
Research Article
Austin J Nanomed Nanotechnol. 2025; 13(1): 1076.
Electrochemical Synthesis, Characterization, and Antimicrobial Activity of Nickel Nanowires
Yawar Abbas; Masooma Jawad*; Ali Hassan; Bashir Ahmad
Department of Biological Sciences, International Islamic University, H-10, Islamabad, Pakistan
*Corresponding author: Masooma Jawad, Department of Biological Sciences, International Islamic University, H-10, Islamabad, Pakistan. Email: masoomajawad@bs.qau.edu.pk
Received: December 04, 2024; Accepted: December 26, 2024; Published: January 02, 2025
Abstract
The growing interest in nanotechnology has opened avenues for fresh research into the capabilities, uses and applications of this technology. With many advancements and greater understanding of the internal and external workings of nanotechnology, a demand for the discovery of versatile applications of the nano technological products has given rise to new avenues of research. The unique properties of nanowires allow for the control of structure and function of these nanostructures. With the growing increase in antibiotic resistance among bacteria, a new method for the control of bacterial infections and bacterial growth must be developed which can minimize the risk of development of resistance while providing therapeutic effects in case of bacterial infections. The use of nanowires for the control of bacterial growth is an interesting prospect and must be investigated as it provides a method for the mechanical or physical control of bacterial growth while minimizing side effects such as resistance. The study investigates the synthesis of Nickel nanowires followed by their characterization through various analytical methods and finally the study of their effects as an antimicrobial agent on a number of gram positive and gram-negative bacterial species in order to determine the effectiveness of the nanowires against common disease-causing bacteria.
Keywords: Nanowires; nickel; electrochemical; synthesis; characterization; application; antibacterial; antiseptic; nanotechnology
Introduction
In recent years, much scientific attention has been directed towards the development and application of nanomaterials in various fields. The unique properties of nanomaterials as compared to their bulk counterparts, establish them as prime candidates for future research.
Nanomaterials provide a range of interesting properties with which to experiment and test with the aim of discovering novel applications and benefits. Physically, nanomaterials provide various benefits as compared to bulk materials such as a higher surfaceto- volume ratio, high tensile strength, sustainability and ease of production, environmentally friendly synthesis and applications, conservation of finite natural resources and their applicability to a large host of purposes [18].
The past decade has witnessed a notable increase in nanotechnology products and an increase in understanding and capabilities of the associated technology. Recent advancements in nanotechnology have made possible the miniaturization of electronic circuits and devices and has helped tackle age old problems in the scientific community. In the field of biotechnology, nanomaterials such as nanoparticles, nanofilms and nanowires have opened new avenues for the treatment and cure of various ailments and diseases. Nanomaterials have formed the basis of a new generation of biosensors due to their applicability in the synthesis of thin flexible films whereas nanoparticles have emerged as prime candidates in the field of targeted medicine, cancer treatment and other such fields where traditional technologies have failed to produce results [19].
Keeping in mind these advancements we can explore hitherto unexplored facets of applicability and experimentation with regards to nanomaterials and nanoparticles. One such pressing and concerning problem faced by the medical community in recent years is the growing resistance of bacteria and other micro-organisms to antibiotic and other such anti-microbial agents. This has led to an increase in the severity of common diseases with a resultant increase in the threat of global epidemics arising due to the immunity of microorganisms to traditional medical agents such as anti-biotic.
In the face of this challenge, the possibility of the use of nanomaterials for the targeted extermination of micro-organisms through various mechanisms has arisen as a promising avenue of research and analysis. The use of nanomaterials for the treatment of cancerous cells has also been an object of intensive research in the past decade and has shown promising results. The growing need for alternative methods of microbial control have led to the recent interest in the synthesis, characteristics and application of nanomaterials such as nanoparticles and nanowires. Nickel is a transition metal that has long been in use traditionally as a coinage metal due to its non-corrosive and lustrous nature. It is one of the four such elements that are ferromagnetic at room temperature. Furthermore, nickel also serves as an essential nutrient in plants and animals that have nickel active sites in their enzymes [13]. Nickel is also highly conductive to heat and electricity and Nickel nanomaterials display a very high compressive strength as compared to its bulk material.
