
Review Article
J Immun Res. 2022; 8(1): 1047.
Macrophage Glucose Metabolism Reprogramming and Cardiovascular Complications of Diabetes
He Yuqi¹, Chen Cong¹, Haitao X¹* and Jingsong C²*
1Department of Laboratory Medicine, The First Affiliated Hospital, University of South China, China
2Department of Endocrinology and Metabolism, The First Affiliated Hospital, Institute of Clinical Medicine, University of South China, China
*Corresponding author: Cao Jingsong, Xie Haitao, Institute of Clinical Medicine, The First Affiliated Hospital of University of South China, Hengyang City, 421000, Hunan Province, China
Received: July 18, 2022; Accepted: August 17, 2022; Published: August 24, 2022
Abstract
Macrophages are the main participants in the pathogenesis of many chronic inflammatory and autoimmune diseases, including diabetes, and cardiovascular complications mediated by abnormal activation of macrophages caused by various reasons are the main causes of morbidity and death in patients with diabetes. Different environmental stimuli can easily lead to glucose metabolism reprogramming in macrophages, resulting in a series of changes in metabolization-related enzymes, metabolites and metabolic pathways. Glucose metabolism reprogramming is closely related to M1 polarization and M2 polarization of macrophages, for example, M1 macrophages tend to anaerobic glycolysis while M2 macrophages tend to aerobic metabolism, and different polarization of macrophages is closely related to various diseases. Therefore, this paper reviews the mechanism and inducing factors of reprogramming of glucose metabolic pathways, as well as the effect of these pathways on macrophage polarization, so as to provide services for clinical research and treatment of the occurrence and development of cardiovascular complications in the context of chronic inflammation of diabetes.
Keywords: Glucose metabolism reprogramming; Macrophage polarization; Diabetes; Cardiovascular complications
Introduction
Diabetes is a group of chronic metabolic diseases worldwide characterized by chronic hyperglycemia, which easily induces the activation of the immune system and leads to chronic inflammation, eventually leading to a series of serious cardiovascular diseases and other complications [1,2]. The World Health Organization predicts diabetes as the seventh leading cause of death in the world by 2035 [3], and the International Diabetes Federation estimates that the number of people diagnosed with T2DM worldwide will rise to 640 million by 2040 [4,5]. Among diabetes-related deaths, cardiovascular complications are a major contributing factor. Among diabetes-related deaths, cardiovascular complications are a major contributing factor. Therefore, the study of diabetes and its main complications cardiovascular complications is of far-reaching significance for its clinical treatment [6,7]. However, at present, there is no cure for this type of disease, so in-depth research on diabetes treatment is very important to the development of diabetes patients and medical services around the world [8]. Macrophages are a very important class of innate immune cells, widely found in various tissues and organs of the body. They have major functions such as phagocytosis, chemotaxis, regulating of inflammation, and clearing pathogenic microorganisms; they are usually divided into classically activated M1 type and alternative activated M2 type [9-11]. Studies have shown that metabolic reprogramming is a core component of macrophage plasticity and polarization [12], and changes in glucose metabolism pathways can support macrophage polarization into different subtypes, contributing to their immune homeostasis and immune system in vivo. It plays an important function in the inflammatory response [13]. Normally, the metabolism of M1 macrophage is biased towards glycolysis, while M2 macrophage metabolism is biased towards the oxidative phosphorylation pathway to obtain its required adenosine triphosphate [14,15]. Therefore, this article reviews the related research on macrophage polarization with glucose metabolism as an entry point, and provides a systematic and comprehensive understanding of the related research on the involvement of macrophage glucose metabolism reprogramming in the complications of diabetes and cardiovascular disease.
Mechanisms of Glucose Metabolism Reprogramming
Restriction Enzymes Related To Glucose Metabolism
Glucose metabolism is a complex and systematic regulation process, mainly including glycolysis, oxidative phosphorylation, and pentose phosphate pathway. The process of glucose catabolism and energy production requires the participation of a variety of enzymes, and this review mentions some of the enzymes that play the main role. For example, the activity changes of hexokinase, 6-phosphate kinase-1, pyruvate kinase, glucose-6-phosphate dehydrogenase and glyceraldehyde 3-phosphate dehydrogenase exerts different regulatory effects on glucose metabolism.
Hexokinase (HK) has 4 isoforms, of which HK2 is considered to be the prototypical inducible isoform of HK family members because it can be up-regulated by various environmental factors and signaling pathways [16,17]. Studies have proved that 2-Deoxygenation-DGlucose (2-DG) is a glycolytic inhibitor targeting HK2, which can significantly reduce the ATP concentration of M1 macrophages, and effectively inhibit the aerobic glycolytic activity of macrophage [18,19]; promotes oxidative phosphorylation, induce macrophage polarization to M2, and thus produce serial metabolic effects such as glucose metabolism reprogramming [20,21].
The 6-Phosphate Kinase-1 (PFK) is activated by its strongest allosteric activator, fructose-2, 6-diphosphatase 3 (PFKFB3), which then promotes the glycolytic pathway [22,23]. There are PFK1 and PFK2, and PFK2 has L-PFK2 (liver) and U-PFK (universal) forms, in which L-PFK2 causes PFKFB3 degradation, and U-PFK shows high phosphokinase activity and catalyzes the production of more PFKFB3, so glycolysis pathway enhanced sugars cause metabolic reprogramming [24,25]. In M1 macrophages, U-PKF is induced by pro-inflammatory stimulators and growth factors, and down regulation of U-PFK2 expression suppresses the activity of M1 macrophages [26,27]. Because PFK2 can regulate the properties of glycolytic metabolism under various physiological conditions, it plays a clinical therapeutic role as a therapeutic target of glucose metabolism [28].
