
Research Article
Austin J Infect Dis. 2024; 11(2): 1101.
Operations and Implementation of Outpatient COVID-19 Therapeutics at a Large Health Care System
Chungyun Kim, PharmD1*; Andrea Pallotta, PharmD1; Alice Kim, MD, MBA2; Steven Gordon, MD3; Chanda Mullen, PhD4; Jill Wesolowski, PharmD5; Cassandra Calabrese, DO6; Stephanie McGuire, MSDA7; Dominique Loparo, PharmD8; Tricia Bravo, MD, FIDSA9
1Department of Pharmacy, Cleveland Clinic Health System, USA
2President/CEO at ID Care, Cleveland Clinic Health System, USA
3Department of Infectious Disease and Staff Member, Transplantation Center, Cleveland Clinic Health System, USA
4Department of Pharmacy, Cleveland Clinic Health System, USA
5Department of Pharmacy Information Technology, Cleveland Clinic Health System, Cleveland Clinic Health System, USA
6Department os Infectious Disease, Department of Rheumatologic and Immunologic Disease, Cleveland Clinic Health System, USA
7Department of Lead Systems Analyst in the Pharmacy Informatics, Cleveland Clinic Health System, USA
8Department of Pharmacy Clinical Specialist in Medication Safety, Cleveland Clinic Health System, USA
9Department of Infectious Disease, Cleveland Clinic Health System, USA
*Corresponding author: Chungyun Kim, PharmD, is a Pharmacy Clinical Specialist in infectious diseases and Outpatient Parenteral Antimicrobial Therapies in the Department of Pharmacy at the Cleveland Clinic Health System, Cleveland Clinic Health System, Department of Pharmacy, 9500 Euclid Avenue, Cleveland, OH 44195, USA. Tel: 216-403-5927; Fax: 216-445-9446 Email: kimc2@ccf.org
Received: November 26, 2024; Accepted: December 16, 2024; Published: December 23, 2024
Abstract
Coronavirus Disease 2019 (COVID-19) monoclonal antibodies (mAb) and oral antiviral agents received authorization for the treatment of non-hospitalized persons with mild-to-moderate COVID-19 at high-risk of progression to severe infection. We describe the operational workflow and implementation of an enterprise outpatient COVID-19 therapy taskforce with a focus on the creation of advanced standardized order sets for COVID-19 therapeutics. We evaluated clinical outcomes of 25,615 outpatient prescriptions originating from these order sets and the impact of order set use on 28-day hospitalization, ICU admission and all-cause mortality rates. This study described the success stories and challenges throughout the pandemic on outpatient COVID-19 therapeutic operations and demonstrated a low rate of 28-day hospitalization using standardized ordering tools.
Keywords: COVID-19; Quality improvement; Process
Introduction
During the Coronavirus Disease 2019 (COVID-19) pandemic, monoclonal antibodies (mAb) and oral antivirals received U.S. Food and Drug Administration (FDA) Emergency Use Authorization (EUA) for the treatment of non-hospitalized persons with mild-tomoderate COVID-19 who are at high-risk of progression to severe infection. These therapies have been supported in the Infectious Diseases Society of America (IDSA) and National Institutes of Health (NIH) COVID-19 guidelines for patients with mild-tomoderate COVID-19 [1,2]. Several mAb received FDA EUA, including bamlanivimab (11/09/2020), casirivimab/imdevimab (11/21/2020), bamlanivmab/etesevimab (02/09/2021), sotrovimab (SOT, 05/26/2021) and bebtelovimab (BEB, 02/11/2022) [3-5]. Although mAbs have shown reduction in hospitalization and death, the SARS-CoV-2 virus variants evolved over time, leaving no mAbs authorized for treatment of 2024 variants. In addition to mAbs, two oral antivirals with FDA EUA include nirmatrelvir plus ritonavir (PaxlovidTM, NMV/r, EUA 12/22/2021, full FDA approval 5/25/2023) and molnupiravir (LagevrioTM, MOL, EUA 12/23/2021) [3,6-10].