Due to its ferromagnetic structure, the nanomaterials of nickel provide the option to be controllable and site directed by localization using magnets or magnetic fields when applied to an experimental setting with regard to micro-organisms where a controllable and regulated action is required. Nickel is a highly biologically active element as it forms a part of many plant and animal enzymes and thus is a crucial part of various biological systems. Nickel also displays high biological toxicity and has also been found to cause cancer in cases where high exposure has been observed [13].
In the form of nanowires, nickel can be used to induce the lysis of biological cells such as bacteria or cancerous tissue through the process of internal entanglement with cellular structures. This property of nickel as a cytotoxic agent paired with the ability to control and confine its activity to a limited area using its ferromagnetic properties has created a niche for the use of nickel nanowires and potent antimicrobial and anticancer agent [17].
This study was aimed at utilizing the most efficient methodology for the synthesis of nickel nanowires, followed by their physical characterization through number of analytical methods and the application of these nanowires as anti-microbial agents was investigated.
Materials and Methods
Synthesis of Nanowires
Aluminum sheet (Whatman, 99.9% pure) was cut 6cm x 6cm with seizer. 6 strips were made each of 1 cm wide. Aluminum sheet was then washed with detergent to remove dirt on it. Then rinsed with distilled water and placed it to dry at room temperature. Electropolishing was performed in a solution of HClO4 (Per chloric acid) and C2H5OH (Ethanol) prepared by mixing HClO4 20% and C2H5OH 80% (1:4 by volume). Before performing the polishing reaction, the solution was placed in a deep freezer to make the solution cool. The Al template was fixed in electrode, acting as anode. The reaction was performed by applying 9 volts DC voltage for 3-4 minutes. During this reaction the surface of aluminum sheet was made smooth and shiny. At the end of the reaction, Al template was removed from the solution, rinsed with distilled water immediately and placed it to dry at room temperature. After getting the polished aluminum, it is then treated with an anodizing solution consisting of phosphoric acid (5ml) in 95 ml of distilled water. The solution was first placed in freezer to make the solution cool for better performance. Aluminum template was fitted in the electrode and then dipped in chilled anodizing solution. The beaker was then placed on stirrer plate which was placed in the refrigerator. Battery was connected in such a way that the positive terminal of the battery was connected to the Al template and negative terminal of the battery was connected to the electrode. The first anodization was performed for 5 hours under constant DC voltage of 60 volts (V). at current 0.06 A. The Al2O3 membrane with vertical pores formed in the aluminum surface during 1st anodization. The pores were a little bit irregular. The regular pores were made in the 2nd anodization reaction. After 5 hours, the connections were removed. The template was removed from the solution. Rinsed with distilled water and kept it to dry. To remove the irregular pores that were made during 1st anodization the template was treated with the heating solution containing chromic acid and phosphoric acid. The template was placed in a clean petri dish. The solution was poured on it to completely dip the template. This petri dish which contains template dipped in heating solution was then placed in oven at 60 degrees Celsius for three hours. After three hours, the template was removed from the solution and then rinsed with distilled water properly. Put the template in a supporting material to dry. During 2nd anodization, the reaction is carried out for 20 hours. The conditions of voltage, current, constant stirring, and the solution was same as in the 1st anodization reaction i.e. 5 ml H3PO4 in 95 ml of distilled water. This reaction gave regular and uniform size pores. The regularity of the pores depends upon the duration of the reaction. After second anodization, the barrier of AAO template was removed. For this, Al strip was dipped in the solution used for anodizing reaction. The reaction was same as anodizing at start such as voltage was 60V and current was kept below 0.06A, but after 1 hour, the voltage was stepped down after specific interval of time i.e. after every three minutes. After 3 minutes 3 volts were stepped down. This process was repeated after every 3 minutes until the voltage decreased to 40V. After 40V, the decrease in voltage was 2 volts after every 3 minutes and this process was repeated until the voltage reached 20V. After 19 volts to 4 volts (the least voltage applied by the battery) the decrease in voltage was 1 volt after every 3 minutes. During this process, the barrier layer completely removed from the bottom of the pores in the templates. The Al strips was then removed from the solution and apparatus and rinsed with distilled water and kept it to dry. Now the template is ready for the electrodeposition of nanowires. The aluminum strips were then fixed in electrode in the same way as done for anodization process. This apparatus was dipped in the 0.1 M solution of NiSO4.6H2O (Figure 6).
