
Research Article
Austin J Microbiol. 2025; 10(1): 1056.
Street Vended Foods, A Critical Source of Antimicrobial Resistant Escherichia Coli in Douala, Cameroon
Ziem A ABAH JO1,3, Koro Koro F2*, Plidikoua A2, Akami M2, Tamgue O2 and Etoa FX1
¹Department of Microbiology, University of Yaounde I, P.O. Box 812 Yaounde, Cameroon
²Department of Biochemistry, University of Douala, P.O. Box 24157 Douala, Cameroon
³Department of Hygiene and Environmental, Laboratory of water and foods Microbiology, Pasteur Center, P.O. Box 1274, Yaounde, Cameroon
*Corresponding author: Koro Koro F, department of Biochemistry, University of Douala, P.O. Box 24157 Douala Cameroon Email: korokorogozion@yahoo.fr
Received: March 30, 2025 Accepted: April 16, 2025 Published: April 21, 2025
Abstract
Background: Street vended foods could be defined as ready-to-eat food or beverage especially sold in streets and others public places valued by low-income countries urban populations for their convenience and nutritional importance. These foods represent a serious public health threat due to poor hygienic handling conditions, lack of microbiological quality status and poor food safety measures. In the present study, we assessed for the first time the antimicrobial susceptibility of Escherichia coli strains isolated from some street vended foods sold and commonly consumed by the urban population of Douala Cameroon.
Methods: A cross-sectional study was conducted in Douala urban council in Littoral region of Cameroon. One hundred and seventeen (117) street foods samples were randomly collected using a stratified sampling method, the E. coli species were isolated and identified by culture-dependent method and biochemical test, and finally, the antimicrobial susceptibility test was assessed according to EUCAST 2021.
Results: The results indicated that 45.3% of street foods were contaminated by β-glucuronidase Positive Escherichia coli and 6% by Extended Spectrum β-lactamase E. coli. The antimicrobial resistance profile of β-glucuronidase positive Escherichia coli and Extended spectrum β-lactamase producing Escherichia coli isolates showed respectively high (20%-80%) and very high (50%-100%) resistance rates to several antibiotics interestingly a very high (93.33%) multidrug resistance rate was observed, even for nine antibiotics classes in E. coli analyzed strains.
Conclusion: These findings revealed that street vended foods may be a critical source of β-glucuronidase positive, Extended Spectrum β-Lactamase and multidrug resistance E. coli strain in Douala.
Keywords: Street vended foods; Antimicrobial resistance; Multidrug resistance; ESβL Escherichia coli; Douala; Cameroon
Introduction
Due to the rapid urbanization and multiple daily constraints, city dwellers couldn’t easily cook their own foods at home. As a result, the street food sale has become one of the most profitable and fastgrowing activity in many developing countries [1]. Street vended food could be defined as any ready-to-eat beverage or drink, especially sold in streets and similar places, sometimes prepared or cooks in outdoor public areas either mobile or fixed at a point of sale [2]. These foods are valued by low-income urban populations for their accessibility and nutritional importance, and therefore constitute an important part of daily dietary intake in Africa [2,3]. In Cameroon, street foods such as bread, dairy products, donuts, salads, chips, juices, vegetables, cassava products, peanut products, fried beef and rusted fish are predominant as daily nutrients intake source among the urban population [4]. However, the poor hygienic conditions under which these foods are made, in addition to the hypothetic microbiological quality of handling materials and equipment raise serious public health concerns in terms of the safety and biosecurity of consumers [2]. Furthermore, most street vended foods are often uncovered, sold around highly congested areas where they are exposed to dust, insects, and flies that may contain pathogenic microorganisms [5]. These conditions increase the risk of microbiological contamination which can lead to foodborne diseases and intoxications. On the basis of the above, street vended foods could constitute a potential major public health concern particularly in low-income countries with increasing outdoors feeding [6,7]. Overall, food-borne diseases are rampant and represent a global significant public health threat. According to a recent report, 1 in 10 people become ill after eating contaminated food resulting in 420,000 deaths yearly [8]. Many studies have highlighted street ready-to-eat foods as carriers of bacterial agents leading to gastroenteritis and diarrheal disease outbreaks [9,10]. Escherichia coli is a subset of coliform bacteria that normally colonize and establish in the human gut flora as commensal symbionts. However, it can easily shift from commensals to pathogenic bacteria when met conducive environmental triggers. For this reason, it is considered as a fecal contaminant indicator and is responsible of a 20% of bacterial foodborne disease outbreaks [11]. The increasing prevalence of foodborne infections in humans has required the use of antibiotics to treat them, while farmers also use antibiotics as growth promoters to control animal diseases [12]. The consequences of the abusive use of these antimicrobials in food chain production and supply is the cause of the emergence and spread of antimicrobial resistance (AMR) bacterial species and antibiotic-resistant genes (ARGs) within the process [13]. The dissemination of ARGs in food chain is a substantial global public health and food safety issue [14,15]. During the food quality control processes, antimicrobial susceptibility tests are rarely conducted, and very little research studies have focused on street vended foods as drivers or carriers of AMR in food chain in Cameroon. This lack of information may hamper our appreciation of the extent to which AMR is disseminated within the Cameroonian food chain. On the basis of One Health perspective, the present study aims at assessing the antimicrobial susceptibility of Escherichia coli strains isolated from some street vended foods sold and commonly consumed by the urban population of Douala Cameroon. Notwithstanding genetic factors, our results highlight that street vended foods constitute a significant vehicle of antimicrobialresistant Escherichia coli in Douala, Cameroon. Therefore, a more systematic approach is required to break the dissemination circuit to optimize antibiotic-based treatments and save lives.
