
Research Article
Austin J Nephrol Hypertens. 2025; 11(1): 1112.
Immune Check-Point Inhibitors and Acute Interstitial Nephritis: A Biopsy-Proven Case Series
Cardoso C¹*; Góis M²; Sousa H²
1Department of Nephrology, Hospital Garcia de Orta, Almada, Portugal
2Department of Nephrology Department, Centro Hospitalar Universitário Lisboa Central, Lisboa, Portugal
*Corresponding author: Cardoso C, Avenida Torrado Da Silva 2805-267 Almada, Portugal. Tel: +351211972677 Email: catarina.cardoso@hgo.min-saude.pt
Received: December 03, 2024; Accepted: December 26, 2024; Published: January o2, 2025
Abstract
Background: Immune Checkpoint Inhibitors (ICI) changed cancer therapy but are associated with immune-related adverse events, including acute kidney injury, particularly Acute Interstitial Nephritis (AIN). This study aims to characterize ICI-associated AIN through a series of biopsy-proven cases.
Methods: We conducted a retrospective analysis of patients with biopsyconfirmed ICI-associated AIN between January 2016 and December 2023. Demographics, cancer type, ICI regimen, co-medications, clinical presentation, biopsy findings, management, and outcomes were analyzed.
Results: Ten patients were included. Most patients (7/10) received singleagent ICIs, predominantly pembrolizumab or nivolumab, and half had a lung adenocarcinoma diagnosis. The median time to AIN diagnosis was four months, with one case presenting nine months post-ICI cessation. At biopsy, median serum creatinine was 3.4 mg/dL. Histology revealed diffuse interstitial inflammation (mononuclear cells alone or with eosinophils) and tubulitis. Coexistent acute tubular necrosis or other nephropathies were observed in 40%. All patients discontinued ICIs and received steroids, with variable regimens. Nine patients achieved partial or complete renal recovery; one patient progressed to end-stage kidney disease.
Conclusions: ICI-associated AIN presents diagnostic challenges due to variable onset and nonspecific clinical features. Kidney biopsy is crucial for diagnosis but is sometimes neglected. Steroid therapy and ICI discontinuation led to favorable renal outcomes in most cases. Further studies are needed to optimize management and guide safe rechallenge strategies, balancing cancer control and adverse effects prevention.
Keywords: Acute Kidney Injury; Acute Interstitial Nephritis; Renal Biopsy; Immune checkpoint inhibitors; Immunotherapy.
Abbreviations: AIN: Acute Interstitial Nephritis; AKI: Acute Kidney Injury; CKD: Chronic Kidney Disease; eGFR: estimated Glomerular Filtration Rate; ICI: Immune Check-point Inhibitors; irAEs: Immune-Related Adverse Events; sCr: Serum Creatinine
Introduction
Immune Check-Point Inhibitors (ICIs) are a class of immunotherapy drugs that have transformed cancer treatment by providing new therapeutic options for several malignancies, including those resistant to conventional therapies or in advanced stages. Currently they are used alone or in combination with other treatments in patients with melanoma, lung cancer, renal cell carcinoma, bladder cancer and Hodgkin lymphoma [1,2]. ICIs are monoclonal antibodies that target inhibitory receptors expressed on T-cells, other immune cells, and tumor cells. They have inhibitory effects on molecules considered important breaks (or checkpoints) of the adaptive immune response, like Cytotoxic T Lymphocyte–Associated protein 4 (CTLA- 4), programmed cell Death Protein 1 (PD-1), and Programmed Death-Ligand 1 (PDL-1) [2]. CTLA-4 is upregulated on the T-cell and competes with the co-stimulatory CD28 ligand molecule, leading to an inhibitory signal and T-cell arrest. Agents blocking CTLA-4 (e.g., ipilimumab) enhance T-cell activation and proliferation. PD-1 and PD-L1 are often upregulated on cancer cells, leading to suppression of T-cell activity and allowing cancer cells to evade immune attack, a common mechanism for cancer cell survival. Agents blocking PD-1 (e.g., nivolumab, pembrolizumab) or PD-L1 (e.g., atezolizumab, durvalumab) restore T-cell function and lead to immune-mediated cancer cell destruction [3]. Although ICIs have recognized benefits and significantly improve prognosis of cancer patients, they also pose some challenges and risks. Immune-Related Adverse Events (irAEs) result from the non-specific overactivation of the immune system with an increase in inflammatory cytokines levels and changes in immune tolerance mechanisms [2]. IrAEs can virtually affect any organ or system, but most affected sites are skin, endocrine glands, gastrointestinal tract, and liver [2,4]. The incidence of irAEs can be as high as 85%, depending on the target and the use of mono- or combination therapy [4,5]. The incidence of Acute Kidney Injury (AKI) in this context has been reported in 2-5% of patients while on ICI therapy and it is most prevalent in patients who receive anti- PDL1-anti-CTLA-4 combination therapy. However, the incidence is rising, and some data report up to 29% of ICI-related AKI [3,6-8].