The ingredients of the solution were:
NiSO4.6H2O = 2.628 g
Distilled water (dH2O) = 100 ml.
Boric Acid = 0.5 g was used as a buffer.
An AC voltage of 15 volts was applied for 1 minute. Nickel deposited in the pores. The electrodeposited nickel nanowires were then etched in 2 molar solution of sodium hydroxide (NaOH). For this, 8 g of NaOH was added in 100 ml of distilled water. The small pieces of aluminum templates containing nickel in its pores was dipped in the NaOH solution. Nickle nanowires were etched from the pores in the solution. A magnet was placed below the beaker. The magnet attracted the nickel nanowires towards itself. The solution of NaOH was then removed with the help of dropper. These nanowires were washed 5 times with distilled water to remove NaOH if present. The nickel nanowires were then put in the Eppendorf tube and the placed for dry to get it in the powdered form.
Characterization of Nanowires
The templates were then characterized by using SEM and EDS for structural and compositional analysis. Different structural, morphological, compositional analysis, surface and moleculer properties were studied by using X-Ray Diffraction (XRD), Scanning Electron Microscope (SEM), and Energy Dispersive X-ray spectroscopy (EDX).
Biological Testing of Nanowires
The nanowires, after physical characterization were found to be of required specification and next step of experiment was conducted by application of these nickel nanowires to microbial agents to test their antimicrobial and cytotoxic properties. Bacteria were selected to represent a wide range of species to investigate the broadspectrum activity of the nanowires. The bacterial strains selected from pre-existing library are in Table 1. Selected bacteria were grown through streaking method on petri dish having nutrient agar of the composition mentioned in Table 2. Prepared colonies were subjected to gram staining to identify and confirm their nature where standard procedure of staining was utilized. Solution of nanowires was prepared by diluting 5mg of nanowires in 1ml distilled water after which the solution was sonicated for 30 minutes to obtain uniform dispersion of nanowires throughout the solution. This concentration was then adjusted to 2.5mg of nanowires in 1ml distilled water and uniformly distributed through sonication. Both these solutions were applied in two separate phases to achieve statistically relevant results. Reference antibiotic was prepared by taking in 9 to 1 ratio distilled water and Levofloxacin. Samples from prepared plates were obtained and serially diluted in 1ml of saline water and shaken to obtain uniform dispersion of bacteria in solution. Then 100miL of dilute solution was used to spread bacteria on agar plates. In this manner, solutions were inserted into the prepared wells as shown in Table 3. The prepared plates were then incubated for 24 hours at 37 degrees Celsius to obtain growth of each of the selected bacteria. After 24 hours growth of bacteria was observed to identify and measure region of growth inhibition.
Bacteria
Diseases
Pseudomonas aeruginosa
UTI, Respiratory, Soft Tissue, Bone/Joint
Proteus
Nausea, Fever, vomiting
Escherichia coli
Pneumonia, Diarrhea
Klebsiella pneumoniae
Pneumonia, Blood infection, wound
Streptococcus pyogenes
Pharyngitis, skin, acute rheumatic fever
Pseudomonas
Blood, skin, lungs, ear
Streptococcus pneumoniae
Pneumonia, ear, blood, meningitis
Table 1: List of Bacteria.
Component
Amount
Peptone
5g
Beef Extract
3g
Sodium Chloride
5g
Agar
15g
Water
1L
p.H
7.4
Table 2: Nutrient Agar Composition.
Wells
Solutions
1
25μl Nanowire Solution
2
50 μl
3
75 μl
4
100 μl
AB
Reference Anti-biotic 15 μl
C
Control – Distilled Water 25 μl
Table 3: Components of Each Well.