These agents came with limitations such as severe drug-drug interactions and requirements for rapid practice changes. With the fast-paced pandemic, prescriber knowledge of the nuances of outpatient therapies was crucial, however the level of knowledge was variable amongst prescribers. To mitigate these safety concerns and support healthcare staff members, we used Computerized Provider Order Entry (CPOE) as a strategy to aid prescribers in decision making and decrease the frequency of medication errors. The use of CPOE for this purpose is a consistent recommendation across many healthcare organizations including The Institute of Medicine (IOM), The Leapfrog Group, the National Quality Forum, and the Joint Commission. One component of CPOE is the incorporation of standardized order sets into the electronic health record (EHR) [11].
Originally written on paper, use of order sets to standardize workflow dates back to the 1980s [12]. This practice upholds a healthcare consensus that well-structured and thought-out order sets have many benefits of playing a role in risk management strategies. Several studies have investigated the improvement of patient-centered outcomes following the incorporation of order sets into the EHR. Previous literature has demonstrated an associated 55% reduction in medical errors following the incorporation of CPOE in the ambulatory setting. Additionally, a study investigating the incorporation of an electronic order set reminding prescribers to order medications for the treatment of heart failure at time of discharge demonstrated an 88-100% improvement in prescription rates [13]. Standardized order sets have been designed to aid healthcare providers with CPOE by preventing errors of commission and omission and help guide in selection of orders that span beyond just medications and incorporate multiple disciplines across various levels of care to ensure best practices are followed during the patient encounter [13,14].
COVID-19 pandemic was an era in which there was an everchanging therapeutic landscape and as a result, a variation in clinical knowledge. To reduce variability in patient care and mitigate risks prescribing COVID-19 therapeutics, two standardized order sets were developed. Epic© SmartSet, a branded term referring to an order set, was used which allowed for medication orders, treatment referrals, documentation tools and education materials to be grouped together for efficient and error-reduced charting. This study describes the implementation, operation and management of an outpatient COVID-19 treatment workflow, focusing on the incorporation of Epic© SmartSets and planning strategies with the COVID-19 taskforce members to implement a mAb clinic. We discuss the importance of facility set up, staffing services and collaboration with enterprise staff members and patients. Continuous review of protocols and procedures and evaluation of clinical outcomes was also conducted with utilization from the SmartSets.
Methods
Study design: This retrospective, multi-site, cohort study aimed to describe the implementation of COVID-19 outpatient order sets at a large healthcare system. Two Epic© COVID-19 SmartSets, one for mAbs and one for oral antivirals, were created along with an outpatient COVID-19 decision tree. Clinical outcomes were evaluated using prescriptions ordered through these SmartSets. Within our healthcare system, all therapeutic agents in this study were ordered exclusively through the SmartSets.
COVID-19 Monoclonal Antibodies
1. Operational Planning
a. A COVID-19 taskforce was created in Quarter 4 of 2020 with leaders nominated by the enterprise Medical Operations committee. The stakeholders included members from hospital leadership, clinical criteria experts, outpatient committees, scheduling and billing, Information Technology (IT), Pharmacy, Executive sponsors, and Medical Operations.
i. Choosing a service site: Among 11 different regional sites, a central hospital location was chosen to serve as the first site to provide mAb infusions within an existing chronic care infusion center. Four hospital leads, the Hospital President, Chief Medical Officer, Chief Nursing Officer and Chief Operating Officer were nominated in addition to two physician leads from the respiratory institute and infectious diseases departments.
ii. Patient criteria experts: Patient criteria and clinical content experts were selected from the infectious disease department including three adult patient leads, and one pediatric patient lead. Five outpatient physician leads were chosen to provide input from the ambulatory setting as main users of the outpatient SmartSet. One IT physician lead was nominated to ensure all IT projects were performed in a timely fashion and information was disseminated as appropriate.
iii. Clinical operations: Two physician leads were chosen to provide insight on research of COVID-19 and assure compliance with healthcare research regulations.
iv. Scheduling and billing: Two staff members were chosen to provide expertise on scheduling workflow and ensure appropriate billing.
v. Pharmacy: Four pharmacy leads were chosen including the Chief Pharmacy Officer, infectious disease clinical coordinator, and Pharmacy Directors (in adult and pediatrics).
vi. Medical ethics: Two ethicists provided input on patient selection criteria.
vii. Executive Sponsors: Chief Medical Operations Officer and the Executive Director Clinical Operations were nominated in addition to two clinical leads from the Medical Operations team, one from main campus and one from a regional site.
b. After the primary infusion center was selected, planning consisted of layout design, nursing/infection prevention/pharmacy support, patient and caregiver needs, environment services, scheduling, IT and reporting and follow-up of serious adverse events.