Pyruvate Kinase (PK) consists of four isoenzymes, commonly PK1 and PK2. At present, muscle-type PKM can be widely studied as a protein kinase of various transcription factors in the nucleus [29]. In the process of macrophage polarization, inhibition of PKM2 activity leads to the formation of dimer of PKM2 and its interaction with HIF-1α, which in turn up-regulates the expression of HIF-1α related glucose metabolism enzyme genes, thereby enhancing the activity of glycolytic metabolism enzyme and inducing the development of glucose metabolism reprogramming [30]. PKM2 can not only regulate gene expression in the nucleus, but also attach to the outer mitochondrial membrane, leading to mitochondrial dysfunction by disrupting the balance of fission and fusion, resulting in abnormal oxidative phosphorylation metabolic pathways [31,32].
Glucose-6-Phosphate Dehydrogenase (G6PD) is a key enzyme in the Pentose Phosphate Pathway (PPP), which promotes intracellular oxidative stress and pro-inflammatory cytokine expression by activating p38 mitogen-activated protein kinase (p38MAPK) and nuclear factor NF-KB signaling pathway. Inhibition of G6PD expression with chemical inhibitors or small interfering RNA can significantly reduce p38MAPK and NF-KB signals, and downregulate the expression of inflammatory factors such as IL-1β and IL-6 [33,34]. Thus, playing a role in regulating the reprogramming of glucose metabolism, and regulating its activity can provide a clinical approach for related treatment.
In addition to the above-mentioned key enzymes of glucose catabolism, other enzymes involved also play important roles in the differentiation, activation and glucose metabolism reprogramming of macrophages. Understanding the role of glucose metabolismrelated enzyme formation or regulated metabolic network in the physiological function of macrophages will facilitate the further study of the molecular mechanism of macrophage polarization and glucose metabolism reprogramming.
Molecular Mechanisms of Glucose Metabolism Reprogramming
PI3K/Akt - mTOR - HIF1-α Signal Enhances Glycolysis
Phosphatidylinositol Kinase (PI3K)/ protein kinase B(Akt) signaling can promote the expression of Glucose Transporter (GLUT) and protein translocation to improve glucose uptake and metabolism [35]. Target rapamycin (mTOR) is a downstream effector of the PI3K/Akt pathway that regulates cell proliferation, growth, survival, and metabolic activity by integrating various signals, including energy, growth factors nutrition and stress [36,37]. It has been demonstrated that activation of pi3K-Akt-MTOR pathway increases HIF-1A synthesis and upregulates HIF-1 regulatory gene mRNA level, while HIF1-α directly activates glycoly-related enzymes to stimulate glycolysis [38,39]. Such as glucose transporters 1 and 3 (GLUT1, GLUT3), HK1 and 2, GAPDH, phosphoglycerate kinase (PGK1), PKM2, Lactate Dehydrogenase (LDHA) and Pyruvate Dehydrogenase Kinase (PDK), etc [40,44]. In addition, enhanced HIF-1 not only blocks TCA cycle and oxidative phosphorylation in mitochondria, but also plays an important role in macrophage migration by promoting the expression of LDHA and PDK migration [45,46], improves cell utilization of acetyl-CoA (CoA) and inhibiting pyruvate dehydrogenase activity to enhance glycolysis [47]. Therefore, regulating mTOR activity to explore the mechanism and effect of aerobic glycolysis is an important strategy to study glucose metabolism reprogramming in macrophages.
NF-KB-CARKL-Enhances Pentose Phosphate Pathway
Nuclear transcription factor (NF-κB) is involved in regulating apoptosis and stress response, and its inactivation or overactivation can lead to abnormal changes in metabolic balance [48,49]. Carbohydrate Kinase-Like Protein (CARKL) is an important factor in the production of setoheptose-7-phosphate (S7P), the intermediate of pentose phosphate pathway [50,51]. LPS can inhibit the expression of CARKL through the NF-κB pathway and up-regulate the PPP pathway, which weakens the restrictive effect on TNF-α and pentose phosphate pathways, thereby promoting the polarization of M1 macrophages [52]. CARKL is expressed at a high level in M2 macrophages, indicating that the metabolic level of the PPP pathway is higher than that of M1. These results suggest that CARKL is an important regulatory factor regulating the balance of glucose reprogramming intermediates in macrophages and may be an important target for glucose reprogramming studies in macrophages.
Mitochondrial Function and Oxidative Phosphorylation OXPHOS Pathway
Mitochondria is key sites for regulating cellular glucose metabolism. Infection, stress and other environmental changes can significantly change the structure and function of macrophages’ mitochondria, and mitochondrial damage can promote the generation of Reactive Oxygen Species (ROS) [53,54], not only promote the stable expression of HIF1-protein, but also promote the production of inflammatory cytokines (such as IL-6 and TNF-α) [55,56]. It has been found that Inducible Nitric Oxide (iNOS) and Nitric Oxide (NO) can modify mitochondrial complex I and IV through nitrosylation, break mitochondrial electron transport chain, and inhibit oxidative phosphorylation of cells [57]. Knockdown of iNOS improves LPSinduced impairment of mitochondrial respiratory function in M1 macrophages [58]; and administration of exogenous NO again leads to mitochondrial dysfunction and glucose metabolism reprogramming, and promotes macrophage inflammatory response [59].