Results
Characterization
Scanning Electron Microscopy
The scanning electron microscopy results taken at 50 thousand and 100 thousand times magnification clearly display finely formed nickel nanowires in a matrix pattern deposited in a tightly wound lattice having clear and distinct unbranched nanowires of atleast 500nm length although some are lesser or greater in length yet average size remains consistent throughout the sample (Figure 1).
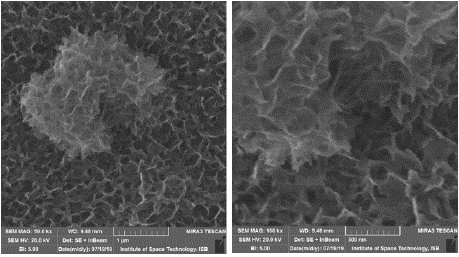
Figure 1: SEM Imaging Results.
X-Ray Diffraction Spectroscopy
The X-ray Diffraction Spectroscopy was performed on the nanowire sample while supported by the anodic aluminum oxide template. The results show a consistent presence of Nickel deposited onto the template along with the presence of the AAO template. The size of the Nickel crystals deposited onto the template are in nano range and comprise the greater part of the sample results (Figure 2 & 3).
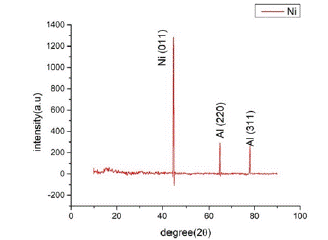
Figure 2: XRD pattern of Nickel sample & Peak list fo XRD analysis.
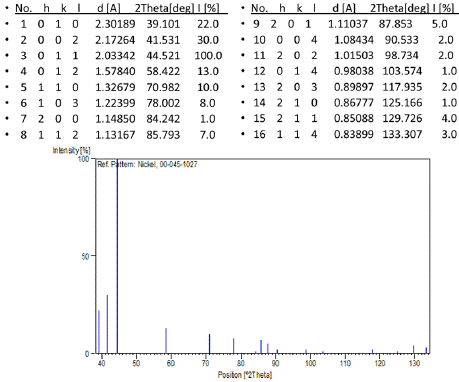
Figure 3: Stick Pattern XRD analysis of Nickel Nanowires.
Energy Dispersive Spectroscopy
Energy Dispersive Spectroscopic analysis or EDX was performed onto the sample to confirm the presence of Nickel Nanowires in the AAO sample and to study the structure of the Nickel nanowires on basis of crystal structure. The results determine the presence of Nickel crystals in the sample where weightage is less due to presence of AAO template nevertheless the Nickel nanowires deposited onto the template are confirmed by the results (Figure 4 & Table 4).
Element
Weight %
Atomic %
Al
59.35
71.49
O
39.28
28.06
Ni
1.37
0.45
Total
100
100
Table 4: Composition of template according to EDX analysis.
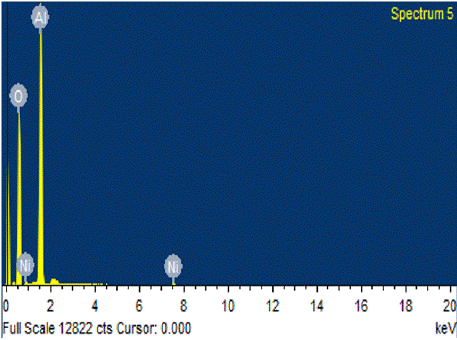
Figure 4: EDX analysis of Nickel Nanowires.
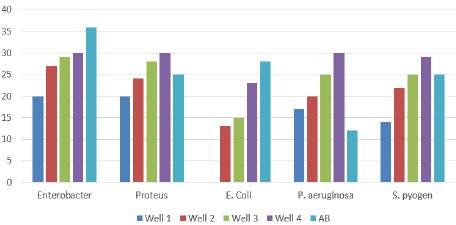
Figure 5: Antimicrobial activity of Nickel nanowires in Phase One.
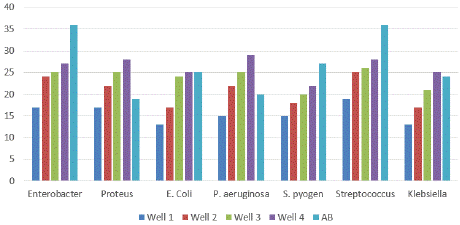
Figure 6: Antimicrobial activity of Nickel nanowires in Phase two.