2. Legal and regulatory compliance
a. Maintaining safety, transparency with EUA and obtaining necessary authorization from patients were important components for legal compliance. The Center for Clinical Research representatives were engaged to aid with chart monitoring and surveillance for adverse reactions following mAb infusions as these events are required to be reported to the FDA per the EUA criteria.
3. Facility setup (mAb Infusion Clinic)
a. A separate entrance and parking lot was assigned for COVID-19 positive patients and designated rooms were created with a bathroom in the room. Each patient was allotted a 3-hr appointment for set up, infusion and post-infusion monitoring. There were up to six appointments starting at 8 am until 4 pm on every weekday. The number of appointments increased throughout the pandemic as demand increased with Ohio locations offering up to 40 appointments per week. Staffing consisted of one registered nurse and one nursing assistant. Support staff of one nurse practitioner was consistently available at the infusion clinic. On-site physician support was available from the intensive care units, with critical care and infectious diseases providers. An oxygen cart and emergency code cart were accessible. Continuous consideration was given to expand sites for adults and pediatric patients at main campus, and/or regional infusion centers based on demand. Emergency Departments (ED) were not selected as a site for mAb infusion due to staffing shortage and the need to focus care on patients with higher acuity.
b. Technological advancement
i. COVID-19 home monitoring program: This population health management program was designed to provide outreach to patients with confirmed or suspected COVID-19 on their day-today health. This allowed early detection of clinical decompensation and timely escalation of patient care to appropriate clinical resources. The goal was to prevent unnecessary utilization of inpatient resources due to prospective surge of inpatient beds and incorporate goals of care discussion for our patients and community. The program also allowed for ongoing patient support and encouraged adherence to recommended health practices and transmission protocols. Epic© MyChart, a branded patient-provider portal and personal health record, was used for daily symptom monitoring and disposition and an escalation pathway was created for the intervention teams to follow.
ii. Pharmacy IT team was mainly responsible for creating the COVID-19 SmartSet, which was accessible to any licensed independent practitioners with privileges under the health system in all ambulatory contexts. Prescribers searched for ‘COVID-19 Monoclonal Antibodies’ or the mAb name to access the SmartSet. The SmartSet included EUA fact sheets with patient instructions and provider documentation with auto-populated enterprise formulary criteria and statements on discussion of investigational therapy use under EUA. The mAb SmartSet included a consult order for COVID-19 infusion referral, medication, IV line care, supportive medication orders including hypersensitivity and anti-emetic medications and a COVID-19 diagnosis code. The mAb orders included four mandatory EUA questions: 1) The patient or caregiver has been given the EUA fact sheet, 2) Patient has been informed of alternatives to receiving mAb, 3) patient has been informed that mAb is an unapproved drug that is authorized for use under EUA, and 4) patient has been advised to continue self-isolation and infection control measures according to CDC guidelines. This was an innovative IT approach to ensure compliance with EUA requirements and to file this information within the order. The EUA questions allowed the providers to reassess the patient criteria and weigh the potential benefits and risks to ensure patient was fit for therapy and all safety and efficacy standards were discussed. There was only one mAb available based on the health system formulary and circulating SARS-CoV-2 variant activity at a given time. A prescriber-facing formulary restriction alert was triggered at each order entry.
iii. Scheduling work queues were created separately for the infusion center administrative assistants by the central scheduling group. Epic© MyChart was used to view appropriate schedules and disseminate pertinent information.