Materials And Methods
Study Design
A cross-sectional study was conducted in Douala urban council in Littoral region of Cameroon from October 2021 to July 2022. The samples were collected in various outlets including makeshift kiosks, fast-food kiosks and street vendors of the 6 districts of the city of Douala [16].
Sampling Collection
This research was carried out on street foods collected from different areas of Douala urban council. A stratified sampling method was chosen to ensure that the sample is representative of the different types of street foods products available in the various outlets. The samples collected for this study consisted of ready to eat lettuces and cabbages, fermented milk “kossam”, hamburgers and peeled fruits (pineapple, papaw and watermelon). One hundred and seventeen (117) samples were aseptically and randomly collected on individual zipper sterile plastic bag from forty-five (45) sales outlets and street vendors across the six districts of Douala urban council as presented in Table 1. Sampling was done according to ISO 13307: 2013 [17] and ISO/TS 17728: 2015 [18] standards recommendations. Samples were then transported in refrigerated boxes to the laboratory for microbiological analysis.
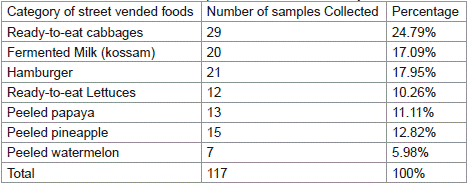
Table 1: Streets vended foods samples collected for this study.
Microbiological Analysis
Samples Preparation: 10 g of each sample was weighed using an electronic weighing scale, added to 90 ml of buffered peptone water diluent (BPW, Biokar TM) and homogenized during 1 min using a peristaltic homogenizer (LB 400 VWR) to obtain a 1/10 dilution following the instructions of ISO 6887-1: 2017 [19] standard. The preparation was then incubated during 24H at 37°C for preenrichment.
Detection of β-glucuronidase Positive Escherichia coli (βGP_Ec): The detection of β-glucuronidase positive Escherichia coli (βGP_Ec) was performed according to the standard operating protocol described in ISO 16649-2: 2001 [20] modified. For practical purposes, 1 mL of pre-enriched suspension was placed in a Petri dish to which 20 ml of sterile Tryptone Bile X Glucuronide Agar (TBX, Biokar, France) were added. Plates were homogenized, left at room temperature and incubated aerobically at 44°C for 24 hours. βGP_ Ec colonies appearing blue-green were seeded onto Tryptone Soy Agar (TSA, Biokar, France) and incubated at 37°C during 24 hours for biochemical identification using API 20E identification system (Bioméreux, France). Confirmed βGP_Ec isolates were stored at -20 °C for further analysis.
Detection of Extended Spectrum β-lactamase Escherichia coli (ESβL_Ec): The detection of ESβL_Ec was performed in two steps: the screening of presumptive ESβL_Ec followed by a confirmation using the double disk synergy test (DDST) method.
For the screening of presumptive ESβL_Ec, Tryptone Bile X Glucuronide Agar (TBX, Biokar, France) supplemented with Cefotaxime 4 mg/L was used. A full inoculation loop (10μL) of preenriched suspension was sewed onto a Petri dish containing TBX + cefotaxime and incubated aerobically at 44 °C for 24 h. Presumptive ESβL_Ec colonies appearing blue or blue-green were subcultured on Tryptone Soy Agar (TSA, Biokar, France) and incubated at 37°C during 24 hours for biochemical identification assays according to the API 20E identification system (Bioméreux, France).
All presumptive ESβL_Ec isolates were confirmed following double disk synergy test method using Amoxicillin-Clavulanic acid 20/10 μg disc and Cefotaxim 5 μg disk. Presumptive ESβL_Ec isolates were confirmed by expansion of third generation cephalosporin (Cefotaxim 5 μg) inhibition zone towards Amoxicillin-Clavulanic acid disk. ESβL production was appreciated through the appearance of a “Champaign cork” image between the clavulanate (Amoxicillin + clavulanic acid) and Cefotaxim disk (Figure 1). ESβL_Ec isolates confirmed were stored at - 20°C for further analysis.