In addition, appropriate mitochondrial division and fusion is also key to determining its structural and functional homeostasis. Excessive mitochondrial division leads to mitochondrial electron transfer disorder and impaired oxidative phosphorylation metabolism, while enhanced fusion promotes the tight connection of mitochondrial complexes and oxidative phosphorylation process [60-62]. It can be seen that the structure and function of mitochondria and the stable expression of related enzymes is conducive to maintaining the glucose metabolism balance of cells, and the occurrence of either tilt may lead to metabolic reprogramming.
Inducible Factors of Glucose Metabolic Reprogramming
The reprogramming of macrophage metabolic pathways is not only caused by nutrition or oxygen, but also by various diseases, intestinal flora disorders, and stimulation of glucose metabolites. In addition, changes in virulence factors, pattern recognition receptors, gene changes, and cytokine receptors can also induce the reprogramming of cellular glucose metabolism.
Intestinal Microflora
With the continuous development of microbial research, the study of intestinal flora, the “hidden organ” of human body, has become hotter and hotter, and it has been found that it plays an important role in a variety of diseases, obesity, diabetes and cardiovascular diseases are closely related to gastrointestinal microbial disorders [63-65]. In recent years, more and more studies have found that metabolites play an important role in the microbiome mediated regulation of glucose metabolism in patients with Type 2 Diabetes Mellitus (T2DM) [66]. For example, propionic acid, butyric acid and succinic acid can promote intestinal gluconeogenesis, affect host appetite and insulin secretion, and inhibit liver gluconeogenesis, thus exerting the effect of glucose metabolism regulation and reprogramming [67,68]. Bile acid derivatives and amino acid derivatives can also promote the synthesis and secretion of glucagon-like peptide 1GLP-1, thus causing insulin secretion to regulate blood glucose and glucose metabolism [69,70]. Therefore, regulating the balance of intestinal flora can regulate glucose metabolism in diabetes and may be an important regulator in macrophages glucose metabolic reprogramming.
Itaconic Acid
It has been suggested that itaconic acid is an immunomodulatory anti-inflammatory metabolite that is synthesized by homeotropic aconite in the TCA cycle in macrophages that are activated by a variety of factors, particularly Lipopolysaccharide (LPS), as well as other Toll-like Receptor (TLR) ligands and cytokine [71,72]. Itaconic acid was found to be significantly higher in women with gestational diabetes, and the current preliminary study found that itaconic acid may have the potential to play an important role as a novel biomarker in predicting the subsequent development of early gestational diabetes [73]. The metabolites itaconic acid and its derivatives inhibit M2 polarization by inhibiting JAK1/STAT6 signaling pathway, and inhibit the transcription of some genes related to M2 polarization process, such as Pparg, CD206 and Fizz1. The mitochondrial oxidative phosphorylation pathway of glucose metabolism is also significantly inhibited [74,75]. It was found that itaconic acid inhibits Succinate Dehydrogenase (SDH), whose activity mediates succinate oxidation to regulate the pro-inflammatory IL-1b-HIF-1α axis, regulates ROS release in vitro and in vivo, and has an effect on mitochondrial function and glucose metabolism TCA cycl [76,77]. The researchers found that itaconate can directly bind to protein Keap1 and promote its alkylation, thus inducing Nrf2 expression, and finally inhibiting inflammation by inhibiting IL-1b expression [76,78]. Itaconate obviously promotes the pentose phosphate pathway, leading to a significantly increased NADPH oxidase activity, and regulating the expression of the gene A20 regulated by the NF-B pathway produces more ROS release [79]. Therefore, the accumulation of chaconic acid can promote the reprogramming of glucose metabolism and also play a role in inducing polarization of macrophages.