Acute Interstitial Nephritis (AIN) is the predominant pathologic lesion found in kidney biopsies [3-5]. It is a form of kidney injury characterized by inflammatory infiltrates and edema of the renal interstitium and tubules, typically without significant involvement of the glomeruli or vasculature. The exact pathophysiology is still unknown but is assumed to involve cell-mediated immunity as T-cell-dominant infiltration of the kidney interstitium occurs [9]. AIN is probably underestimated in this population as many patients do not undergo biopsy (e.g. mild sCr rise) or are already under treatment with steroids, masking the diagnosis. Other forms of kidney manifestations are less frequent, but acute tubular necrosis and glomerular diseases have been reported [10-15]. This review intends to provide an overview of ICI-associated AIN, highlighting its pathophysiology, clinical manifestations, diagnostic challenges, and management strategies. We present our experience with biopsy proven ICI-associated AIN, focusing on the histological features and presenting, when available, the corresponding clinical data.
Materials and Methods
This is a retrospective, observational study. We consulted the electronic records of all patients with a biopsy proven ICI-associated AIN received at this center from January 1st, 2016, to December 31st, 2023. Demographic characteristics, cancer type, concurrent Proton Pump Inhibitors (PPIs) use and chemotherapy regimen were considered. Information of prior and concurrent extrarenal irAEs as documented by care providers was collected. Management of AIN regarding the use of steroids, including dose, duration, and taper regimen used was registered when available. Data on kidney and overall outcomes were recorded. AKI severity was staged according to the Kidney Disease Improving Global Outcomes Work Group criteria [16]. All cases were at least an AKI stage 1 (=1.5-fold increase in sCr).
Results
Baseline Characteristics
We found 10 patients with a biopsy-proven AIN while on ICI treatment, included in this review. Baseline characteristics are detailed in Table 1. Our patients had a median age of 68 (min 46; max 79) years and only two were females. Single agent therapy with ICI (either pembrolizumab, nivolumab or atezolizumab) was used in seven of 10 patients (70%). Three patients had concomitant chemotherapy agents, with cisplatin or carboplatin and pemetrexed. The predominant underlying cancer was lung adenocarcinoma (50%). Three patients had concurrent extrarenal irAEs, with rash, thyroiditis or colitis. Only two patients had known preexisting Chronic Kidney Disease (CKD).
Patient
Age
Gender
Cancer Type
ICI
Concomitant cancer therapy
CKD
PPI
Extrarenal irAEs
No.1
65
F
Lung AdenoCa
Pembrolizumab
Cisplatin, pemetrexed
No
Yes
No
No. 2
58
M
Squamous cell carcinoma of the lung
Pembrolizumab
Monotherapy
No
Yes
Rash
No. 3
75
M
Lung AdenoCa
Pembrolizumab
Carboplatin, pemetrexed
No
No
No
No. 4
77
M
Lung AdenoCa
Pembrolizumab
Carboplatin, pemetrexed
Yes
Yes
No
No. 5
46
M
Melanoma
Nivolumab
Monotherapy
No
No
Thyroiditis
No. 6
65
M
Lung AdenoCa
Nivolumab
Monotherapy
ND
No
No
No. 7
76
M
Hepatocellular Carcinoma
Nivolumab
Monotherapy
ND
No
No
No. 8
79
F
Lung AdenoCa
Atezolizumab
Monotherapy
Yes
No
No
No. 9
71
M
Squamous cell carcinoma of the lung
Pembrolizumab
Monotherapy
No
Yes
Colitis
No. 10
52
M
Melanoma
Nivolumab
Monotherapy; previously ipilimumab
No
Yes
No
AdenoCa: Adenocarcinoma; CKD: Chronic Kidney Disease; F: Female; ICI: Immune Checkpoint Inhibitor; irAE: Immune-Related Adverse Event; M: Male; ND: No Data; PPI: Proton Pump Inhibitor.
