
Research Article
Austin J Musculoskelet Disord. 2015;2(1): 1012.
Presence and Localization of Pro-and Mature Forms of Biglycan and Decorin in Human Costal Cartilage Derived from Chest Wall Deformities
Asmar A1, Werner A3, Kelly RE Jr4, Fecteau A5 and Stacey MW1,2*
1Frank Reidy Research Center for Bioelectrics, Old Dominion University, USA
2Department of Pediatrics, Eastern Virginia Medical School, USA
3Department of Pathology, Eastern Virginia Medical School and Med Director of Laboratories, Children's Hospital of The King's Daughters, USA
4Department of Surgery, Eastern Virginia Medical School and Pediatric Surgery Division, Children's Hospital of the King's Daughters, USA
5Hospital for Sick Children, Canada
*Corresponding author: Michael Stacey, Frank Reidy Research Center for Bioelectrics, 4211 Monarch Way, Suite 300, Norfolk, VA23508, USA
Received: November 03, 2014; Accepted: January 07, 2015; Published: February 10, 2015
Abstract
Costal cartilage is a type of hyaline cartilage that forms rod-like structures that connect the ribs to the sternum. The most common chest wall deformities, pectus excavatum and pectus carinatum involved efective costal cartilage resulting in sternal displacement. Costal cartilage is not widely studied leaving little insight into possible factors involved in the pathogenesis of these pectus deformities. This study focused on the presence and distribution of two important regulators of collagen fibrillogenesis and organization, biglycan and decorin. Immunohistochemical analysis of transverse cross sections of normal and deformed costal cartilage revealed that biglycan and decorin mainly localized in the territorial matrix except for prodecorin which was only found within chondrocytes. Western blot analysis of whole protein extracts demonstrated the presence of both pro and mature forms of biglycan and mature decorin in patients and controls. In normal costal cartilage of different ages, the mature form of decorin was absent in a fetal sample whereas mature biglycan was weakly expressed, suggestive that mature biglycan may play a role in early costal cartilage development. Further studies are needed to determine the functional differences between the pro- and mature forms of biglycan and decorin both in age and disease
Keywords: Decorin; Biglycan; Prodecorin; Probiglycan; SLRP; Chest wall deformities
Introduction
The biology of costal cartilage is much understudied, yet disorders of costal cartilage have major clinical consequences. Pectus excavatum and pectus carinatum show disfigurement of the chest wall primarily resulting in severely concave or convex chest wall with associated heart, lung, and psychological anomalies. The costal cartilage of patients with chest wall deformities is considered abnormally grown and weak, suggestive of underlying structural defects in growth and assembly. Ultrastructural electron microscopy images have shown that human costal cartilage is unlike other cartilage types. Images appear to show that individual fibers are assembled to collectively form very thick, fascicle structures appearing to consist of large numbers of collagen nanostraws that run parallel along the cartilage length [1]. The morphological form of costal cartilage and how this relates to function is currently unknown.
Small leucine rich proteoglycans (SLRPs) are extra cellular matrix molecules that bind strongly to collagen and other matrix molecules. They are associated with collagen fibril formation and therefore important in the proper formation of ECMs. The cooperation, sequential, timely, orchestrated action of SLRPs that shape architecture and mechanical properties of the collagen matrix and overall importance in disease is reviewed [2,3]. Indeed, SLRP knockout mice exhibit disorganized collagen fibers and loss of connective tissue function [4]. SLRPs are approximately 40kDa, compared to ~200kDa for the large aggregating proteins aggrecan and versican, possess numerous adjacent leucine rich repeats, and one or very few glycosaminoglycan (GAG) side chains. SLRPs are generally expressed in a very tissue-specific manner. Mechanistically, it is accepted that horseshoe-shaped SLRPs interact with collagen molecules by their concave surfaces, and the space inside their curved shape accommodates a single triple helix of collagen [5,6]. Mutations in SLRPs may be important as predisposing genetic factors for diseases of the ECM.