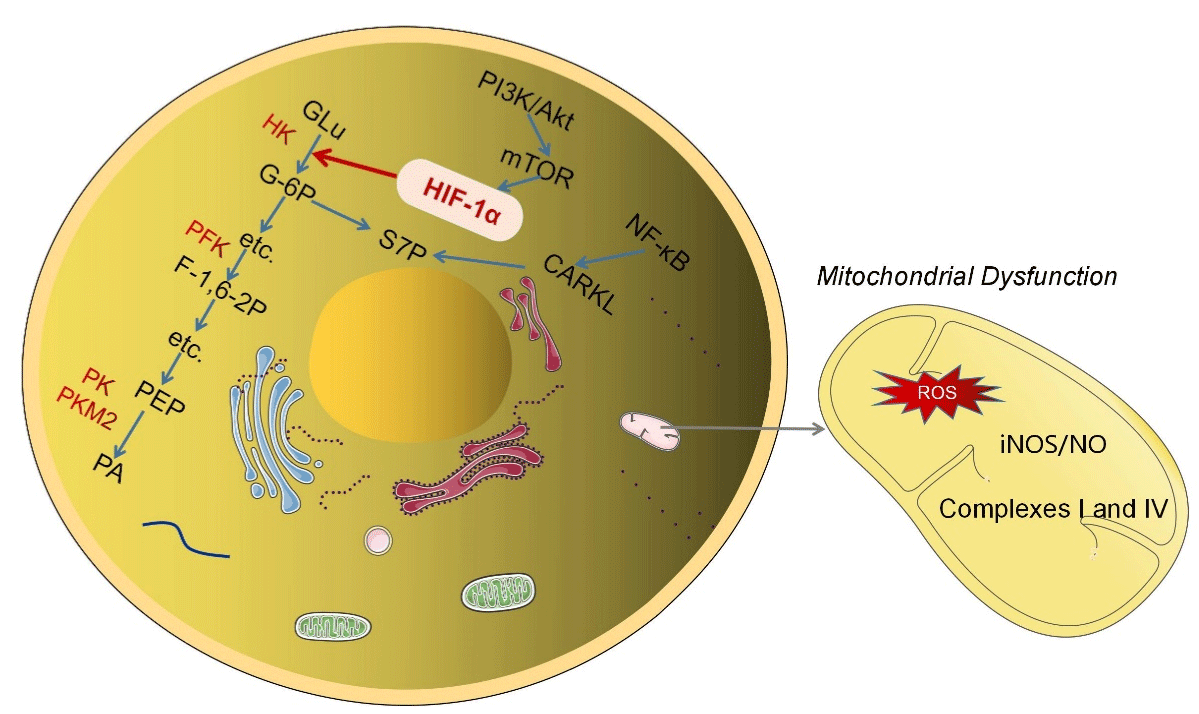
Figure 1: The mechanism of glucose metabolism reprogramming. The mechanism of glycolysis key enzymes in glucose reprogramming and the regulation of
HIF-α on it; The signaling pathway in which intermediate S7P of pentose phosphate pathway plays a role; TCA cycling in mitochondria and its effect on glucose
metabolism reprogramming during dysfunction.
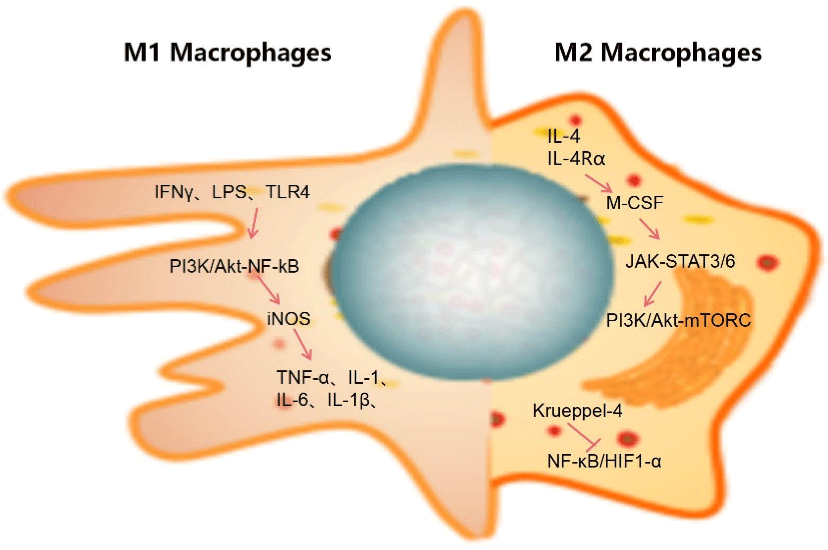
Figure 2: Polarization of macrophages. On the left are the inducing factors of M1 polarization and the expression of signal pathway and final secretory factor; The
figure on the right shows the related signal pathways of M2 polarization. The process of glucose metabolism was different with the polarization of M1M2.
Costimulatory Molecular Ligand (4-1BBL)
4-1BBL is a member of the tumor necrosis factor superfamily, which the 4-1BBL signaling pathway may be a valid target for controlling macrophage-mediated chronic inflammation in obesity and metabolic diseases [80,81]. 4-1BBL stimulates macrophage to increase Akt phosphorylation through the Akt/mTOR signalmediated pathway, resulting in increased glucose uptake, GLUT1 and glycolysis HK1, PFK, LDH transcription/protein levels, and lactic acid production, enhancing the reprogramming process of glucose metabolism in cells. It also enhances the pentose phosphate pathway by regulating G6PD and PGD [82,83]. 4-1BBL can stimulate the induction of polarization of M2 macrophages through the reduction of heme oxygenase HO-1, increasing the transcription level of M2 macrophages in macrophages. HO-1 is a microsomal enzyme that is induced in response to oxidative stress and inflammatory stimulation and has a powerful anti-inflammatory effect on macrophagemediated inflammation by preferentially promoting the M2 phenotype [84,85]. Therefore, the signaling pathway regulated by 4-1BBL is also an effective target for macrophage-mediated chronic inflammation regulating glucose metabolism reprogramming and metabolic diseases.
FoxO1
FoxO1 (FoxO1), as an important transcription factor in human body, plays an important role in oxidative stress, DNA damage repair, metabolism, cell cycle and homeostasis [86,87]. FoxO1 expression is inhibited by insulin and induced by glucagon [88,89]. It was found that transcription factor FoxO1 is involved in the regulation of key enzymes of glycolysis, such as PKM2, LDH and GIUT1, suggesting that FoxO1 plays an important role in the regulation of glucose metabolism and glucose reprogramming in macrophages [90]. According to the results of professor Guangfucheng’s team of USTC in 2020, glycol-related genes were significantly downregulated in macrophages lacking transcription factor FoxO1, and macrophages lacking FoxO1 tended to be M2 polarized, and glucose intake and glycolysis levels decreased significantly, while oxidative phosphorylation levels increased. These results indicate that FoxO1 can affect the polarization and function of macrophages by regulating glucose metabolism reprogramming, but the specific downstream genes and related mechanisms need to be discovered.