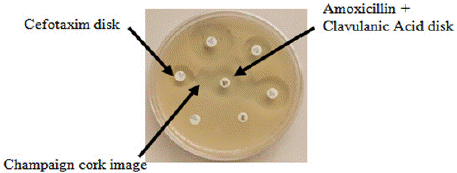
Figure 1: Analytical design of double disk synergy test plate presenting a “Champaign cork” image.
Phenotypic Detection of Antimicrobial Resistance: Antimicrobial susceptibility testing was carried out on all the isolates using the Kirby Bauer disk diffusion method and the results were interpreted according to European Committee on Antimicrobial Susceptibility [21] recommendation breakpoints. A panel of 14 antibiotics belonging to 5 families, namely β-lactam (Amoxicillin 20μg, amoxicillin-clavulanic acid 20/10 μg, Ticarcilline 75 μg, Cefotaxim 5 μg, Ceftriaxone 30 μg, Aztreonam 30 μg and Imipenem 10 μg), phenicols (Chloramphenicol 30 μg), aminoglycosides (Tobramycin 10 μg, Amikacin 30 μg), Macrolides (Azithromycin 15 μg) and quinolone (Ofloxacin 5 μg, Nalidixic acid 30 μg, Ciprofloxacin 5 μg) were tested. The stored strains of E. coli were subcultured and purified on a non-selective agar (TSA, Biokar, France). Culture suspension was made by inoculating 3 to 5 purified colonies into 4-5ml of sterilized saline solution in a test tube. The turbidity of the suspension was then adjusted to 0.5 McFarland standard turbidity using a densitometer (Densimat, Den-1B Biosan). The standardized inoculum was inoculated into prepared Mueller Hinton Agar using a swab stick to cover the whole surface of the plate and obtain a dense and uniform growth. Sterile forceps was used to place antibiotic disks onto the surface of the inoculated plates and incubated at 37°C for 24 hours. After incubation period, the inhibition zone produced by antibiotics disks were measured in mm using a caliper gauge and the results were interpreted according to EUCAST 2021 recommendation breakpoints. Escherichia coli ATCC 25922 was included as quality control strain during analysis (Table 2).
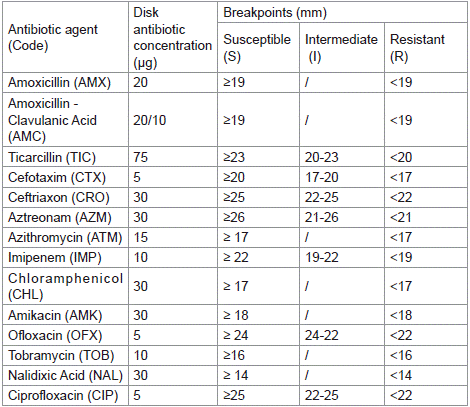
Table 2: Concentrations and interpretation breakpoints of antibiotics disks used
in this study EUCAST 2021.
Data Analyses
Data obtained from the detection of Escherichia coli were analyzed using IMB SPSS version 25.0 software package (SPSS Inc., Armonk, NY, United States). Chi-square tests was used to analyze the association between categorical variables. Statistical significance was set at P < 0.05. The resistance rate per 100 for each antibiotic was calculated as the percentage of isolates showing resistance to a specific antibiotic with the following formula.
![]()
According to Papadopoulos et al. [22], resistance rates were classified as extremely high (% rate > 70%), very high (% rate: > 50 to 70), high (% rate > 20 to 50), moderate (% rate > 10 to 20), low (% rate > 1 to 10), very low (% rate 0.1 to 1), and rare (% rate < 0.1). The isolates which were resistant to more than three classes of antimicrobials were considered as multidrug-resistant.
Results
Occurrences of β-glucuronidase Positive Escherichia coli (βGP_Ec) Contamination in Street Vended Foods
Among the 117 samples collected, 53 (45.3%) were tested positive for βGP_Ec test. The highest occurrence of βGP_Ec contamination was recorded in lettuces samples with 10/12 of positives samples representing 83.3%, followed by hamburger with 13/31 of positives samples representing 61.9%, peeled fruits with 17/35 (48.6%) of positives samples, cabbages with 12/29 (41.4%) of positives samples and fermented milk with 5% of positives samples. Concerning peeled fruits βGP_Ec contamination, peeled papaw with 69.2% of positives samples was the most contaminated fruit followed by peeled watermelon with 42.9% of positives samples and finally peeled pineapple with 33.3% of positive samples. Furthermore, the distribution of βGP_Ec amongst street vended foods samples was significantly different (khi-2 of Pearson: 22.782; p: 0.0001 < 0.05). The occurrences of βGP_Ec contamination of different street vended foods is presented in Figure 2
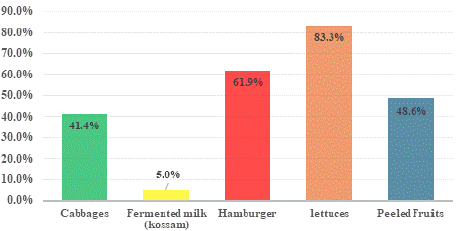
Figure 2: Occurrences of β-glucuronidase Positive Escherichia coli
contamination of street vended foods.