Biological Application
The nanowires were then used to create a nanowire solution which was applied to biological agents i.e. bacteria in two phases and the resultant antimicrobial activity of the nanowires on the selected bacteria was studied on the basis of size of zone of inhibition created by the bacteria in each of the bacterial samples in two phases, the results of which are displayed below:
Phase One: The phase one results showed clear antimicrobial activity against the selected microbes being comparable in strength to the reference antibiotic and in few cases even surpassing the effects of the reference antibiotic having much greater antibiotic effect on the selected bacteria in terms of greater zone of inhibition as compared to the reference antibiotic in some cases. Thus, the phase one results showed promising effectiveness of nanowires solution in combating the bacteria present in each sample and results of zone of inhibition showed clear effectiveness of the nanowire solution in combating the bacterial samples (Table 5a & b).
Sr. No.
Name of Bacteria
Front side
Back side
1
P. aeriginosa


2
E. coli


3
Enterobacter


4
Proteus


5
S. pyogenes


Table 5(a): Antimicrobial activity of Ni nanowires in Phase 1.
Name of
BacteriaWell 1 (mm)
Well 2
(mm)Well 3
(mm)Well 4
(mm)Antibiotic
(mm)Control
(mm)P. aeriginosa
17
20
25
30
12
0
E. coli
0
13
15
23
28
0
Enterobacter
20
27
29
30
36
0
Proteus
20
24
28
30
25
0
S. pyogenes
12
22
25
29
25
0
Table 5(b): Antimicrobial activity of Ni nanowires in Phase 1.
Phase Two: In phase two the samples were again treated with the nanowire solution but in this case the concentration of the nanowires in the solution was reduced to compare the effect of reduced concentration of the nanowires with the effect of the reference antibiotic with regards to the zone of inhibition created within the samples. The results of phase two are displayed below:
The results from phase two show excellent activity of the nanowire solution on the bacterial samples even at a lower concentration as compared to phase one (Table 6a & b). The nanowires still display a very potent effectiveness on the bacterial agents very much surpassing at times the effectiveness of the reference antibiotic and having activity comparable to the antibiotic in all other cases. The results display a very promising effectiveness of the nanowires in inducing cell death of the bacterial samples selected in both phase one and phase two.
Sr. No.
Name of bacteria
Front side
Back side
1
P. aeriginosa


2
E. coli


3
Enterobacter


4
Klebsiela


5
Proteus


6
S. pyogenes


7
Streptococcus


Table 6 (a): Antimicrobial activity of Ni nanowires in Phase 2.
Name of
bacteriaWell 1
(mm)Well 2
(mm)Well 3
(mm)Well 4
(mm)Antibiotic
(mm)Control
(mm)P. aeriginosa
15
22
25
29
20
0
E. coli
13
17
24
25
25
0
Enterobacter
17
24
25
27
36
0
Klebsiela
13
17
21
25
24
0
Proteus sp.
17
22
25
28
19
0
S. pyogenes
15
18
20
22
27
0
Streptococcus
19
25
26
28
36
0
Table 6 (b): Antimicrobial activity of Ni nanowires in Phase 2.
The results of both Phase One and Phase Two can be compared graphically and are displayed below in the following charts:
Discussion
The results obtained from the characterization of the Nickel nanowire samples provided proof of the synthesis of clear and unbranched nickel nanowires deposited onto the pores of the AAO template [29]. The SEM imaging displayed clear deposited nickel nanowires onto the template and results from the spectroscopic analysis of the samples on one hand provided clear quantitative proof of the presence of the Nickel nanowires on the sample and also provided insight into the crystal structure of the Nickel nanowires.
The application of the nanowires onto the selected bacterial species was noted for the effectiveness of the nanowires when compared the zone of inhibition created by the nanowires solution with the zone created by the reference antibiotic solution.