Glucose Metabolism and Macrophage Polarization
Aerobic Glycolysis and Macrophage M1 Polarization
Under normal circumstances, cells generally produce energy by means of aerobic oxidation, and only when the level of oxidative phosphorylation decreases under conditions such as hypoxia or mitochondrial dysfunction, can ATP be produced for energy by means of anaerobic colysis. However, for some unknown reasons and mechanisms, the rapidly increasing cells, such as tumor cells and macrophages, still tend to obtain energy through glycolysis rather than oxidative phosphorylation under aerobic conditions, which is the Warburg effect [91-94].
Under various stimuli such as interferon (IFN-γ), LPS and TLR4, the intracellular metabolism of macrophages is changed, glycolysis pathway and pentose phosphate pathway are enhanced, and TCA cycle and oxidative phosphorylation pathway are weakened, leading to M1 polarization [95,96]. When macrophage M1 polarization, TCA cycle is weakened and two interruption occurs [97]: The first interruption involved a significant decrease in Isocitrate Dehydrogenase (IDH) expression, resulting in a substantial increase in citrate production and succinate levels, as well as reducing mitochondrial respiration (SDH comprises mitochondrial respiratory chain complex II) [98-100]. When succinic acid is transported to the cytoplasm, the degradation of hypoxia-inducible factor HIF1-α by Proline Hydrogenase (PDH) is reduced (PDH promotes HIF1-α degradation to regulate glucose metabolism reprogramming and macrophage polarization [101,102].
Moreover, PI3K activation can inhibit downstream Akt1 activation, and the activation of PI3K/Akt pathway plays an antiinflammatory role in TLR-stimulated macrophages and is a negative regulator of TLR and NF-κB signaling in macrophages [103,104]. The activation or over expression of PI3K/Akt protein kinase resulted in reduced stimulation of macrophages by LPS, while TLR activated non-specific chemical inhibition of PI3K signal in cells and enhanced the activation of NF-KB and the expression of iNOS, thus promoting m1-type polarization of macrophages [104]. Therefore, the metabolic state of macrophages is closely related to its polarization, which affects the function of macrophages and the occurrence and development of diseases.
M1 macrophages are an important source of many inflammatory cytokines, including TNF-α, IL-1, IL-12, IL-18, and IL-23, which have been identified as important mediators and drivers of chronic inflammation and autoimmune diseases. In cardiovascular complications of diabetes, M1-type macrophages are significantly increased, leading to myocardial hypertrophy and myocardial interstitial fibrosis, aggravating cardiac remodeling, and ultimately leading to apoptosis [105,107]. Therefore, targeted regulation of glucose metabolism reprogramming to regulate M1 polarization level of macrophages in vivo can effectively improve cardiovascular complications of diabetes.
Aerobic Oxidation of Sugar and M2 Polarization of Macrophages
Aerobic oxidation is usually mainly divided into three parts: glycolytic pathway, pyruvate metabolism to acetyl-CoA, and tricarboxylic acid cycle (TCA). M2 macrophages mainly obtain the required energy through this metabolic method. At this time, the TCA cycle and mitochondrial oxidative phosphorylation metabolic pathways are all enhanced [108,109].
In the process of M2 polarization, PI3K-MTORC2 and STAT6 signaling pathways are crucial. MTORC operates in parallel with IL- 4RA-Stat6 pathway and promotes the activation of M2 polarization by inducing transcription factors (IRF4), at which time the glycolysis pathway in the aerobic oxidation of glucose is enhanced [110]. The pro-polarizing factor IL-4 causes non-receptor tyrosine protein kinase/signal transducer and activator of transcription (JAK/ STAT) by binding to interleukin-4 receptor alpha (IL-4Rα) on the cell membrane A pathway is initiated in which activation of STAT3 and STAT6 leads to the polarization of macrophages towards the M2 phenotype, and its downstream Krueppel-like factor 4 can participate in macrophage M2 polarization by inhibiting NF-κB/ HIF1-α-dependent signaling transcription [111]. At the same time, macrophage colony stimulating factor (M-CSF) and IL-4 can synergically induce signal transduction by activating AKT and mTORC, leading to the polarization of macrophage M2, and the involved Akt-MTORC pathway may be the target of regulating the substitution activation of macrophages for glucose metabolism reprogramming [112].
Studies show that cardiovascular complications in diabetes, macrophage polarization is closely related to atherosclerosis, probably by reducing the macrophages of the absorption of atherosclerotic plaque or increase the polarization to M2 macrophage phenotypes, promote the secretion of anti-inflammatory cytokine, M2 should play the role of anti-inflammatory, adjacent cells proliferation and repair of damaged heart tissue, reduce inflammation and improve cardiac function, which plays a beneficial role in clinical treatment [113,114].
Conclusion and Outlook
Glucose metabolism is an important cellular physiological process that controls the energy balance of the whole body, and its dysregulation is associated with the occurrence of various diseases. The state of glucose metabolism is closely related to the functional state of macrophages. The reprogramming of glucose metabolism caused by various reasons causes the polarization of macrophages to have different biological roles, and finally leads to the development of diabetes cardiovascular disease which with macrophages as the core of the disease, such as vascular complications. Therefore, this paper hopes to review the occurrence and development of macrophage glucose metabolism reprogramming, and hope to provide a reference for the in-depth study of cardiovascular complications of diabetes and its clinical treatment.