Occurrences of Extended Spectrum β-lactamase Escherichia coli (ESβL_Ec) Contamination in Street Vended Foods
ESβL_Ec was detected on 7samples (out of the 117) representing an occurrence rate of 5.98%. Amongst the street vended foods, the highest occurrence was recorded in lettuces samples with 25.0% of positive ESβL_Ec, followed by peeled fruits with 5.7%, hamburger with 4.8% and finally cabbages with 3.4% of positive samples (Figure 3). ESβL_Ec was not detected on fermented milk and watermelon. The highest ESβL_Ec contamination of peeled fruits was noticed on peeled papaw with 7.7% followed by peeled pineapple with 6.7% (Figure 3). The distribution of ESβL_Ec amongst street vended foods samples was not significantly different (khi-2 of Pearson: 9.379; p: 0.052 > 0.05).
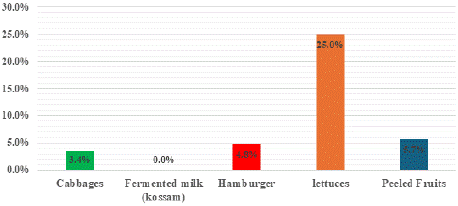
Figure 3: Occurrences of Extended Spectrum β-lactamase Escherichia coli
contamination of street vended foods.
Antimicrobial Resistance Pattern of β-glucuronidase Positive Escherichia coli (βGP_Ec)
βGP_Ec isolates showed extremely higher resistance rate for Amoxicillin, Ticarcillin and Cefotaxim with 81.1%, 77.4% and 77.4%, respectively (Figure 4). Higher resistance rate was observed for Amoxicillin-clavulanic acid, Aztreonam and Ofloxacin with respectively 50.9%, 56.6% and 66.0%. High resistance rate was observed with Ceftriaxon, Amikacin, Tobramycin, Nalidixic Acid and Ciprofloxacin respectively with 26.4%, 24.5%, 32.1%, 35.8% and 39.6% respectively. And finally, low resistance rate was observed with Azithromycin, Imipenem and Chloramphenicol respectively with 5.7%, 7.5% and 3.8% of resistance rate (Figure 4).
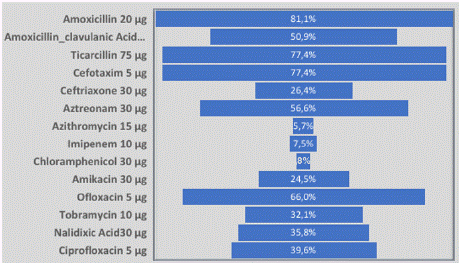
Figure 4: Phenotypic resistances rates of βGP_Ec isolated in street vended
foods.
Antimicrobial Resistance Pattern of Extended Spectrum β-lactamase Producing Escherichia coli (ESβL_Ec)
ESβL_Ec isolates showed extremely higher resistance rate for Amoxicillin, Amoxicillin-clavulanic acid, Ticarcillin and Cefotaxim and Ofloxacin with 100.0%, 71.1%, 85.7%, 85.7% and 100.0%, respectively. Very high resistance rate was observed for Amikacin and Tobramycin with a resistance rate of 57.1% each. Additionally, higher resistance rate was observed with Ceftriaxon, Aztreonam, Nalidixic acid and Ciprofloxacin with 28.6%, 42.9%, 42.9% and 42.9% rate, respectively and moderate resistance rate was observed with Azithromycin and Imipenem with 14.3% and 14.3%, respectively (Figure 5).
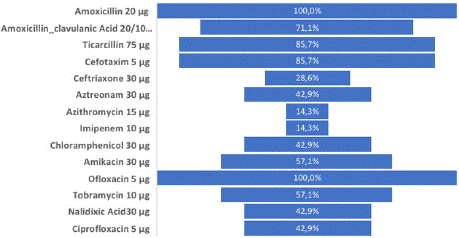
Figure 5: Phenotypic resistance rate of ESβL_Ec isolated in street vended
foods.
Occurrences of Multidrug Resistant Escherichia coli Isolates
Of the 60 isolates tested in this study, 56 (93,33%) isolates both βGP_Ec and ESβL_Ec isolates showed resistance to at least three different classes of antibiotics and were considered as multidrug resistant isolates. The main multidrug resistant profile amongst βGP_Ec isolates was AMC,AMX,TIC,CFX,CEF,AZM,OFX,NAL,C IP (15.7%) (Table 3) whereas the main multidrug resistant profile of ESβL_Ec isolates was AMC,AMX,TIC,AMK,OFX,TOB,NAL (28.6%) (Table 4). Furthermore, the occurrences of multidrug resistant profile amongst βGP_Ec and ESβL_Ec isolates were significantly different (khi-2 of Pearson: 48.577; p: 0.023 < 0.05) (Table 4).