Table 1: Baseline characteristics before development of AKI/AIN.
Clinical Presentation
Latency between drug initiation and the development of ICI-AIN was highly variable, with the median time being 4 (min 2; max 18) months. One patient (patient 7) had a delayed presentation, with a diagnosis of AIN nine months after cessation of nivolumab. At the time of diagnosis (considered as the time of kidney biopsy), the median sCr was 3.4 (min 1.9; max, 10.1) mg/dl. The median random urine protein/creatinine ratio was 0.4 (min 0; max 20.5) g/g. Five patients had urinalysis available for consultation; four had white cells on urine microscopy (sterile pyuria); one had no changes in urinalysis.
Kidney Biopsy Results
Kidney biopsies showed diffuse interstitial inflammatory infiltrates with interstitial edema (Figure 1) and several degrees of tubulitis (in three cases tubular basement membrane disruption was present – Figure 2). The median number of glomeruli obtained was 7 (min 4; max 15). The infiltrating cells were mononuclear cells only (n=6), mononuclear cells plus eosinophils (n=2), and mononuclear and plasma cells (n=2). Mean percentage of tubular atrophy and interstitial fibrosis was 0% (seen in eight patients; two had 30%). No glomerular lesions were described. One biopsy showed a granuloma with multinucleated giant cells (Figure 3). Immunofluorescence studies were negative in nine patients (one showed albumin lining the tubular basement membrane and capillary walls, consistent with diabetic nephropathy). Final diagnosis was interstitial nephritis only (n=5), interstitial nephritis plus acute tubular necrosis (n=3), or interstitial nephritis plus a non-related diagnosis (n=2; one patient with concurrent diabetic nephropathy and one patient with Congo red positive deposits and positive serum amyloid A in immunohistochemistry studies). One patient with acute tubular necrosis was concurrently being treated with carboplatin.
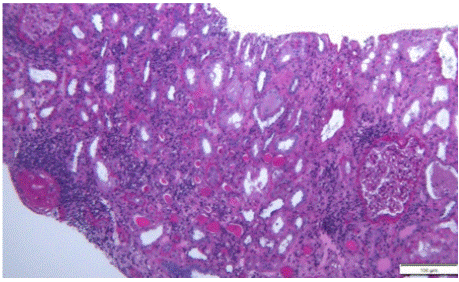
Figure 1: Kidney biopsy fragment on optical microscopy (Periodic Acid-Schiff
stain) showing diffuse interstitial inflammatory infiltrates with mononuclear
cells, suggestive of acute interstitial nephritis. Tubulitis and acute tubular
necrosis are also seen. The glomeruli show normal morphology (one
sclerotic).
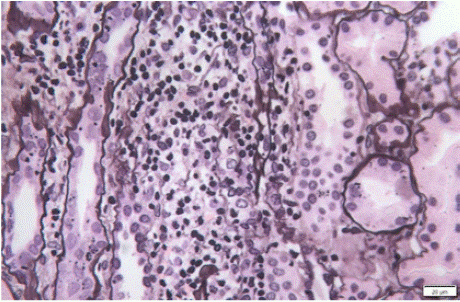
Figure 2: Kidney biopsy fragment on optical microscopy (Silver stain) showing severe tubulitis with tubular basement membrane disruption.
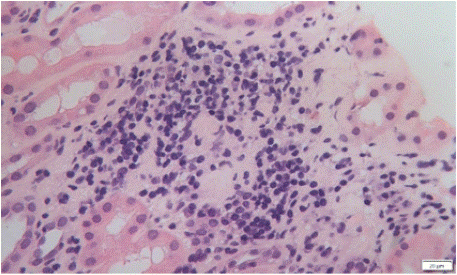
Figure 3: Kidney biopsy fragment on optical microscopy (Hematoxylin and
Eosin stain) suggesting the presence of a granuloma with multinucleated
giant cells.