Data Availability Statement
All data in the article can be requested from the corresponding author.
Competing Interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Sources
This work was supported by Natural Science Foundation of Hunan Province, China (No. 2021JJ40490 and No. 2021JJ70113); Scientific Research Fund Project of Hunan Provincial Health Commission, China (No. 20201981).
References
- Burger D, Turner M, Xiao F, Munkonda MN, Akbari S, Burns KD. High glucose increases the formation and pro-oxidative activity of endothelial microparticles. Diabetologia. 2017; 60: 1791-1800.
- Wang L, Peng W, Zhao Z, Zhang M, Shi Z, Song Z, et al. Prevalence and Treatment of Diabetes in China, 2013-2018. JAMA. 2021; 326: 2498-2506.
- Polonsky WH, Fisher L, Hessler D, Edelman SV. Investigating Hypoglycemic Confidence in Type 1 and Type 2 Diabetes. Diabetes technology & therapeutics. 2017; 19: 131-136.
- Aschner P. New IDF clinical practice recommendations for managing type 2 diabetes in primary care. Diabetes research and clinical practice. 2017; 132: 169-170.
- Ogurtsova K, Fernandes JDDR, Huang Y, Linnenkamp U, Guariguata L, Cho NH, et al. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes research and clinical practice. 2017; 128: 40-50.
- Plein A, Fantin A, Denti L, Pollard JW, Ruhrberg C. Erythro-myeloid progenitors contribute endothelial cells to blood vessels. Nature. 2018; 562: 223-228.
- Cao J, Zu X, Liu J. The roles of immune cells in atherosclerotic calcification. Vascular. 2021: 170853812110327.
- Astha Soni, Neil Wright, Juliana Chizo Agwu, Josephine Drew, Melanie Kershaw, Christopher Moudiotis, et al. Fifteen-minute consultation: Practical use of continuous glucose monitoring. Archives of disease in childhood. Education and practice edition. 2022; 107: 188-193.
- Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014; 41: 14-20.
- Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013; 496: 445-455.
- Funes SC, Rios M, Escobar-Vera J, Kalergis AM. Implications of macrophage polarization in autoimmunity. Immunology. 2018; 154: 186-195.
- Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. The Journal of clinical investigation. 2012; 122: 787-795.
- Viola A, Munari F, Sánchez-Rodríguez R, Scolaro T, Castegna A. The Metabolic Signature of Macrophage Responses. Frontiers in Immunology. 2019; 10.
- Wang S, Liu R, Yu Q, Dong L, Bi Y, Liu G. Metabolic reprogramming of macrophages during infections and cancer. Cancer letters. 2019; 452: 14- 22.
- Peterson KR, Cottam MA, Kennedy AJ, Hasty AH. Macrophage-Targeted Therapeutics for Metabolic Disease. Trends in pharmacological sciences. 2018; 39: 536-546.
- Garcia SN, Guedes RC, & Marques MM. Unlocking the Potential of HK2 in Cancer Metabolism and Therapeutics. Current medicinal chemistry. 2019; 26:7285-7322.
- Bian X, Jiang H, Meng Y, Li Y, Fang J, Lu Z. Regulation of gene expression by glycolytic and gluconeogenic enzymes. Trends in cell biology. 2022. 32: 786-799.
- Pajak B, Siwiak E, Sołtyka M, Priebe A, Zieliński R, Fokt I, et al. 2-Deoxyd- Glucose and Its Analogs: From Diagnostic to Therapeutic Agents. International Journal of Molecular Sciences. 2019; 21: 234.
- Zhang T, Zhu X, Wu H, Jiang K, Zhao G, Shaukat A, et al. Targeting the ROS/PI3K/AKT/HIF-1α/HK2 axis of breast cancer cells: Combined administration of Polydatin and 2-Deoxy-d-glucose. Journal of Cellular and Molecular Medicine. 2019; 23: 3711-3723.
- Aft RL, Zhang FW, Gius D. Evaluation of 2-deoxy-D-glucose as a chemotherapeutic agent: mechanism of cell death. British Journal of Cancer. 2002; 87: 805-812.
- Li L, Fath MA, Scarbrough PM, Watson WH, Spitz DR. Combined inhibition of glycolysis, the pentose cycle, and thioredoxin metabolism selectively increases cytotoxicity and oxidative stress in human breast and prostate cancer. Redox Biology. 2015; 4: 127-135.
- Trojan SE, Markiewicz MJ, Leśkiewicz K, Kocemba-Pilarczyk KA. The influence of PFK-II overexpression on neuroblastoma patients’ survival may be dependent on the particular isoenzyme expressed, PFKFB3 or PFKFB4. Cancer Cell International. 2019; 19: 292.
- Cao Y, Zhang X, Wang L, Yang Q, Ma Q, Xu J, et al. PFKFB3-mediated endothelial glycolysis promotes pulmonary hypertension. Proceedings of the National Academy of Sciences. 2019; 116: 13394-13403.
- Rodríguez-Prados J, Través PG, Cuenca J, Rico D, Aragonés J, Martín-Sanz P, et al. Substrate Fate in Activated Macrophages: A Comparison between Innate, Classic, and Alternative Activation. The Journal of Immunology. 2010; 185: 605-614.