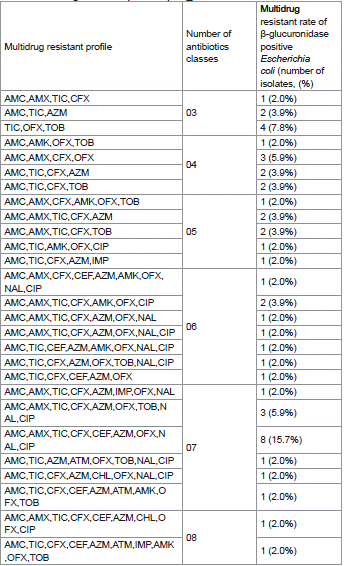
Table 3: Multidrug resistance profile of βGP_Ec isolates.
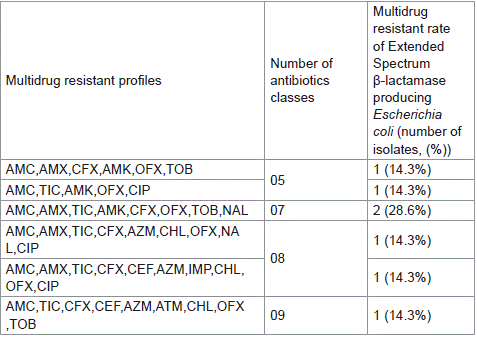
Table 4: Multidrug resistance profil of EsβL_Ec isolates.
Discussion
The rapid urbanization process and the fast-growing economic activities in developing countries astrained populations in urban areas to long working hours and multi-tasking. This pattern does not offer city dwellers in low- and middle-income countries such as Cameroon enough time for cooking and therefore constrain them to eat street foods [1]. However, the quality of street foods sold is wanting and expose the consumers to gastroenteritis. Several previous studies highlighted the low microbiological quality of street vended foods in some municipalities of Yaounde, Cameroon [23,24], but none of them explored the antimicrobial susceptibility of Escherichia coli strains, major factor that could explain the dissemination of resistance of antibiotics in humans and mammals at large which hampers the control and treatments of bacteria-based infections. To the best of our knowledge, our study is the first to investigate the antimicrobial susceptibility of isolates from street foods consumed by the urban population in Douala, Cameroon and Escherichia coli strains that sustain antimicrobial resistance. Our results revealed that 45.3% of street foods samples were contaminated by β-glucuronidase positive Escherichia coli among other, ready-to-eat lettuces followed by hamburger and peeled fruits. These results correlate with Moussé et al. [25] study where a prevalence of 44% was found in street vended foods collected in different localities of Cotonou and Abomey in Benin. In contrary, our results were different to previous studies done by Nguendo Yongsi [23] who found an occurrence of 24.6% from street vended foods collected in Yaounde. The high occurrence of β-glucuronidase positive Escherichia coli in streets vended foods samples is an indication of poor hygiene process conditions during foods preparation. It has been proven in previous studies that unsafe food preparation conditions, lack of formal food safety training and poor personal hygienic practices amongst vendors could be causes of the poor microbial quality of street vended foods [26]. In the present study, a low percentage of β-glucuronidase positive Escherichia coli contamination was observed in fermented milk. This low occurrence could be explained by the low PH value of artisanal fermented milk “Kossam” (3.1±0.2) as mentioned in previous studies which does not deal with mesophilic bacterial growth [27]. The high percentage of β-glucuronidase positive Escherichia coli contamination in lettuces is in contrast with previous studies [28,29] where occurrences of 8% and 17% were noted respectively in south Africa and Pennsylvania. The differences of βGP_Ec contamination of lettuces could be due to poor hygienic process conditions. In fact, it has been demonstrated that poor hygienic handling conditions, poor water quality as well as exposition to dust, insects, and flies are important sources of street foods contamination in our environment [2,4]. The rise of EsβL Escherichia coli in food chain is becoming a challenging public health threat for food safety authorities and the contribution of the food chain to the occurrence of ESβL bacteria has a large influence on its emergence and spread in the general population, decreasing therapeutic options, increasing mortality, and extending hospital stay [30-32]. Our findings revealed that 6% of street foods samples were contaminated by ESβL producing Escherichia coli amongst which lettuces were the most contaminated samples followed by peeled fruits, hamburger and cabbages without statistical difference. These results are different to those of Sivakumar et al. study [1] who found a prevalence of 19.71% in street foods in Delhi in India. ESβL Escherichia coli are important food safety issue and a public health significance. Listed as a priority research pathogen by the World Health Organization, E. coli is the most important gene pool encoding extended-spectrum β-lactamases [33]. The presence of ESβL producing E. coli in street food samples may indicate their continued widespread distribution from farm to fork continuum and poses a serious public health threat to human populations [34]. Antimicrobials have been used intensively without proper respect of therapy guidelines, mostly in low- and middleincome countries [35] resulting in the development and spread of antimicrobial resistance (AMR) bacteria and antibiotic-resistant genes (ARGs) throughout the food chain [13]. Antibiotic resistance in E. coli is a key public health concern because of its diverse origin, and its ability to acquire and share antibiotic resistance genes not only within E. coli strains but also between other bacterial species [36]. While analyzing the drug resistance