Reports of decreased biomechanical stability of costal cartilage in pectus excavatum patients and a suggested disorderly arrangement and distribution of collagen fibers in chest wall deformities [7,9] led us to examine the size and distribution of two SLRPs expressed in cartilage, decorin and biglycan. Both regulate ECM organization and fibrillogenesis. Interestingly, both sequester transforming growth factor beta (TGFβ), controlling availability of this growth factor and thus growth of cartilage, suggesting a mechanistic role for these two SLRPs in disorders of cartilage growth and assembly [10,11]. Decorin and biglycan are prominent class I members of the SLRP family and are homologous (55% identity at the amino acid level) but with divergent patterns of expression [12]. Despite their similarities, biglycan and decorin have distinct functions which may partially result from differences in GAG chains; decorin having one, and biglycan two O-linked GAG chains respectively and three and two N-linked oligosaccharides[13,14].Biglycan deficiency has been shown to cause spontaneous aortic dissection and rupture in mice [15], a characteristic of Marfan syndrome,a syndrome known to exhibit chest wall deformities. Decorin function is consistent with functions related to fibrillogenesis[2-16].
Decorin and biglycan each occur in two different forms, the pro- and mature (or processed) forms. Proforms of biglycan and decorin have N-terminal pro-peptides that are14 and 21 amino acid residues respectively which are cleaved in the mature forms by bone morphogenic protein (BMP-1) [17,18]. The abundance of proforms of both SLRPs is tissue and age-dependent with the mature form being present in both juvenile and adult tissue and the proform mainly in adult tissue[19,20]. The complex arrangement of fibers observed in costal cartilage and the role of decorin and biglycan in these structures has not been explored. In this study we investigated the presence and distribution of the different forms of decorin and biglycan, hypothesizing that both mature and proforms are functionally required in our late-juvenile samples. This was achieved by immunohistochemistry of costal cartilage from adolescent patients with pectus carinatum and an age-matched control. Our results show the localization of both forms of biglycan and mature form of decorin in the territorial matrix in patient and control samples. Prodecorin was only seen within chondrocytes. Proforms of decorin and biglycan are maintained evolutionarily, suggesting they play an important, but as yet undetermined functional role.
Materials and Methods
Subjects
Human costal cartilage was obtained from 3 patients with Pectus Carinatum (PC) severe enough to warrant surgical repair. Informed consent was obtained following IRB approval of the protocol at EasternVirginiaMedicalSchool. The IRB protocol currently prevents disclosure of many clinical features, and thus close correlation of clinical phenotype with expression is not possible. A consenting case of pectus excavatum (PE) was obtained from the Hospital for Sick Children, Toronto, Canada. Costal cartilage samples were collected from ribs 6-8 at surgery. Experiments were performed on the round, rod-like, mid-sections of cartilage. All patients were male, with an age range of late-teen to early 20's. Normal costal cartilage was obtained from an age-matched control, a 15 year old male, as well as a 36 week and 81 year oldsample and processed within 24 hours. Resected samples were snap frozen in liquid nitrogen and stored at -80oC until use.
Immunohistochemical analysis
Confirmation of protein distribution was made by immunohistochemistry. Frozen cartilage from 3 cases of PC and an age-matched control were mounted in CRYO-OCTC ompound (Tissue-Tek, CA, USA) and sections (5μm) generated using a Microm HM525 cryostat (Microm International, Walldorf, Germany). Sections were fixed in ice-cold 3:1Methanol:Acetone.Sections were digested with 1.25U/ml Chondroitinase ABC (C3667, Sigma- Aldrich, St. Louis, MO, USA) at 37°C for 2 hours before staining to remove O-linked glyocosaminoglycans and expose the epitope for antibody binding. Blocking, incubation with secondary antibodies, and washes were performed following manufacturer's guidelines for the ImmunoCruz rabbit LSAB staining system (sc-2051, Santa Cruz, Santa Cruz, CA, USA). Tissues were incubated with rabbit polyclonal antibodies specific for probiglycan (LF104), mature biglycan (LF112), prodecorin (LF110), or mature decorin (LF136). Negative controls were produced using normal mouse IgG included in the ImmunoCruz rabbit LSAB staining system.Electronic images were captured using an Olympus DP70 CCD camera through an Olympus BX51 microscope(Olympus America Inc., Center Valley USA). LF-# antibodies were kindly provided by Dr L. Fisher, NIDCR, Bethesda, MD [21].