4. Staffing and services offered
a. Nursing: One nurse and one nursing assistant was available during all infusion appointments. Nurses took vital signs and established intravenous access and provided continuous monitoring throughout infusion as per EUA recommendations. Pharmacy members provided infusion education, including monitoring for infusion-related reactions. Discharge education was reinforced in written and verbal format, including continued isolation at home, wearing a mask, social distancing and frequent handwashing and disinfecting.
b. Infection prevention guidance: Compliance with Personal Protective Equipment (PPE) was mandatory and followed institutional and national guideline recommendations.
c. Pharmacy: All mAb orders were reviewed by a pharmacist. Pharmacists documented each patient’s eligibility prior to verifying the order and releasing for scheduling. Once mAb was prepared in the in-patient pharmacy, it was delivered to the infusion center at time of patient arrival. Inventory was performed daily to validate number and for state reporting purposes.
d. Patient’s caregiver needs: All appropriate information was given to patient’s caregiver regarding therapy (obtained from FDA fact sheet). All caregivers were asked to give emergency contact number for patient and asked to wait outside until patient pick-up.
e. Reporting compliance: All adverse events or medication errors were reported to the COVID-19 taskforce group and to FDA per EUA guideline recommendations. Reporting all errors were a fundamental component of risk management as it helped our healthcare system and researchers detect, understand and mitigate risks, ultimately leading to safer patient care.
f. Scheduling and billing: A system was created on the COVID-19 SmartSet for all mAb orders to route to a pharmacy Epic© In Basket message, a message-based task management system to streamline communication and coordinate action, for review and verification. Once pharmacy verified patient criteria, the order was released to enable scheduling at the infusion center. Due to limited slots, all appointments were made on a first-come, firstserved basis. Patients were given instructions for parking, check-in and infection control measures and were contacted one day prior to confirm appointment. Throughout the duration of the study, all COVID-19 outpatient therapies were provided free of charge through the government sponsored supply, therefore, no information on insurance coverage was provided until recently, when the products became commercially available.
5. Education, communication, and outreach
a. Timely education and unification of information was important in this large healthcare system. A training document was updated based on national guidelines and/or enterprise formulary restriction criteria changes. Training documents with a provider section described the use of COVID-19 SmartSet including how to create a new encounter, search for the COVID-19 SmartSet and complete required documentations. Training also included a script for patient consent for mAb therapy, key points of consideration, infection control measures and appointment information. Additionally, the pharmacist training section included management of Epic© In Basket on how to verify patient criteria and release consult referral order for infusion clinic. The nursing documents included information on mAb administration, evaluation of vital signs prior to, during and after infusion, and releasing orders in the SmartSet. It included notes for schedulers on location, driving and parking directions and U.S. Centers for Medicare and Medicaid Services coding and billing information. Lastly, it included clinical trial information on the formulary agent of choice, EUA information and reporting of serious adverse drug reaction to the Center for Clinical Research team and FDA.
b. Dissemination of information: Enterprise communication was provided through news articles on the enterprise intranet page, e-mail to department leads for dissemination, specifically the primary care institutes and targeted specialty institutes, and weekly verbal presentations for the enterprise. Throughout the pandemic, the COVID-19 taskforce team held recurring meetings to discuss latest updates, provide training and education and share workflow challenges while tracking metrics.
c. Patient identification: When a prescriber opened an electronic medical record of a patient with a recent positive COVID-19 test, an alert appeared with the available COVID-19 therapies, patient eligibility criteria, and instructions on how to order the therapies. The alert also included product availability, links to training materials and buttons to the mAb SmartSet and COVID-19 oral therapies SmartSet.
6. Monitoring and evaluation
a. All adverse effects were reported through contacting the clinical compliance team through phone call or pager. The Center for Clinical Research staff proactively screened patients following mAb infusion and assisted with reporting eligible events to the FDA. Reporting was an important part of risk management for our health system to ensure standards were met and take proactive measures when a suspected or confirmed adverse event occurred. Reporting to FDA was also a key component in ensuring continued safety and effectiveness of novel COVID-19 therapeutics and to be a part of protecting the public health.
b. Pharmacy completed continuous evaluation on weekly utilization of monoclonal antibodies. Drug Use Evaluation (DUE) was also conducted one year after implementation of outpatient COVID-19 therapeutics. This DUE included baseline demographics including age, sex, co-morbidities and zip codes, infusion characteristics such as time to infusion from symptom onset or positive COVID-19 test, and adverse reactions. Outcomes such as post infusion ED visits and hospital admissions were measured. Data was presented to Medical Operations teams and enterprise Antimicrobial Stewardship Steering Committee.