profile of 53 βGP_E. coli and 7 ESβL producing E. coli isolates in the present study, βGP_E. coli and ESβL producing E. coli showed significantly higher resistance rate to amoxicillin, amoxicillin + clavulanic acid, ticarcillin and cefotaxime with few variation (with resistance ranging from 71.1% to 100%). These results corroborate those of Abdullah et al. [37] in which 70%- 100% of E. coli resistant strains to ampicillin, amoxicillin, nalidixic acid, tetracycline and erythromycin were recorded in street vended foods in Bangladesh. Furthermore, very high and high resistance rate was observed with Tobramycin, Nalidixic Acid, Ciprofloxacin, Amikacin, Ceftriaxon and Aztreonam for βGP_E. coli and ESβL producing E. coli. It is alarming to note that, the E. coli isolates in the current study showed higher resistance rate to aminoglycosides and quinolone antibiotics families. Similar findings were observed with Sivakumar et al. [1] study and Kar et al [38] study in street foods in India. The variability of resistance rate phenotypes in this study could be explained by the acquisition of resistance to several classes of antibiotics (co-resistance), as exchanged plasmids often have multiple resistance genes, such as those resistant to cephalosporins, penicillin, chloramphenicol, tetracycline, and fluoroquinolones [39]. The results from this study show that street vended fast food samples are contaminated with various bacteria most of which are resistant to commonly used antibiotics and therefore represent risks and public health hazards.
The present study reports a high prevalence of multidrugresistant (MDR) both on βGP_E. coli and ESβL_producing E. coli isolates with occurrences of 96.22% and 100%, respectively. These results are similar with those of Adzitey, [40] who reported MDR prevalence of 100% in Lettuces and cabbages samples in Tamale in Ghana. Conversely, the MDR prevalence recorded here was compared to that reported by Loandi Richter et al. [28] in street vended foods in South Africa. The difference could be due to the misuse and uncontrolled use of antimicrobials in the environment. It is demonstrated that the abusive use of antimicrobial emphasizes the spread of antimicrobial resistance bacteria in food chain including street foods in developing countries. The most common βGP_E. coli MDR profile was made up of AMC,AMX,TIC,CFX,CEF,AZM,OF X,NAL,CIP (15.7%) while the ESβL_producing E. coli MDR profile was made up of AMC,AMX,TIC,AMK,OFX,TOB,NAL (28.6%). These results are alarming and a call for a regulation of street foods vending activities because the risk of the emergence of antimicrobial resistance in both commensal and pathogenic bacteria in street foods could spread along food-chain, thereby limiting treatment options to infections [41].
Despite the above observations, the present study has some limitations. The results of the present study could not be generalized because of the limited sample size and diversity of street foods products from one region to another region. Furthermore, rather than using combined disc diffusion as an ESβL confirmatory test, the Double-Disc Synergy Test (DDST) was used due to cost and resource limitations. Similarly, the confirmation of ESβL E. coli isolates was based only on phenotypic and biochemical assays.
Conclusion
This study investigated the antimicrobial resistance profile of E. coli strains isolated from street vended foods collected in Douala urban council. The results indicated that street foods were contaminated by multi-drug-resistant E. coli with higher occurrences. The antimicrobial resistance profile of β-glucuronidase positive Escherichia coli and Extended spectrum β-lactamase producing Escherichia coli isolates showed higher resistance rates with several antibiotics families. Eventually, the street vended foods may be the potential source of ESβL and multidrug resistance E. coli.
Authors’ Contributions
This manuscrit was written through contributions of all authors. All authors gave approval to the final version of the manuscrit. Olivier Ziem, Francioli Koro koro and Francois-Xavier Etoa conceived and designed the experiments. Olivier Ziem and Amandine Plidikoua performed the experiments. Francioli Koro koro, Olivier Ziem and Francois-Xavier Etoa analysed the data. Olivier Ziem wrote the first draft of the paper and designed figures. All authors provided critical input. Francioli Koro Koro and Francois-Xavier Etoa supervised the research, edited and approved the final manuscript.
References
- Sivakumar M, Abass G, Vivekanandhan R, et al. Extended-spectrum beta lactamase (ESBL) producing and multidrug resistant Escherichia coli in street foods: a public health concern. J Food Sci Technol. 2021; 58: 1247–1261.
- Fao H. Les bonnes pratiques d’hygiène dans la préparation et la vente des aliments de rue en Afrique: Outils pour la formation. Ed. Food & Agriculture Org, Italie. 2007; 175.
