhasz C, Asano E, Sood S, Muzik O, et al. Objective detection of epileptic foci by 18F-FDG PET in children undergoing epilepsy surgery. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2010; 51: 1901-1907.
- Duncan JS. Imaging in the surgical treatment of epilepsy. Nature reviews. Neurology. 2010; 6: 537-550.
- Salamon N, Kung J, Shaw SJ, Koo J, Koh S, et al. FDG-PET/MRI coregistration improves detection of cortical dysplasia in patients with epilepsy. Neurology. 2008; 71: 1594-1601.
- Lee KK, Salamon N. [18F] fluorodeoxyglucose-positron-emission tomography and MR imaging coregistration for presurgical evaluation of medically refractory epilepsy. AJNR. American journal of neuroradiology. 2009; 30: 1811-1816.
- O'Brien TJ, Miles K, Ware R, Cook MJ, Binns DS, et al. The cost-effective use of 18F-FDG PET in the presurgical evaluation of medically refractory focal epilepsy. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2008; 49: 931-937.
- Vinton AB, Carne R, Hicks RJ, Desmond PM, Kilpatrick C, et al. The extent of resection of FDG-PET hypometabolism relates to outcome of temporal lobectomy. Brain: a journal of neurology. 2007; 130: 548-560.
- Wakamoto H, Chugani DC, Juhasz C, Muzik O, Kupsky WJ, et al. Alpha-methyl-l-tryptophan positron emission tomography in epilepsy with cortical developmental malformations. Pediatric neurology. 2008; 39: 181-188.
- Chassoux F, Rodrigo S, Semah F, Beuvon F, Landre E, et al. FDG-PET improves surgical outcome in negative MRI Taylor-type focal cortical dysplasias. Neurology. 2010; 75: 2168-2175.
- Goffin K, Dedeurwaerdere S, Van Laere K, Van Paesschen W. Neuronuclear assessment of patients with epilepsy. Seminars in nuclear medicine. 2008; 38: 227-239.
- Mazzuca M, Jambaque I, Hertz-Pannier L, Bouilleret V, Archambaud F, et al. 18F-FDG PET reveals frontotemporal dysfunction in children with fever-induced refractory epileptic encephalopathy. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2011; 52: 40-47.
- Akimura T, Yeh HS, Mantil JC, Privitera MD, Gartner M, et al. Cerebral metabolism of the remote area after epilepsy surgery. Neurologia medico-chirurgica. 1999; 39: 16-25.
- Joo EY, Hong SB, Han HJ, Tae WK, Kim JH, et al. Postoperative alteration of cerebral glucose metabolism in mesial temporal lobe epilepsy. Brain: a journal of neurology. 2005; 128: 1802-1810.
- Spanaki MV, Kopylev L, DeCarli C, Gaillard WD, Liow K, et al. Postoperative changes in cerebral metabolism in temporal lobe epilepsy. Archives of neurology. 2000; 57: 1447-1452.
- Muzik O, da Silva EA, Juhasz C, Chugani DC, Shah J, et al. Intracranial EEG versus flumazenil and glucose PET in children with extratemporal lobe epilepsy. Neurology. 2000; 54: 171-179.
- Rosenow F, Luders H. Presurgical evaluation of epilepsy. Brain. 2001; 124: 1683-700.