Protein extraction
Protein extraction was performed using the Fisher-Termine procedure [22] adapted for non-mineralized tissue. All extraction steps were performed with the addition of COMPLETE mini EDTAfree protease inhibitor cocktail (Roche, Mannheim, DE) to prevent the degradation of proteins due to proteases. Transverse segments of whole tissue approximately 0.2g were frozen in liquid nitrogen then crushed into a fine powder. All samples were rinsed with an extraction buffer (4M guanidine-HCl and 50mM Tris, pH 7.5), spun, and the supernatant removed. The samples were then tumbled overnight in a large volume of extraction buffer. The remaining pellets were rinsed twice in extraction buffer before being transferred into Spectra/ Por Dialysis Membranes (Spectrum Labs, Rancho Dominguez, CA, USA), with a 10mm flat width and 12-14kDa molecular weight cut off, and dialyzed in tubes containing extraction buffer for two days. The contents of the dialysis membranes were removed and the supernatants dialyzed against water overnight. The resulting supernatants were then lyophilized.
Western blot analysis
Lyophilized extracts were reconstituted, and approximately 100 μg of each total extractwas separated by electrophoresis on 12.5% Tris-Glycine gels (Bio-Rad, Hercules, CA, USA). Extracts were treated with 1.25 U/ml Chondroitinase ABC (Sigma-Aldrich) at 37°C for 2 hours before loading to remove O-linked glyocosaminoglycans. To investigate cleavage of N-linked deglycosylationfrom core proteins, ABCase treated extracts were denatured at 100°C for 5 minutes then digested with PNGase-F using a N-Glycanase kit (ProZyme, Hayward, CA, USA) for 16 hours at 37°C following manufacturer's guidelines. After electrophoresis, the samples were transferred to nitrocellulose membranes (Bio-Rad). The nitrocellulose membranes were washed, blocked, andincubated with primary and secondary antibodies following manufacturer's guidelines for Odyssey IRDye fluorescent antibodies (LI-COR, Lincoln, NE, USA).Membranes were incubated with rabbit polyclonal antibodies specific for probiglycan (LF104), mature biglycan (LF112), prodecorin (LF110), or mature decorin (LF136). The stained membraneswere imaged using the Odyssey Infrared Imaging System (LI-COR).Positive controls of purified recombinant biglycan and decorin proteoglycans,derived from mouse and human respectively, were kindly provided by Dr M. Young of the NIDCR, Bethesda, MD.
Results
To better understand the function and importance of decorin and biglycan in defective costal cartilage biology, immunohistochemistry and western blot analysis were performed on a limited sample group in order to observe where the protein localizes within the tissue and if any isoforms of the protein exist. The tissue sections and protein extracts were all digested with ABC Chondroitinase to remove O-linked glycosaminoglycans in the N-terminus, a prerequisite to expose the epitope for antibody binding. Examples of patients with defective costal cartilages are shown in (Figure1).
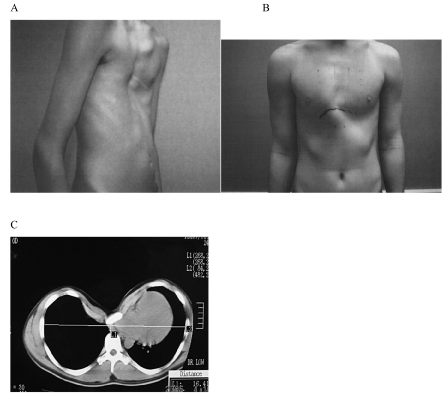
Figure 1: Patients with A) pectus excavatum and B) pectus carinatum. C) A CT scan of a severe case of pectus excavatum showing displacement of the sternum towards the spinal column with heart and lung compression.
Probiglycan
Immunohistochemical analysis using LF-104 showed strong localization of probingly canaround the lacunae in the territorial matrix in PC3, 4, 5, and control. (Figure 2A) shows representative staining. Staining specificity was confirmed with controls using normal rabbit IgG.