COVID-19 Oral therapeutics (nirmatrelvir/ritonavir, molnupiravir)
1. Of note, all operations and processes were identical as the mAbs, except for the SmartSet build as described below.
2. Operational planning: Similar stakeholders were included with additional members from the ambulatory care team, continuous improvement and COVID-19 testing team. The medical operations team kept up to date with the U.S. Department of Health and Human Services for allocation and distribution of EUA agents.
3. IT setup
a. Technological advancement: An outpatient COVID-19 Oral Therapeutics SmartSet was created in late 2021. This oral antiviral SmartSet provided the best available therapy(ies) based on the patient’s age (greater than or equal to 18 years), gender, renal function (eGFR 30 to <60 ml/min or eGFR > or equal to 60 ml/min) and pregnancy and lactation status. The SmartSet highlighted all contraindicated medications based on patients active medication list to guide decision making and provided a link to the EUA fact sheet for additional drugdrug interactions. The oral antiviral orders included four mandatory EUA questions: 1) The patient or caregiver has been given the EUA fact sheet, 2) Patient is within 5 days of symptom onset, 3) The patient has a confirmed positive COVID-19 test, and 4) Provider agrees to report serious adverse reactions per EUA requirements. If any question was answered “no”, a restricted medication warning alert fired to either reevaluate patients’ eligibility and adjust accordingly or remove order. The prescription defaulted to the healthcare system’s Home Delivery pharmacy dispensing and were delivered to the patients’ residence same day or next day. Prescriptions had to be signed by a certain time for shipment and weekend workflows were slightly different due to staffing resources. As the pandemic continued and supplies increased, providers were allowed to send the electronic prescriptions to select outside pharmacies. Continuous updates on the training materials were made based on new clinical outcomes and recommendations, outpatient pharmacy locations/hours, and availability of EUA supply.
Study Population and Outcomes
The study included all outpatient prescriptions for adult patients 18 years and older with a BEB, NMV/r, MOL, or SOT prescriptions from the Epic© COVID-19 SmartSets during January 1, 2022 to December 31, 2022. The ordering provider ensured formulary restriction criteria were met at the time of order by completing documentation. If patients had multiple COVID-19 episodes, the study included only the first episode of COVID-19 during 2022. Patients were excluded if they received duplicate prescriptions with mAb and/or oral antiviral agents for a single COVID-19 episode. The primary clinical endpoint was the rate of hospitalization within 28 days of treatment following mAb or oral antiviral in outpatients with COVID-19. Secondary endpoints included the rate of ICU admission or all-cause mortality within 28 days of treatment.
Statistical Analysis
Patient demographic, disease, and treatment summary measures were presented using frequencies and percentages for categorical factors, and medians with interquartile range (IQR)s non-parametric continuous measures. Multivariable logistic regression was performed to assess what factors of interest independently predicted hospitalization after adjustment for other factors and reported as odds ratio (OR) and 95% confidence interval (CI). Analyses were performed using R version 4.2.3 (R Core Team, 2023) and STATA version 16.1. A p-value < 0.05 was considered statistically significant for all tests.
Results
Between January 1, 2022 and December 31, 2022, 28,628 prescriptions for COVID-19 mAb or antivirals were prescribed. All prescriptions were screened for eligibility and 3,013 prescriptions were excluded as they were duplicate prescriptions or same patient with more than one COVID-19 episodes. A total of 25,615 patients were included for analysis and baseline characteristics are featured in Table 1. The median age was 65 years (IQR 53 to 73), majority female (58%), White race (87%) and non-Hispanic ethnicity (95%). Patients received a mAb (10%) or an oral antiviral agent (90%).