In both phases of the experiment, the nickel nanowire solution displayed extremely promising effectiveness against the selected bacterial species where in most cases the zone created by the nanowires was greater than the zone created by the antibiotic, in other cases the zone was comparable to that of the antibiotic and in only few cases the activity of the antibiotic was greater than the nanowires.
This effectiveness of the nanowires displays their great potential to be used as antibiotic agents against a wide range of gram negative and gram-positive bacteria as their inhibitory effects to the growth of the bacteria are incredibly promising and comparable or even surpassing the effectiveness of the commonly used antibiotic solutions present for use today.
In this light, the use of the Nickel nanowires synthesized through the AAO Template Assisted Electrodeposition method shows not only the possibility to create functioning and high quality nanowires in one dimension, but also their application onto various biological agents shows their effectiveness for the combating of antimicrobial resistance prevalent among bacteria today across the world by providing a physical methodology for the lysis and control of bacterial cells and populations without having the risk of creating a possibility for resistance in the target bacteria.
Conclusion & Future Prospect
The study investigated the synthesis of Nickel nanowires followed by their characterization through various analytical methods i.e. XRD, SEM and EDAX, and finally the study of their effects as an antimicrobial agent on a number of gram positive and gramnegative bacterial species in order to determine the effectiveness of the nanowires against common disease causing bacteria. The results showed greater effectiveness as compare to the available antibiotic against the bacterial species available in the laboratory.
Now the next aim is to study the anti-cancerous activity of these electrochemically synthesized Nickel nanowires. For this purpose, the nanowires should be tested on cancer cell-lines. Two major results would be drawn from testing the anticancer activity of these nanowires. Firstly, what is the action of nanowires on cancer cells. Secondly, what is the effect of nanowires on normal cells.
Author Statements
Acknowledgements
The authors wish to acknowledge the help and support of International Islamic University, Islamabad specifically Department of Biological Sciences and Department of Physics along with Higher Education Commission, Pakistan.
Conflict of Interest
The authors declare no conflict of interest with any organization, party or individual.
References
- Ahmad J, Alhadlaq HAA, Siddiqui MA, Saquib Q, Al-Khedhairy AA, Musarrat J, et al. Concentration-dependent induction of reactive oxygen species, cell cycle arrest and apoptosis in human liver cells after nickel nanoparticles exposure. Environmental Toxicology. 2005; 30: 137–148.
- Ahmed M, Akhtar MJ, Siddiqui MA, Ahmad J, Musarrat J, Al-Khedhairy AA, et al. Oxidative stress mediated apoptosis induced by nickel ferrite nanoparticles in cultured A549 cells. Toxicology. 2011; 283: 101–108.
- Alarifi S, Ali D, Alakhtani S, Al Suhaibani ES, Al-Qahtani AA. Reactive oxygen species-mediated DNA damage and apoptosis in human skin epidermal cells after exposure to nickel nanoparticles. Biological Trace Element Research. 2014; 157: 84–93.
- Beganskiene A, Sirutkaitis V, Kurtinaitiene M, Juškenas R, Kareiva A. FTIR, TEM and NMR investigations of Stöber silica nanoparticles. Mater Sci (Medžiagotyra). 2004; 10: 287–290.
- Bentley AK, Ellis AB, Lisensky GC, Crone WC. Suspensions of nickel nanowires as magneto-optical switches. Nanotechnology. 2005; 16: 2193.
- Bera D, Kuiry SC, Seal S. Synthesis of nanostructured materials using template-assisted electrodeposition. Jom. 2004; 56: 49–53.
- Buhr E, Senftleben N, Klein T, Bergmann D, Gnieser D, Frase C, et al. Characterization of nanoparticles by scanning electron microscopy in transmission mode. Measurement Science and Technology. 2009; 20: 084025.
- Chen P-C, Shen G, Shi Y, Chen H, Zhou C. Preparation and characterization of flexible asymmetric supercapacitors based on transition-metaloxide nanowire/single-walled carbon nanotube hybrid thin-film electrodes. ACS Nano. 2010; 4: 4403–4411.