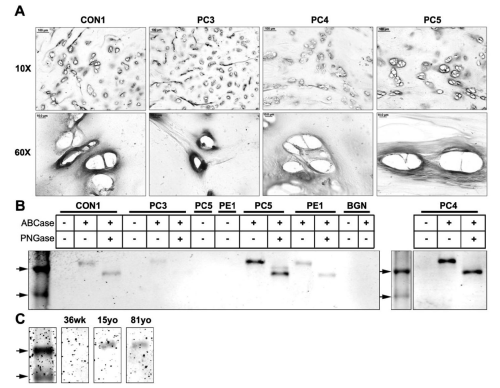
Figure 2: Western blot and immunohistochemical analysis of probiglycan in cartilage tissues with pectus deformities and varying ages. (A) Immunohistochemical analysis of probiglycan in transverse cross sections of a control sample (CON1) and pectus carinatum samples (PC3, 4, 5) at 10x and 60x (Scale bars: 10x = 100μm, 60x = 10μm). (B) Western blot analysis of probiglycan in an age- matched control sample (CON1), pectus carinatum samples (PC3, 4, and 5), pectus excavatum (PE1), and purified recombinant biglycan proteoglycan (BGN). Marker arrows are 44kDa and 37kDa. (C) Western blot analysis of probiglycan in normal samples of the ages 36 weeks (36wk), 15 years old (15yo), and 81 years old (81yo). Top arrow is 44kDa.
Western blot analysis with 100μg of protein extracts from CON1, PC 3, 4, 5, and PE1 are shown in Figure 2B. Purified recombinant mouse biglycan proteoglycan is shown in lane BGN. As expected, undigested (-/-) samples did not show stained protein bands as the LF104 antibody epitope is unexposed. PC samples and control all showed bands at ~50kDa after digestion to remove O-linked GAG's, and a further reduction in size (~40kDa) following digestion of N-linked GAG's. Even though the same amount of protein was loaded, different intensities of bands are present. PC3 showed very light staining compared to Con1 and PE1. PC4 and PC5 appeared to express comparatively more protein, with a doublet present in PC5 at ~40kDa-42kDa. The more intense band at ~40kDacorresponds to the biglycan core protein whereas the lighter ~42kDa band may be due to incomplete digestion of the N-glycans. The recombinant mouse biglycan (BGN) did not stain, possibly due to the epitope not being recognized or unmasked. Western blot analysis of costal cartilage from three different age groups showed staining in only the 15yo and 81yo samples, with no staining in the 36wo, consistent with expression of preforms expressed primarily in adult tissue.
Mature biglycan
Mature biglycan, the processed form of probiglycan, where the 140 amino acid chain is cleaved, was studied using immunohistochemistry and western blotting. Immunohistochemical analysis for mature biglycan using LF-112 revealed positive staining in the territorial matrix (Figure 3A) in patient and control samples.
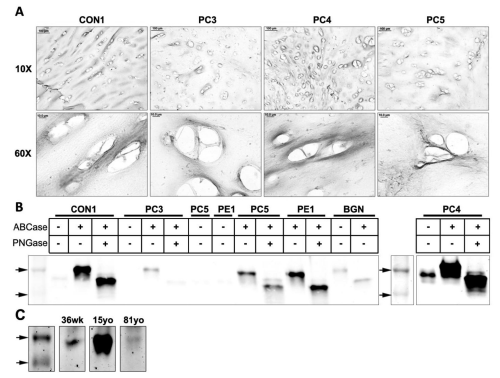
Figure 3: Western blot and immunohistochemical analysis of mature biglycan in tissues with pectus deformities and varying ages. (A) Immunohistochemical analysis of mature biglycan in transverse cross sections of a control sample (CON1) and pectus carinatum samples (PC3, 4, 5) at 10x and 60x (Scale bars: 10x = 100μm, 60x = 10μm). (B) Western blot analysis of mature biglycan in a control sample (CON1), pectus carinatum samples (PC3, 4, and 5), pectus excavatum (PE1), and purified recombinant biglycan proteoglycan (BGN). Marker arrows are 44kDa and 37kDa. (C) Western blot analysis of mature biglycan in normal samples of the ages 36 weeks (36wk), 15 years old (15yo), and 81 years old (81yo). Top arrow is 44kDa.