Factor
N = 25,615a
Age (Median, IQR) a
65 (53, 73)
65 and older
13,099 (51%)
55 to 64 years old
5,328 (21%)
Sex
Female
14,900 (58%)
Male
10,713 (42%)
Unknown
2 (<1%)
Race
White
22,215 (87%)
Black
1,741 (7%)
Asian
456 (2%)
Multiracial
492 (2%)
Others, unknown or declined
711 (3%)
Ethnicity
Hispanic
1,160 (5%)
Treatment agents received
Oral antivirals
22,991 (90%)
Nirmatrelvir/ritonavir (NMV/r)
18,136 (71%)
Molnupiravir (MOL)
4,855 (19%)
Monoclonal antibodies
2,624 (10%)
Sotrovimab (SOT)
766 (3%)
Bebtelovimab (BEB)
1,858 (7%)
Oxygen management
236 (<1%)
Non-invasive ventilation
21 (<1%)
Mechanical ventilation
26 (<1%)
Tracheostomy
1 (<1%)
Pre-existing conditions
Hypertension
2,176 (8%)
Neoplasm
1,689 (7%)
Disease of arteries, and veins
1,569 (6%)
Ischemic heart disease
868 (3%)
Type 2 Diabetes mellitus
813 (3%)
Asthma
774 (3%)
Cerebrovascular disease
501 (2%)
COPD
440 (2%)
Heart failure
414 (2%)
Emphysema
187 (<1%)
Transplanted organ or tissue
185 (<1%)
Type 1 Diabetes mellitus
95 (<1%)
Epilepsy and recurrent seizures
86 (<1%)
Pregnancy
59 (<1%)
Multiple sclerosis
58 (<1%)
aMedian (IQR); n (%)
Table 1: Patient demographics.
Figure 1 illustrates the number of 28-day hospitalization in proportion to the number of COVID-19 therapeutic agents during the study period. A total of 482 patients were hospitalized within 28 days of treatment (2%, Table 2). Among those hospitalized, the 28- day ICU admission rate was 21%, while all-cause mortality within 28 days was less than 1%. Table 3 presents patient demographics grouped by patients with or without hospitalization within 28 days. A multivariable regression model was performed and showed increasing to analyze the relationship between hospitalization and high-risk patient characteristics showed increased odds of hospitalization with every 10-year increase in age (OR 1.12, 95% CI 1.05 to 1.19), Black race versus White (OR 1.81, 95% CI 1.34 to 2.40), and in patients with history of transplanted organ or tissue (OR 2.76, 95% CI 1.51 to 4.80).
Entire Cohort
Factor
N
n (%)
95% CI a
Hospitalization within 28 days
25,615
482 (2%)
1.7%, 2.1%
ICU admission within 28 days
482
103 (21%)
-
All-cause mortality within 28 days
25,615
30 (<1%)
-
aCI = Confidence Interval
Table 2: Proportion of 28-day clinical outcomes.
Hospitalization within 28 Daysa
Factor
No, N = 25,131
Yes, N = 482
Age (Median, IQR)a
65 (53, 73)
69 (54, 78)
Sex
Female
14,629 (58%)
270 (56%)
Race
White
21,830 (87%)
383 (80%)
Black
1,686 (7%)
55 (11%)
Others
1,615 (7%)
44 (9%)
Treatment agents received
Oral antivirals (NMV/r and MOL)
22,644 (90%)
345 (72%)
Nirmatrelvir/ritonavir (NMV/r)
17,921 (71%)
213 (44%)
Molnupiravir (MOL)
4,723 (19%)
132 (27%)
Monoclonal antibodies (SOT and BEB)
2,487 (10%)
137 (28%)
Sotrovimab (SOT)
728 (3%)
38 (8%)
Bebtelovimab (BEB)
1,759 (7%)
99 (20%)
Number of Hospitalizations within 28 days
None
-
-
One
-
412 (86%)
Two
-
66 (14%)
Three
-
4 (<1%)
Time from treatment to hospitalization (Median days, IQR)
-
11.71
(5.5 to 18.5)Oxygen management
0 (0%)
235 (49%)
Non-invasive ventilation
0 (0%)
21 (4%)
Mechanical ventilation
0 (0%)
26 (5%)
Tracheostomy
0 (0%)
1 (<1%)
Any h/o neoplasm
1,643 (7%)
46 (10%)
Solid neoplasm
1,574 (6%)
42 (9%)
Hematologic malignancy
221 (<1%)
11 (2%)
Other neoplasm
436 (2%)
21 (4%)
Any h/o transplanted organ or tissue
168 (<1%)
17 (4%)
Solid transplant
149 (<1%)
16 (3%)
Blond marrow transplant
28 (<1%)
2 (<1%)
Other transplant
26 (<1%)
4 (<1%)
ICU admission within 28 days
0 (0%)
103 (21%)
All-cause mortality within 28 days
114 (<1%)
34 (7%)
aMedian (IQR); n (%)
Table 3: Patient demographics of hospitalized patients versus those who were not hospitalized.