- Bartrons R, Rodríguez-García A, Simon-Molas H, Castaño E, Manzano A, Navarro-Sabaté. The potential utility of PFKFB3 as a therapeutic target. Expert Opinion on Therapeutic Targets. 2018; 22: 659-674.
- He Q, Ma Y, Liu J, Zhang D, Ren J, Zhao R, et al. Biological Functions and Regulatory Mechanisms of Hypoxia-Inducible Factor-1α in Ischemic Stroke. Frontiers in Immunology. 2021; 12.
- Kim SY, Choi YJ, Joung SM, Lee BH, Jung Y, Lee JY. Hypoxic stress upregulates the expression of Toll-like receptor 4 in macrophages via hypoxiainducible factor. Immunology. 2010; 129: 516-524.
- Geeraerts X, Bolli E, Fendt S, Ginderachter JAV. Macrophage Metabolism As Therapeutic Target for Cancer, Atherosclerosis, and Obesity. Frontiers in Immunology. 2017; 8.
- Park B, et al. Pyruvate kinase M1 regulates butyrate metabolism in cancerous colonocytes. Scientific reports. 2022; 12: 8771.
- Palsson-McDermott EM, Curtis AM, Goel G, Lauterbach MAR, Sheedy FJ, Gleeson LE, et al. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell metabolism. 2015; 21: 65-80.
- Zhang Z, Deng X, Liu Y, Liu Y, Sun L, Chen F. PKM2, function and expression and regulation. Cell & Bioscience. 2019; 9.
- Dayton TL, Jacks T, Heiden MGV. PKM2, cancer metabolism, and the road ahead. EMBO reports. 2016; 17: 1721-1730.
- Ham M, Lee J, Choi AH, Jang H, Choi G, Park J, et al. Macrophage Glucose- 6-Phosphate Dehydrogenase Stimulates Proinflammatory Responses with Oxidative Stress. Molecular and Cellular Biology. 2013; 33: 2425-2435.
- Nagy C, Haschemi A. Time and Demand are Two Critical Dimensions of Immunometabolism: The Process of Macrophage Activation and the Pentose Phosphate Pathway. Frontiers in Immunology. 2015; 6.
- Liu GY, Sabatini DM. mTOR at the nexus of nutrition, growth, ageing and disease. Nature Reviews Molecular Cell Biology. 2020; 21: 183-203.
- Hua H, Kong Q, Zhang H, Wang J, Luo T, Jiang Y. Targeting mTOR for cancer therapy. Journal of Hematology & Oncology. 2019; 12: 71.
- Li H, Prever L, Hirsch E, Gulluni F. Targeting PI3K/AKT/mTOR Signaling Pathway in Breast Cancer. Cancers. 2021; 13: 3517.
- Tewari D, Patni P, Bishayee A, Sah AN, Bishayee A. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: A novel therapeutic strategy. Seminars in cancer biology. 2019; 80: 1-17.
- Everts B, Amiel E, Huang SC, Smith AM, Chang C, Lam WY, et al. TLRdriven early glycolytic reprogramming via the kinases TBK1-IKKɛ supports the anabolic demands of dendritic cell activation. Nature Immunology. 2014; 15: 323-332.
- Zhang J, Zhang F, Hong C, Giuliano AE, Cui X, Zhou G, et al. Critical protein GAPDH and its regulatory mechanisms in cancer cells. Cancer Biology & Medicine. 2015; 12: 10-22.
- Wong BW, Marsch E, Treps L, Baes M, Carmeliet P. Endothelial cell metabolism in health and disease: impact of hypoxia. The EMBO Journal. 2017; 36: 2187-2203.
- Vaupel P, Schmidberger H, Mayer A. The Warburg effect: essential part of metabolic reprogramming and central contributor to cancer progression. International Journal of Radiation Biology. 2019; 95: 912-919.
- Semenza GL. Targeting HIF-1 for cancer therapy. Nature Reviews Cancer. 2003; 3: 721-732.
- Tin-Lok Wong, Kai-Yu Ng, Kel Vin Tan, Lok-Hei Chan, Lei Zhou, Noélia Che, et al. CRAF Methylation by PRMT6 Regulates Aerobic Glycolysis-Driven Hepatocarcinogenesis via ERK-Dependent PKM2 Nuclear Relocalization and Activation. Hepatology (Baltimore, Md.). 2020; 71: 1279-1296.
- Infantino V, Santarsiero A, Convertini P, Todisco S, Iacobazzi V. Cancer Cell Metabolism in Hypoxia: Role of HIF-1 as Key Regulator and Therapeutic Target. International Journal of Molecular Sciences. 2021; 22: 5703.
- Semba H, Takeda N, Isagawa T, Sugiura Y, Honda K, Wake M, et al. HIF-1α- PDK1 axis-induced active glycolysis plays an essential role in macrophage migratory capacity. Nature Communications. 2016; 7: 11635.
- Kim J, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell metabolism. 2006; 3: 177-185.
- Barnabei L, Laplantine E, Mbongo W, Rieux-Laucat F, Weil R. NF-κB: At the Borders of Autoimmunity and Inflammation. Frontiers in Immunology. 2021; 12.
- Yu H, Lin L, Zhang Z, Zhang H, Hu H. Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduction and Targeted Therapy. 2020; 5. 209.
- Haschemi A, Kosma P, Gille L, Evans CR, Burant CF, Starkl P, et al. The Sedoheptulose Kinase CARKL Directs Macrophage Polarization through Control of Glucose Metabolism. Cell Metabolism. 2012; 15: 813-826.