(Figure 3B) shows western blot analysis of costal cartilage protein extracts. All samples showbands at ~50kDa after digestion to remove O-linked GAG's, and a further reduction in size (~40kDa) following digestion of N-linked GAG's. Similar to pro biglycan, PC3 showed only faint bands for mature biglycan, possibly indicating this patient is producing less biglycan compared to other samples. PC4 appeared to be overloaded, which may account for the 50kDa band in the undigested sample, rather than changed protein confirmation exposing the antibody epitope. We performed Western blot analysis of samples of different ages (Figure 3C). Both the 36 week old and 15yo showed clear 50kDa bands.A faint band wasobserved in the 81 yr. old but results are consistent with stronger expression of the mature form in the younger age group.
proforms
Immunohistochemical analysis with LF-110 revealed protein localization in small spots within the lacunae (Figure 4A). These small intense spots are likely to be chondrocytes which have shrunken due to the fixation process. Pro-decorin appears to be an intracellular protein.
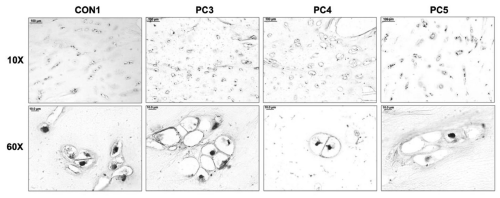
Figure 4: Immunohistochemical analysis of prodecorin in tissues with pectus deformities. (A) Immunohistochemical analysis of prodecorin in transverse cross sections of a control sample (CON1) and pectus carinatum samples (PC3, 4, 5) at 10x and 60x (Scale bars: 10x = 100μm, 60x = 10μm).
Western blot analysis showed no bands, presumably due to the high ratio of extracellular matrix protein to cellular protein (data not shown).
Mature decorin
Immunohistochemical analysis for mature decorin using LF- 136 revealed strong staining in the territorial matrix (Figure 5A) in patient and control samples.
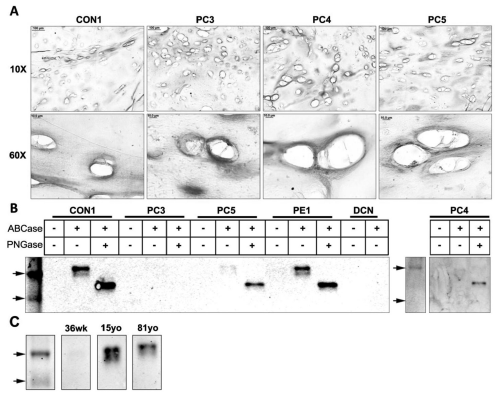
Figure 5: Western blot and immunohistochemical analysis of mature decorin in tissues with pectus deformities and varying ages. (A) Immunohistochemical analysis of mature decorin in transverse cross sections of a control sample (CON1) and pectus carinatum samples (PC3, 4, 5) at 10x and 60x (Scale bars: 10x = 100μm, 60x = 10μm). (B) Western blot analysis of prodecorin in a control sample (CON1), pectus carinatum samples (PC3, 4, and 5), pectus excavatum (PE1), and purified recombinant decorin proteoglycan (DCN). (C) Western blot analysis of mature decorin in normal samples of the ages 36 weeks (36wk), 15 years old (15yo), and 81 years old (81yo).
Western blot analysis revealed an apparent core protein doublet ~44kDa-47kDa in Con1, PE1 and to a lesser extent in PC4 and PC5. PC3 showed little staining for mature decorin (Figure 5B). Digestion of N-GAG's revealed a ~40kDa band in all but PC3. Western blot analysis of samples of different ages showed mature decorin core protein to be present in adolescent (15yo) and adult (81yo) tissue but absent in fetal (36wk) tissue (Figure 5C). More fetal samples need to be investigated to determine the biological relevance of this result. It is not clear why a doublet was not observed in the human decorin recombinant control.
Discussion
Pectus excavatum and pectus carinatum are chest wall deformities with unidentified etiology resulting in displacement of the sternum. The organization and regulation of the ECM in costal cartilage is obscure and requires further investigation in order to understand what leads to the presentation of these deformities. Costal cartilage of patients with pectus deformities have been described as having cartilage that is weak and abnormally grown [23], which led us to investigate two important regulators of ECM fibrillogenesis, biglycan and decorin. This work is the first description of the presence and distribution of biglycan and decorin in their different forms in human costal cartilage.Our results show the presence of mature form of decorin and pro-and mature forms of biglycan in the inter-territorial matrix in patient and control samples. Prodecorin was localized cellularly only. There appeared to be no correlation of presence and localization of these proteins to chest wall deformities in the small samples investigated.