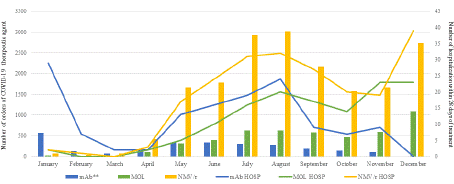
Figure 1: Number of orders of COVID-19 therapeutic agent (bar graphs)
on 28-day hospitalization by month (line graphs). Monoclonal antibody
agent on formulary were sotrovimab from January to March 2022 and
bebtelovimab from April to November 2022.
Limitations
There are limitations that exist in this study. First, the study did not objectively assess the utility of a COVID-19 outpatient therapy focused order set and the effectiveness of using an order set over ordering independently, as independent ordering was not available at our health system. Second, the study was a retrospective, chart review design with all available information obtained from a COVID-19 registry and EHR based on patient records, diagnosis codes and medications ordered. Third, all prescriptions identified were pulled using the order set and presumed to be in outpatient status based on the department location. Fourth, the time to delivery, initiation of first dose and adherence rate of oral antivirals were not confirmed.
Discussion
The Epic© COVID-19 SmartSets were designed to provide a proactive approach to ordering, managing, and monitoring COVID-19 therapeutics with a goal to ensure standardized care while enhancing patient safety. The SmartSets allowed for timely and continuous updates from the multidisciplinary COVID-19 taskforce team based on national recommendations, FDA approvals and procurement issues. Additionally, limiting the selection of therapeutic agent orderable on the SmartSets created a safeguard protecting from variation in practice and adherence to EUA. High-risk patient criteria were managed and revised in phased approaches by the taskforce team members and auto populated into the SmartSet progress notes for prescriber awareness. The SmartSets also functioned as a valuable location to link EUA and patient education materials along with communication relating to supply issues of EUA agents. Overall, this intuitive SmartSet facilitated a streamlined decision-making process and potentially reduced the risk of errors by providing clear, and structured instructions to all staff members during the heightened pressures of a pandemic.
As of December 2023, our health system included over 5,700 physicians and 3,600 advanced practice providers who had access to the COVID-19 therapeutics SmartSets. Given the scale of our enterprise, a standardized, unified approach was important to provide consistent practice according to the most recent guideline changes. One of the highlights of our SmartSet was the built-in, cascading clinical decision support tool with the ability to consider patient criteria such as age, renal and hepatic impairment, and pregnancy and lactation. This functionality supported efficient risk stratification and autonomous decision making for all SmartSet users. Moreover, the ability to display active contraindicated prescriptions due to drugdrug interactions provided a safety net for prescribers at ordering. This was a critical component with some COVID-19 therapeutics, such as NMV/r as severe interactions with anti-arrhythmic, anticonvulsants and/or other immunosuppressants could lead to detrimental effects [15]. The SmartSet provided links to all drug-drug interaction consideration documents, however, pharmacists were often consulted to evaluate significant interactions and dispensing.
Documentation was also streamlined as a structed template for all providers and included the formulary restriction criteria and EUA information. The SmartSet facilitated communication for all healthcare team members, fostering coordinated care between the prescriber, nurses, pharmacists, patients, and caregivers.
In addition to the operational aspect, our study reported reallife outcomes during the 2022 Omicron era treated with a mAb or oral antivirals ordered through the SmartSets. The overall low 28- day hospitalization rate of 2% in our study was comparable to other landmark trials for both mAbs and oral antivirals, although the predominant COVID-19 variants differed during our study. As per CDC, older age remains the strongest risk factor for severe COVID-19 outcomes with hospitalization and death by age group showing risk of death up to 25 times higher in those aged 50 to 64 years, and 60 times higher in 65 to 74 years [16,17].