- World Health Organization. Essential safety requirements for street-vended foods (No. WHO/FNU/FOS/96.7 Rev1). World Health Organization. 1996.
- Edima HC, Tem Nnam R, Awono Enama T, Biloa D & Ndjouenkeu R. Case study of the street food sector in the metropolitan areas of a Cameroonian City, Yaounde. Int J Curr Microbiol App Sci. 2014; 3: 740-751.
- Marras S, Bendech MA & Laar A. Street food vending in Accra, Ghana. Meng, J., & Doyle, M. P. (2002). Introduction. Microbiological food safety. Microbes and Infection. 2016; 4: 395-397.
- Danikuu FM, Baguo FB & Azipala O. Hygiene practices among street food vendors in Tamale Metropolis. Journal of Medical and Biomedical Sciences. 2015; 4: 25-30.
- Alamo-Tonelada C, Silaran FY & Bildan MCA. Sanitary conditions of food vending sites and food handling practices of street food vendors: Implication for food hygiene and safety. International Journal of Education and Research. 2018; 6: 31-34.
- Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B, et al. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS medicine. 2015; 12: e1001921.
- Mensah P, Yeboah-Manu D, Owusu-Darko K & Ablordey A. Street foods in Accra, Ghana: how safe are they? Bulletin of the World Health Organization. 2002; 80: 546-554.
- Adu-Gyamfi A & Nketsia-Tabiri J. Microbiological studies of macaroni and vegetable salads in Waakye, a local street food. Ghana Journal of science. 2007; 47: 3-9.
- Getaneh DK, Hordofa LO, Ayana DA, Tessema TS, Regassa LD. Prevalence of Escherichia coli O157:H7 and associated factors in under-five children in Eastern Ethiopia. PLoS One. 2021; 16: e0246024.
- Aslam B, Khurshid M, Arshad MI, Muzammil S, Rasool M. Antibiotic resistance: one health one world outlook. Front. Cell. Infect. Microbiol. 2021; 11: 771510.
- Ayukekbong JA, Ntemgwa M, Atabe AN. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. Infect. Contrib. 2017; 6: 47.
- Chang Q, Wang W, Regev-Yochay G, Lipsitch M, Hanage WP. Antibiotics in agriculture and the risk to human health: How worried should we be? Evol. Appl. 2015; 8: 240–247.
- Founou LL, Founou RC, Essack SY. Antibiotic resistance in the food chain: a developing country-perspective. Front. Microbiol. 2016; 23: 1881.
- Blaise NYH. An assessment of hygiene practices and health status of street-food vendors in Yaoundé, Cameroon. International Journal of Tropical Disease & Health. 2014; 4: 1153-1170.
- ISO 13307: Microbiology of food and animal feed – Primary production stage -Sampling techniques. 2013-03, ISO/TC 34/SC 9 Microbiology. Technical committee: ISO/TC 34/SC 9 Microbiology. ICS: 07.100.30 Food Microbiology. 2013.
- ISO/TS 17728: Microbiology of the food chain – Sampling techniques for microbiological analysis of food and feed samples, 2015-06. Technical committee: ISO/TC 34/SC 9 Microbiology. ICS: 07.100.30 Food Microbiology. 2015.
- ISO 6887-1: Microbiology of the food chain - Preparation of test samples, initial suspension and decimal dilutions for microbiological examination - Part 1: General rules for the preparation of the initial suspension and decimal dilutions. 2017-03. Technical committee: ISO/TC 34/SC 9 Microbiology. ICS:07.100.30 Food Microbiology. 2017.
- ISO 16649-2: Microbiology of food and animal feeding stuffs-Horizontal method for the enumeration of Beta_glucuronidase-positive Escherichia coli - Part 2: Colony-count technique at 44 degrees °C using 5-bromo-4- chloro-3-indolyl beta D-glucuronide. Technical committee: ISO/TC 34/SC 9 Microbiology. ICS: 07.100.30 Food Microbiology. 2001.
- European Committee on Antimicrobial Susceptibility Testing. Calibration of zone diameter breakpoints against MIC values. 2021.
- Papadopoulos D, Papadopoulos T, Papageorgiou K, Sergelidis D, Adamopoulou M, Kritas SK, et al. Antimicrobial resistance rates in commensal Escherichia coli isolates from healthy pigs in Greek swine farms. J. Hell. Vet. Med. Soc. 2021; 72: 2909–2916.
- Nguendo Yongsi HB. Eating to Live or Eating to Damage One’s Health: Microbiological Risks Associated with Street-Vended Foods in a Subtropical Urban Setting (Yaoundé-Cameroon). Nutri Food Sci Int J. 2018; 6.
- Maffouo STM, Mouafo HT, Mouokeu RS, Manet L, Tchuenchieu AK, Simo BN, et al. Evaluation of sanitary risks associated with the consumption of street food in the city of Yaoundé (Cameroon): case of braised fish from Mvog-Ada, Ngoa Ekélé, Simbock, Ahala and Olézoa. Heliyon. 2021; 7.