Processing of functional ECM proteins prior to secretion is complex and often age-dependent [24]. Spatial and temporal processing of proforms of decorin and biglycan forms a dilemma as differential functions of pro and mature forms are not well understood. Immunohistochemistry clearly show the distribution of biglycan and decorin. Prodecorin was the only protein investigated that localized intra cellularly. This is in agreement with reports in the literature that secretion of prodecorin from cells has not been observed suggesting that prodecorin acts as an intermediate in intracellular processing [16]. It is effectively removed in vivo and is rarely detected, even in decorin rich tissues [19]. The remaining proteins were found at high levels surrounding the lacunae. This is indicative of the orchestration of the ECM production occurring soon after proteins are secreted from the cell. Biglycan likely initiates collagen fibril organization that is further assembled by decorin to form the large nanostraw-like structures characteristic of costal cartilage. Many other proteins will play a crucial role in the final network [25]. Decorin and biglycan sequester TGFβ, controlling availability of this growth factor to chondrocytes. This needs to be proven in the environment of costal cartilage.
The components of decorin and biglycan, (leucine rich repeats, absence or presence of propeptide, and variable O-and N-linked glycosylated side chains), allow for multiple interactions with collagen fibrils and may be an important factor in cartilage stability in relation to formation of chest wall deformities.Careful analysis of the composition of GAG side-chains may be warranted as the sugar content of such chains has been suggested to play a role in binding of collagen fibers and pathology [26].Increasing attention is being given to the glycosylated side chains of proteins, particularly with relevance to disease [27]. Differences in side chains of decorin and biglycan have been described from different cartilage sites, including non-glycosylated decorin and biglycan in nucleus pulposus of inter vertebral discs [28]. Fragmentation of SLRPs is associated with degeneration of matrix has been described in meniscus, knee and hip cartilage [29].Approximately 50kDa and 40kDa bands are typically observed in biglycan and decorin following removal of O-linked and N-linked glycans respectively, which we observed in probiglycan, mature biglycan, and mature decorin. Bands at ~50kDa are O-linked digested core proteins with N-glycans still attached, and the doublets observed for mature decorin are attributed to variable N-glycanation. Following removal of N-linked glycans, the ~50kDa bands, and the doublets,are replaced by a 40kDaband thought to be the core fragment.
The ultra-structural morphology of costal cartilage appears to be complex, with large straw-like structures reported [1]. In the same study, decorin gene expression was higher than that of biglycan. At the protein level observed here, biglycan is more predominant than decorin, suggesting that differential post-translational processing of these proteins is occurring. Alternatively, a high turnover rate results in more decorin is being produced as the tissue matures. Additionally, the presence of both the mature and pro forms of biglycan is present in our late teen to early 20's samples. Although mature forms are reported to be more prevalent in juvenile tissues, juvenile may be defined to include our age range. The functional interaction of pro and mature forms in this tissue remains to be determined. There are no apparent differences between our age-matched control and patient samples.
The inheritance of chest wall deformity is extremely complex [30,31]. It is of interest that males are predominantly affected (4 male: 1 female) and that biglycan is located on the X-chromosome. A single copy of this gene in males makes biglycan a possible candidate gene where mutations may have pathological consequences however the importance of the role of enzymes responsible for glycosylation of biglycan cannot be overlooked.
Overall, the results clearly show the presence of both pro-and mature forms of decorin and biglycan in human costal cartilage. There appears to be no difference in patient samples with an age-matched control for either types of decorin. The presence of fragments of mature biglycan at 40-44kDa suggests that the complex structures of costal cartilage includes different glycosylated forms of the protein that need further clarification. Glycanated side chains show length variation, with shorted chains being associated with tighter collagen fiber configuration [32]. More recent work [33] has shown that decorin may bind to one collagen fibril by its core protein and to another by its side chain. Additionally, they demonstrate that closely related side chain molecules affects fusion and layout of collagen fibers. This exemplifies the importance of recognizing subtle variations in these molecules and their biological consequences in addition to enzyme systems that are responsible for synthesis and assembly of these molecules.Conversely, the presence of pro- and mature forms of these two proteins may be a reflection of their exposure to cleaving enzymes like BMP-1, and their presence is more a reflection of enzyme exposure and activity, rather than differential function of the two forms. The formation of large nanostraws in costal cartilage may hinder proper visualization of collagen-associated proteins by immunohistochemical analysis in the inter-territorial matrix as the proteins may be embedded deep within the structures.