- Chen Y, Peng D-L, Lin D, Luo X. Preparation and magnetic properties of nickel nanoparticles via the thermal decomposition of nickel organometallic precursor in alkylamines. Nanotechnology. 2007; 18: 505703.
- Chidchob P, Sleiman HF. Recent advances in DNA nanotechnology. Current Opinion in Chemical Biology. 2018; 46: 63–70.
- Corchero JL, Villaverde A. Biomedical applications of distally controlled magnetic nanoparticles. Trends in Biotechnology. 2009; 27: 468–476.
- El-Sherik A, Erb U. Synthesis of bulk nanocrystalline nickel by pulsed electrodeposition. Journal of Materials Science. 1995; 30: 5743–5749.
- Gerendas J, Sattelmacher B. Influence of Ni Supply on Growth and Nitrogen Metabolism of Brassica napusL. Grown with NH4NO3or Urea as N Source, Annals of Botany. 1999; 83: 65-71.
- Gu H, Xu K, Xu C, Xu B. Biofunctional magnetic nanoparticles for protein separation and pathogen detection. Chemical Communications. 2006; 9: 941–949.
- Guo D, Wu C, Li X, Jiang H, Wang X, Chen B. In vitro cellular uptake and cytotoxic effect of functionalized nickel nanoparticles on leukemia cancer cells. Journal of Nanoscience and Nanotechnology. 2018; 8: 2301–2307.
- Haun JB, Yoon T-J, Lee H, Weissleder R. Magnetic nanoparticle biosensors. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2010; 2: 291–304.
- Hossain MZ, Kleve MG. Nickel nanowires induced and reactive oxygen species mediated apoptosis in human pancreatic adenocarcinoma cells. International journal of nanomedicine. 2011; 6: 1475.
- Huber DL. Synthesis, properties, and applications of iron nanoparticles. Small. 2005; 1: 482-501.
- Jianrong C, Yuqing M, Nongyue H, Xiaohua W, Sijiao L. Nanotechnology and biosensors, Biotechnology advances. 2005; 22: 505-518.
- Kanczler JM, Sura HS, Magnay J, Green D, Oreffo ROC, Dobson JP, El Haj AJ. Controlled differentiation of human bone marrow stromal cells using magnetic nanoparticle technology. Tissue Engineering Part A. 2010; 16: 3241– 3250.
- Klug HP, Alexander LE. X-ray diffraction procedures: For Polycrystalline and Amorphous Materials, 2nd Edition. 1974: 992.
- Rogers JA. Wearable electronics: Nanomesh on-skin electronics. Nature Nanotechnology. 2017; 12: 839.
- Schaefer H-E, Kisker H, Kronmüller H, Würschum R. Magnetic properties of nanocrystalline nickel. Nanostructured Materials. 1992; 1: 523–529.
- Seil JT, Webster TJ. Antimicrobial applications of nanotechnology: Methods and literature. International Journal of Nanomedicine. 2012; 7: 2767.
- Stepniowski WJ, Salerno M. Fabrication of nanowires and nanotubes by anodic alumina template-assisted electrodeposition. Manufacturing Nanostructures. 2014; 12: 321–357.
- Utsunomiya S, Ewing RC. Application of high-angle annular dark field scanning transmission electron microscopy, scanning transmission electron microscopy-energy dispersive X-ray spectrometry, and energy-filtered transmission electron microscopy to the characterization of nanoparticles in the environment. Environmental Science & Technology. 2003; 37: 786–791.
- Volokitin Y, Sinzig J, De Jongh LJ, Schmid G, Vargaftik MN, Moiseevi II. Quantum-size effects in the thermodynamic properties of metallic nanoparticles. Nature. 1996; 384: 621.
- Xu C, Liao J, Yang C, Wang R, Wu D, Zou P, et al. An ultrafast, high capacity and superior longevity Ni/Zn battery constructed on nickel nanowire array film. Nano Energy. 2016; 30: 900–908.
- Trafela Š, Zavašnik J, Šturm S, Rožman KŽ. Formation of a Ni (OH) 2/ NiOOH active redox couple on nickel nanowires for formaldehyde detection in alkaline media. Electrochimica Acta. 2019; 309: 346-353.