- Moussé W, Baba-Moussa F, Adjanohoun A, Noumavo PA, Sina H, Assogba S, et al. Virulence profiles of pathogenic Escherichia coli strains isolated from street foods in Benin. International Journal of Biotechnology and Food Science. 2016; 4: 52-64.
- Kaptso KG, Tchabo W, Chebelem Mbafor B, Asoba NG, Amungwa AF and Mbofung CMF. Assessment of Food Hygienic and Vending Practices among Street Food Vendors in Buea and Kumba City Council (South-West Region Cameroon). Food Sci & Nutri Tech. 2021; 6.
- Djoulde DR, Lendzemo V, Essia-Ngang JJ & Etoa FX. Processing of” Kossam” an African sour fermented milk beverage from northern Cameroon. 2013.
- Richter L, Plessis ED, Duvenage S & Korsten L. High prevalence of multidrug resistant Escherichia coli isolated from fresh vegetables sold by selected formal and informal traders in the most densely populated Province of South Africa. Journal of Food Science. 2021; 86: 161-168.
- Scheinberg JA, Dudley EG, Campbell J, Roberts B, Marzio MDI, Roy CDEB, et al. Prevalence and phylogenetic characterization of Escherichia coli and hygiene indicator bacteria isolated from leafy green produce, beef, and pork obtained from farmers’ Markets in Pennsylvania. Journal of Food Protection. 2017; 80: 237–244.
- Lavilla S, Gonzalez-Lopez JJ, Miro E, Dominguez A, Llagostera M, Bartolome RM, et al. Dissemination of extended-spectrum β-lactamaseproducing bacteria: the food-borne outbreak lesson. Journal of antimicrobial chemotherapy. 2008; 61: 1244-1251.
- Dsani E, Afari EA, Danso-Appiah A, et al. Antimicrobial resistance and molecular detection of extended spectrum β-lactamase producing Escherichia coli isolates from raw meat in Greater Accra region, Ghana. BMC Microbiol. 2020; 20; 253.
- Samtiya M, Matthews KR, Dhewa T & Puniya AK. Antimicrobial resistance in the food chain: trends, mechanisms, pathways, and possible regulation strategies. Foods. 2022; 11: 2966.
- Patil S, Chen X, Lian M & Wen F. Phenotypic and genotypic characterization of multi-drug-resistant Escherichia coli isolates harboring blaCTX-M group extended-spectrum β-lactamases recovered from pediatric patients in Shenzhen, southern China. Infection and Drug Resistance. 2019; 12: 1325– 1332.
- Founou LL, Founou RC, Ntshobeni N, Govinden U, Bester LA, Chenia HY, et al. Emergence and Spread of Extended Spectrum β-Lactamase Producing Enterobacteriaceae (ESBL-PE) in Pigs and Exposed Workers: A Multicentre Comparative Study between Cameroon and South Africa. Pathogens. 2019; 8: 10.
- Mouiche MMM, Moffo F, Akoachere JFTK, et al. Antimicrobial resistance from a one health perspective in Cameroon: a systematic review and metaanalysis. BMC Public Health. 2019; 19; 1135.
- Österblad MHakanen A, Manninen R, Leistevuo TPeltonen R, Meurman O, Huovinen P, Kotilainen P. A between-Species Comparison of Antimicrobial Resistance in Enterobacteria in Fecal Flora. Antimicrob Agents Chemother. 2000; 44.
- Abdullah Al Momen Sabuj, Zobayda Farzana Haque, Nanda Barua, Md. Alimul Islam and Sukumar Saha. Assessment of Bacteriological Quality of Street Vended Fast Foods and their Antimicrobial Resistance. Int. J. Curr. Microbiol. App. Sci. 2018; 7: 3049-3059.
- Kar D, Bandyopadhyay S, Bhattacharyya D, Samanta I, Mahanti A, Nanda PK, Singh RK. Molecular and phylogenetic characterization of multidrugresistant extended spectrum betalactamase producing Escherichia coli isolated from poultry and cattle in Odisha, India. Infect Genet Evolut. 2015; 29: 82–90.
- Barour D, Berghiche A & Boulebda N. Antimicrobial resistance of Escherichia coli isolates from cattle in Eastern Algeria. Veterinary world. 2019; 12: 1195– 1203.
- Adzitey F. Antibiotic resistance of Escherichia coli and Salmonella enterica isolated from cabbage and lettuce samples in Tamale metropolis of Ghana. International Journal of Food Contamination. 2018; 5.
- Freitag C, Michael GB, Li J, Kadlec K, Wang Y, Hassel M & Schwarz S. Occurrence and characterization of ESBL-encoding plasmids among Escherichia coli isolates from fresh vegetables. Veterinary Microbiology. 2018; 219: 63–69.