The development, organization, and regulation of the ECM by SLRPs are complex and understudied in costal cartilage. The two SLRPs biglycan and decorin are key players in this process and while the structures and general function have been observed, the importance of the different forms and glycanation are unknown. The lack of apparent differences between patients and controls suggest, at least at this level of investigation, that decorin and biglycan may not be causative agents of chest wall deformities. It has even been suggested that rib abnormalities may be secondary to events of the thorax, with costal cartilage responding to micro-environmental factors, changing their biological characteristics as a result [34].
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institute of Health under the award number R21AR063334. The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH. The authors would also like to thank Dr. M. Young and Dr. L. Fisher of the NIDCR for their invaluable input in experimental design, recombinant proteins and use of antibodies.
References
- Stacey MW, Grubbs J, Asmar A, Pryor J, Elsayed-Ali H, Cao W, et al. Decorin expression, straw-like structure, and differentiation of human costal cartilage. Connect Tissue Res. 2012; 53: 415-421.
- Ameye L,Young MF. Mice deficient in small leucine-rich proteoglycans. novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular, dystrophy and corneal diseases. Glycobiology. 2002;12:107R-16R.
- Halper J. Proteoglycans and diseases of soft tissues. Adv Exp Med Biol. 2014; 802: 49-58.
- Young MF1, Bi Y, Ameye L, Chen XD. Biglycan knockout mice: new models for musculoskeletal diseases. Glycoconj J.2002; 19: 257-262.
- Weber IT, Harrison RW, Iozzo RV. Model structure of decorin and implications for collagen fibrillogenesis. J Biol Chem. 1996; 271: 31767-31770.
- Scott JE. Proteodermatan and proteokeratan sulfate (decorin, lumican/fibromodulin) proteins are horseshoe shaped. Implications for their interactions with collagen.Biochemistry. 1996; 35: 8795-9.
- Feng J, Hu T, Liu W, Zhang S, Tang Y, Chen R, et al. The biomechanical, morphologic, and histochemical properties of the costal cartilages in children with pectus excavatum. J Pediatr Surg. 2001; 36: 1770-6.
- Rupprecht H, Hummer HP, Stoss H, Waldherr T. Pathogenesis of chest wall abnormalities--electron microscopy studies and trace element analysis of rib cartilage. Z Kinderchir. 1987; 42: 228-9.
- Fokin AA, Steuerwald NM, Ahrens WA, Allen KE. Anatomical, histologic, and genetic characteristics of congenital chest wall deformities.SeminThoracCardiovasc Surg. 2009; 21: 44-57.
- Schaefer L, Iozzo RV. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J Biol Chem2008; 283: 21305-9.
- Boivin WA, Shackleford M, VandenHoek A, Zhao H, Hackett TL, Knight DA, et al . Granzyme B cleaves decorin, biglycan and soluble betaglycan, releasing active transforming growth factor-beta1. PLoS One. 2012; 7: 33163.
- Fisher LW, Termine JD, Young MF. Deduced protein sequence of bone small proteoglycan I (biglycan) shows homology with proteoglycan II (decorin) and several nonconnective tissue proteins in a variety of species. J Biol Chem. 1989; 264: 4571-6.
- Heinegard D. Proteoglycans and more--from molecules to biology. Int J ExpPathol. 2009; 90:575-86.
- Krishman P, Hocking AM, Scholtz JM, Pace CN, Holik KK, McQuillan DJ. Direct secondary structures of the leucine-rich repeat proteoglycans decorin and biglycan. The J. of Biol. Chem. 1999; 274; 10945-10950
- Heegaard AM, Corsi A, Danielsen CC, Nielsen KL, Jorgensen HL, Riminucci M, et al. Biglycan deficiency causes spontaneous aortic dissection and rupture in mice. Circulation. 2007; 115: 2731-8.