Additionally, race and ethnicity were also shown to impact care and equitable use of effective medications [18]. It is important to note that racial and ethnic disparities with COVID-19 treatments received are also known, with only 15.2% of Black or African Americans receiving mAb compared to74.7% for White (national patientcentered clinical research network).
Understanding that elderly patients have higher rates of comorbidities and are more vulnerable to negative predictive outcomes helps us obligate better preventative measures such as vaccination and appropriate and timely therapeutic care. Systemic factors such as limited access to transportation to medical sites, lack of insurance coverage for medical care and variations in treatment distribution and education are areas to work on.
Conclusion
In conclusion, our study demonstrated a unified and innovative implementation of COVID-19 order sets at a large healthcare system to allow for standardized ordering of COVID-19 therapeutics with readily accessible information. We identified a low 28-day hospitalization rate of 2% for patients receiving COVID-19 mAb or antiviral therapy using this strategy. Further studies are warranted to evaluate the effect of standardized order sets on other disease states subject to deviations from standard of care.
References
- Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Infectious Diseases Society of America. 2022.
- National Institutes of Health COVID-19 treatment guidelines panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. 2023.
- US Food and Drugs Administration. Emergency use authorization for drugs and non-vaccine biological products. 2022.
- Gupta A, Gonazlez-Rojas Y, Juarez E, Casal MC, Moya J, Falci DR, et al. Early treatment for covid-19 with sars-coV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021; 385: 1941-1950.
- Westendorf, K, Zentelis S, Wang L, Mascola JR, Jones BE, Barnhart BC, et al. LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants. Cell Rep. 2022; 39: 1-17.
- Paxlovid (nirmatrelvir and ritonavir). Package insert. Pfizer. 2023.
- Lagevrio (molnupiravir). Package insert. Merck Sharp & Dohme LLC. 2023.
- Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, et al. Oral nirmatrelvir for high-risk, nonhospitalizaed adults with Covid-19. N Engl J Med. 2022; 386: 1397-1408.
- Bernal AJ, Gomes da Silve MM, Musngaie DB, Kovalchuk E, Gonzalez A, Reyes VD, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022; 386: 509-520.
- Butler C, Hobbs FDR, Gbinigie OA, Rahman NM, Hayward G, Richards DB, et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platformadaptive randomized controlled trial. Lancet. 2023; 401:281-293.
- Mahoney CD, Berard-Collins CM, Coleman R, Amaral JF, Cotter CM. Effects of an integrated clinical information system on medication safety in a multihospital setting. Am J Health Syst Pharm. 2007; 64: 1969-77.
- Devine EB, Hansen RN, Wilson-Norton JL, Lawless NM, Fisk AW, Blough DK, et al. The impact of computerized provider order entry on medication errors in a multispecialty group practice. J Am Med Inform Assoc. 2010; 17: 78-84.
- Agrawal A. Medication errors: prevention using information technology systems. Br J Clin Pharmacol. 2009; 67: 681-6.
- Institute for Safe Medication Practices (ISMP)’s Guidelines for Standard Order Sets. Institute for Safe Medication Practices. 2024.
- Marzolini C, Kuritzkes DR, Marra F, Boyle A, Gibbons S, Flexner C, et al. Recommendations for the management of drug-drug interactions between the COVID-19 antiviral nirmatrelvir/ritonavir (Paxlovid) and comedications. Clin Pharmacol Ther. 2022; 112: 1191-1200.
- Centers for Disease Control and Prevention. Underlying medical conditions associated with higher risk for severe COVID-19: Information for healthcare professionals. 2023.
- Centers for Disease Control and Prevention. COVID-19 death data and resources. 2023.
- Wiltz JL, Feehan AK, Molinari NM, Ladva CN, Truman BI, Hall J, et al. Racial and ethnic disparities in receipt of medications for treatment of COVID-19 – United States, March 2020 – August 2021. MMWR Morb Mortal Wkly Rep. 2022; 71: 96-102.