Management of Patients with Confirmed Ici–Related Ain
All patients had their ICI withheld and were closely monitored. Half of the patients were on a PPI at the time of diagnosis, which was discontinued in all five. No patient was taking nonsteroidal antiinflammatory drugs or was treated with antibiotics at the time of diagnosis.
After the confirmed diagnosis of ICI-related AIN, all patients were treated with steroids. One patient (patient 2) received intravenous pulse steroids (250 mg times three). Steroid dose and taper regimens were highly variable. Most patients started with a 1 mg/kg/day dose (max 80 mg in one patient) for two weeks with a taper regimen over the course of four to six weeks (two months in total in most cases). Two patients received a starting dose of 0.5 mg/Kg/day. Management details are described in Table 2.
Patient
Drug
Time to AIN (months)
ICI withheld
IV Pulse Steroids
Oral Prednisone Starting Dose (mg)
Peak sCr before AIN treatment (mg/dl)
sCr after AIN treatment (mg/dl)
Outcome
No.1
Pembrolizumab
6
Yes
No
60
2.2
1
Patient alive, vigilance
No. 2
Pembrolizumab
3
Yes
Yes (0.75g)
30
10.1
3.8
Progression of disease; patient deceased
No. 3
Pembrolizumab
2
Yes
No
60
6.3
0.9
Patient alive, ICI suspension
No. 4
Pembrolizumab
13
Yes
No
40
4.8
2.2
ND
No. 5
Nivolumab
4
Yes
No
80
1.9
1.2
Rechallenge with stable sCr
No. 6
Nivolumab
2
Yes
No
ND
3
ND
ND
No. 7
Nivolumab
11 (9 months after cessation)
Yes
No
60
3.6
ND
Progression of disease; patient deceased
No. 8
Atezolizumab
3
Yes
No
60
3.2
1.9
ND
No. 9
Pembrolizumab
11
Yes
No
ND
1.9
ND
ND
No. 10
Nivolumab
10
Yes
No
40
4.4
2.7
Hemodialysis
AIN: Acute Interstitial Nephritis; ICI: Immune Checkpoint Inhibitor; IV: Intravenous; sCr: Serum Creatinine; ND: No Data.
Table 2: Management of patients with confirmed ICI–related AIN.
Most patients (9/10) improved their renal function (either completely or partially) with ICI withdrawal and steroids except one patient (patient 10) that initially responded to AIN treatment but later progressed to end-stage kidney disease. This patient had concurrent amyloidosis A diagnosis with persistent urine protein/creatinine ratio > 10 g/day.
Outcome data was available for six patients: in two there was disease progression leading to their death; one patient started hemodialysis (died within the first six months of dialysis); two patients kept stable renal function with ICI suspension and are under surveillance without cancer therapy; one patient was rechallenged with the same agent ICI implicated in the initial AKI episode and did not recur.
Discussion
Renal toxicities of ICI are not considered common irAEs, but their incidence is rising, as these drugs are being more used in clinical practice. AIN is the most common form of ICI associated AKI, and our results are consistent with the literature [5,17]. It poses an important challenge and differs from other causes of drug-associated AIN as ICIs strongly impact the prognosis of cancer patients and their suspension is not a straightforward decision.
Clinical presentation and laboratory findings are not specific and as with other drug-associated AIN, a strong clinical suspicion is necessary for diagnosis. The median time to develop clinically significant AIN in our study was four months, but it is highly variable, and it might happen even after cessation of the drug, as we also reported, making the diagnosis even harder [5,17,18].
Other forms of AKI, namely secondary to hypovolemia, concurrent medications, obstruction, or contrast are frequent in cancer patients and should be excluded [19].
The classic triad of AIN (fever, rash, and peripheral eosinophilia) is rarely seen, and our patients did not present it. Kidney biopsy plays a significant role in confirming the diagnosis, but many patients are considered unsuitable. As with other forms of AIN, the diagnosis is underestimated (in large kidney biopsy registries, AIN accounts for only 1–3%) [20]. Reasons for not doing a kidney biopsy vary, and it can be related with patient characteristics, as high bleeding risk, hemodynamic instability, active infection, comorbidities or increased risk for complications (solitary kidney, obesity), empiric start of steroids with stable or improving kidney function or if the results will not change management plans.