- Bianco P, Fisher LW, Young MF, Termine JD, Robey PG. Expression and localization of the two small proteoglycans biglycan and decorin in developing human skeletal and non-skeletal tissues. J HistochemCytochem. 1990 ; 38: 1549-63.
- von Marschall Z, Fisher LW. Decorin is processed by three isoforms of bone morphogenetic protein-1 (BMP1). BiochemBiophys Res Commun. 2010; 391: 1374-8.
- Scott IC, Imamura Y, Pappano WN, Troedel JM, Recklies AD, Roughley PJ, et al. Bone morphogenetic protein-1 processes probiglycan. J. Biological Chem. 2000; 275: 30504-30511
- Roughley PJ, White RJ, Mort JS. Presence of pro-forms of decorin and biglycan in human articular cartilage.Biochem J. 1996; 318:779-84.
- Götz W, Barnert S, Bertagnoli R, Miosge N, Kresse H, Herken R. Immunohistochemical localization of the small proteoglycans decorin and biglycan in human intervertebral discs. Cell Tissue Res.1997; 289: 185-190
- Fisher LW, Stubbs III JT, Young MF. (1995). Antisera and cDNA probes to human and certain animal model bone matrix noncollagenous proteins. ActaOrthop Scand.199; 66:61-65
- Fisher LW, Whitson SW, Avioli LV, Termine JD. Matrix sialoprotein of developing bone. J Biol Chem. 1983; 258: 12723-7.
- Brochhausen C, Turial S, Muller FK, Schmitt VH, Coerdt W, Wihlm JM, et al. Pectus excavatum: history, hypotheses and treatment options. Interact CardiovascThorac Surg. 2012; 14: 801-6.
- Moses J, Oldberg A, Eklund E, Fransson LA. Biosynthesis of the proteoglycan decorin -- identification of intermediates in galactosaminoglycan assembly.Eur J Biochem. 1997; 248: 767-74.
- Roughley PJ. Articular cartilage and changes in arthritis. noncollagenous proteins and proteoglycans in the extracellular matrix of cartilage. Arthritis Res.2001; 3: 342-347
- Freeze HH, Schachter H. Genetic Disorders of Glycosylation. Chapter 42. Essentials of Glycobiology. 2nd edn. Varki A, Cummings RD, Esko JD, et al. editors. In: Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press. 2009.
- Rhodes J, Campbell BJ, Yu LG. Glycosylation and disease. Encyclopedia of Life Sciences (eLS). Wiley and Sons Ltd, Chichester. 2010.
- Johnstone B, Markopoulos M, Neame P, Caterson B.Identification and characterization of glycanated and non-glycanated forms of biglycan and decorin in human intervertebral discs.Biochem J. 1993; 292: 661-6
- Melrose J, Fuller ES, Roughley PJ, Smith MM, Kerr B, Hughes CE, et al. Fragmentation of decorin, biglycan, lumican and keratocan is elevated in degenerate human meniscus, knee and hip articular cartilages compared with age-matched macroscopically normal and control tissues. Arthritis Res Ther 2008; 10: R79.
- Creswick H, Stacey M, Kelly R, Burke B, Gustin T, Mitchell K, et al. Family studies on the inheritance of pectus excavatum. J. Pediatr. Surg. 2006; 41: 1699-1703
- Horth L, Stacey MW, Benjamin T, Segna K , Proud VK, Nuss D, et al. Genetic analysis of inheritance of Pectus excavatum J. Pediatric Genet. 2012; 1: 161-173.
- Scott JE. Morphometry of cupromeronic blue-stained proteoglycan molecules in animal corneas, versus that of purified proteoglycans stained in vitro implies that tertiary structures contribute to corneal ultrastructure. J. Anat. 1992;180: 155-164
- Raspanti M, Viola M, Forlino A, Tenni R, Gruppi C, Tira ME. Glycosaminoglycans show a specific periodic interaction with type I collagen fibrils. J. Struct. Biol. 2008; 164: 134-139
- Goretsky MJ, Kelly RE, Croitoru D, Nuss D. Chest wall anomalies:pectus excavatum and pectus carinatum. Adolesc.Med. 2004; 15: 455-471.