Some authors defend that renal biopsy is generally not recommended unless AKI is refractory to steroids.21 However, considering the possibility of an alternative diagnosis (e.g., acute tubular necrosis or other histologic lesions), the absence of kidney biopsy puts patients at risk of inappropriate exposition to steroids. The need for kidney biopsy should be individualized, weighing both risks and benefits.
Concurrent use of PPIs and presence of extrarenal irAEs were considered independent risk factors for ICI-associated AKI [5,17]. Lower baseline eGFR was considered an independent risk factor in some studies [5,17], but not in others [8]. Cisplatin, carboplatin and pemetrexed are known causes of kidney injury, but generally through toxic effects on tubular epithelium cells with consequent acute tubular necrosis and variable forms of Fanconi syndrome [22].
There is still no consensus as to how to manage these patients. European Society for Medical Oncology (ESMO) Guidelines consider different approaches depending on the severity of kidney injury [3]. For mild AKI (stage 1), it is accepted to maintain ICI therapy, discontinuing all nephrotoxic therapies and monitor kidney function. In a more severe AKI (stage 2 or 3), it is recommended to withhold the ICI therapy, consider kidney biopsy and start steroids (either oral or intravenous pulses). Other studies suggest better outcomes when using high oral doses of steroids (at least 0.8 mg/kg) in the initial period for patients with AKI even in mild cases and pulse intravenous steroids for patients with more severe kidney dysfunction [23]. Early initiation of steroids (up to 14 days following AKI) increases the likelihood of renal recovery [17].
Rechallenge of immunotherapy after recovery from a kidney irAE may be reasonable in the right setting as some authors found that most patients rechallenged with an ICI did not recur, but data is limited and ideal conditions or which patients to rechallenge are yet to be determined [5,17,23,24].
Failure to achieve complete kidney recover after an episode of ICIAKI is a poor prognosis factor, as with other forms of AKI [5,25]. The increased mortality associated with failure to recover from ICI-AKI may also reflect the limited cancer therapeutic options for patients with impaired renal function and reinforces the importance of early recognition and treatment of this entity to allow kidney recovery.
Limitations and Final Considerations
This study has several limitations. It is a retrospective study with a small number of kidney biopsies, limiting the ability to perform statistical analysis for associations or correlations. AIN and other irAEs were not consistently managed and some data, particularly after the kidney biopsy was performed, was not available, limiting the conclusions of management, outcomes and rechallenge of the drug. Also, by only including patients with biopsy-confirmed AIN, our study only has patients with more severe degrees of AKI, and the findings may not be generalizable to mild cases of ICI-associated AKI.
All providers working with ICIs should be aware of irAE development and consider an early nephrology referral and eventually a kidney biopsy when an increase in sCr, abnormalities of urine sediment or persistent electrolyte abnormalities are present. Close monitoring of patients under ICI therapy should be pursued.
There is a need for future prospective studies to accurately identify and characterize kidney irAEs and their response to immunosuppression. More important and considering the critical role these drugs have on patients’ prognosis, there is a need to promptly recognize and treat ICI-AKI, avoiding persistent kidney damage, and develop safe strategies to rechallenge immunotherapy (ideal time, concurrent use of low-dose steroids, considerations on the severity of prior irAEs).
To conclude, and as the clinical experience with these agents increases, multidisciplinary clinical involvement is essential to share insights from different fields of medicine and to fulfill the potential of this treatment approach.
References
- Kim JY, Lee KH, Eisenhut M, van der Vliet HJ, Kronbichler A, Jeong GH, et al. Efficacy of Cancer Immunotherapy: An Umbrella Review of Meta-Analyses of Randomized Controlled Trials. Cancers. 2019; 11: 1801.
- Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018; 378: 158-168.
- Wanchoo R, Karam S, Uppal NN, Barta VS, Deray G, Devoe C, et al. Cancer and Kidney International Network Workgroup on Immune Checkpoint Inhibitors. Adverse Renal Effects of Immune Checkpoint Inhibitors: A Narrative Review. Am J Nephrol. 2017; 45: 160-169.
- Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. ESMO Guidelines Committee: Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up [published correction in Ann Oncol. 2018; 29: iv264–iv266.
- Cortazar FB, Kibbelaar ZA, Glezerman IG, Abudayyeh A, Mamlouk O, Motwani SS, et al. Clinical Features and Outcomes of Immune Checkpoint Inhibitor–Associated AKI: A Multicenter Study. JASN. 2020; 31: 435-446.
- Cortazar FB, Marrone KA, Troxell ML, Ralto KM, Hoenig MP, Brahmer JR, et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 2016; 90: 638-47.
- Perazella MA, Shirali AC. Nephrotoxicity of cancer immunotherapies: past, present and future. J Am Soc Nephrol. 2018; 29: 2039–2052.
- Meraz-Muñoz A, Amir E, Ng P, Avila-Casado C, Ragobar C, Chan C, et al. Acute kidney injury associated with immune checkpoint inhibitor therapy: incidence, risk factors and outcomes Journal for Immuno Therapy of Cancer. 2020; 8: e000467.
- Perazella MA. Checkmate: kidney injury associated with targeted cancer immunotherapy. Kidney Int. 2016; 90: 474-6.
- Mamlouk O, Selamet U, Machado S, Abdelrahim M, Glass WF, Tchakarov A, et al. Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: Single-center experience. J Immunother Cancer. 2019; 7: 2.
- Kishi S, Minato M, Saijo A, Murakami N, Tamaki M, Matsuura M, et al. IgA Nephropathy after Nivolumab Therapy for Postoperative Recurrence of Lung Squamous Cell Carcinoma. Intern Med. 2018; 57: 1259-63.
- Jung K, Zeng X, Bilusic M. Nivolumab-associated acute glomerulonephritis: a case report and literature review. BMC Nephrol. 2016; 17: 188.
- Bickel A, Koneth I, Enzler-Tschudy A, Neuweiler J, Flatz L, Früh M. Pembrolizumab-associated minimal change disease in a patient with malignant pleural mesothelioma. BMC Cancer. 2016; 16: 656.
- Fadel F, El Karoui K, Knebelmann B. Anti-CTLA4 antibody-induced lupus nephritis. N Engl J Med. 2009; 361: 211–212.
- Izzedine H, Mathian A, Champiat S, Picard C, Mateus C, Routier E, et al. Renal toxicities associated with pembrolizumab. Clin Kidney J. 2019; 12: 81- 8.
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012; 120: c179-84.
- Gupta S, Short SAP, Sise ME, Prosek JM, Madhavan SM, Soler MJ, et al. ICPi-AKI Consortium Investigators. Acute kidney injury in patients treated with immune checkpoint inhibitors. J Immunother Cancer. 2021; 9: e003467.
- Shirali AC, Perazella MA, Gettinger S. Association of Acute Interstitial Nephritis With Programmed Cell Death 1 Inhibitor Therapy in Lung Cancer Patients. Am J Kidney Dis. 2016; 68: 287-291.
- Lam AQ, Humphreys BD. Onco-nephrology: AKI in the cancer patient. Clin J Am Soc Nephrol. 2012; 7: 1692-700.
- Goicoechea M, Rivera F, López-Gómez JM; Spanish Registry of Glomerulonephritis. Increased prevalence of acute tubulointerstitial nephritis. Nephrol Dial Transplant. 2013; 28: 112-5.
- Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J Clin Oncol. 2021; 39: 4073-4126.
- Perazella MA. Onco-nephrology: renal toxicities of chemotherapeutic agents. Clin J Am Soc Nephrol. 2012; 7: 1713-21.
- Manohar S, Ghamrawi R, Chengappa M, Goksu BNB, Kottschade L, Finnes H, et al. Acute Interstitial Nephritis and Checkpoint Inhibitor Therapy: Single Center Experience of Management and Drug Rechallenge. Kidney360. 2020; 1: 16-24.
- Herrmann SM. Is Rechallenge Appropriate in Patients that Develop Immune Checkpoint Inhibitor-Associated AKI?: PRO. Kidney360. 2021; 3: 799-802.
- Pannu N, James M, Hemmelgarn B, Klarenbach S; Alberta Kidney Disease Network: Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol. 2013; 8: 194–202.