- Sun L, Zhou F, Shao Y, Lv Z, Li C. Sedoheptulose kinase bridges the pentose phosphate pathway and immune responses in pathogen-challenged sea cucumber Apostichopus japonicus. Developmental and comparative immunology. 2020; 109: 103694.
- Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell metabolism. 2006; 4: 13-24.
- Mills EL, O’Neill LA. Reprogramming mitochondrial metabolism in macrophages as an anti-inflammatory signal. European Journal of Immunology. 2016; 46: 13-21.
- Wu S, Zou M. AMPK, Mitochondrial Function, and Cardiovascular Disease. International Journal of Molecular Sciences. 2020; 21: 4987.
- Corcoran SE, O’Neill LAJ. HIF1α and metabolic reprogramming in inflammation. The Journal of clinical investigation. 2016; 126: 3699-3707.
- Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim K, et al. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). The Journal of Experimental Medicine. 2011; 208: 519-533.
- Bailey JD, Diotallevi M, Nicol T, McNeill E, Shaw A, Chuaiphichai S, et al. Nitric Oxide Modulates Metabolic Remodeling in Inflammatory Macrophages through TCA Cycle Regulation and Itaconate Accumulation. Cell Reports. 2019; 28: 218-230.e7.
- Cleeter M, Cooper J, Darley-Usmar V, Moncada S, Schapira A. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. FEBS Letters. 1994; 345: 50-54.
- Windt GJWVD, Everts B, Chang C, Curtis JD, Freitas TC, Amiel E, et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012; 36: 68-78.
- Youle RJ, Bliek AMVD. Mitochondrial Fission, Fusion, and Stress. Science. 2012; 337: 1062-1065.
- Annesley SJ, Fisher PR. Mitochondria in Health and Disease. Cells. 2019; 8: 680.
- Rambold AS, Pearce EL. Mitochondrial Dynamics at the Interface of Immune Cell Metabolism and Function. Trends in immunology. 2018; 39: 6-18.
- Hills RD, Pontefract BA, Mishcon HR, Black CA, Sutton SC, Theberge CR. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients. 2019; 11: 1613.
- Bozzetti V, Senger S. Organoid technologies for the study of intestinal microbiota-host interactions. Trends in molecular medicine. 2022; 28: 290- 303.
- Aron-Wisnewsky J, Warmbrunn MV, Nieuwdorp M, & Clément K. Metabolism and Metabolic Disorders and the Microbiome: The Intestinal Microbiota Associated With Obesity, Lipid Metabolism, and Metabolic Health-Pathophysiology and Therapeutic Strategies. Gastroenterology. 2021; 160: 573-599.
- Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nature Reviews Microbiology. 2020; 19: 55-71.
- Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013; 498: 99-103.
- Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012; 490: 55-60.
- Murakami M, Une N, Nishizawa M, Suzuki S, Ito H, Horiuchi T. Incretin secretion stimulated by ursodeoxycholic acid in healthy subjects. SpringerPlus. 2013; 2: 20.
- Kumar DP, Rajagopal S, Mahavadi S, Mirshahi F, Grider JR, Murthy KS, et al. Activation of transmembrane bile acid receptor TGR5 stimulates insulin secretion in pancreatic β cells. Biochemical and biophysical research communications. 2012; 427: 600-605.
- Michelucci A, Cordes T, Ghelfi J, Pailot A, Reiling N, Goldmann O, et al. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proceedings of the National Academy of Sciences. 2013; 110: 7820-7825.
- Strelko CL, Lu W, Dufort FJ, Seyfried TN, Chiles TC, Rabinowitz JD, et al. Itaconic acid is a mammalian metabolite induced during macrophage activation. Journal of the American Chemical Society. 2011; 133: 16386- 16389.
- Seymour JV, Conlon CA, Sulek K, Bôas SGV, McCowan LME, Kenny LC, et al. Early pregnancy metabolite profiling discovers a potential biomarker for the subsequent development of gestational diabetes mellitus. Acta Diabetologica. 2014; 51: 887-890.
- Runtsch MC, Angiari S, Hooftman A, Wadhwa R, Zhang Y, Zheng Y, et al. Itaconate and itaconate derivatives target JAK1 to suppress alternative activation of macrophages. Cell metabolism. 2022; 34: 487-501.e8.
- Hooftman A, O’Neill LAJ. The Immunomodulatory Potential of the Metabolite Itaconate. Trends in immunology. 2019; 40: 687-698.
- Mills EL, Ryan DG, Prag HA, Dikovskaya D, Menon D, Zaslona Z, et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature. 2018; 556: 113-117.
- Lampropoulou V, Sergushichev A, Bambouskova M, Nair S, Vincent EE, Loginicheva E, et al. Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cell metabolism. 2016; 24: 158-166.
- Song H, Xu T, Feng X, Lai Y, Yang Y, Zheng H, et al. Itaconate prevents abdominal aortic aneurysm formation through inhibiting inflammation via activation of Nrf2. EBioMedicine. 2020; 57: 102832.
- Zhu X, Guo Y, Liu Z, Yang J, Tang H, Wang Y. Itaconic acid exerts antiinflammatory and antibacterial effects via promoting pentose phosphate pathway to produce ROS. Scientific Reports. 2021; 11: 18173.
- Choi BK, Lee DY, Lee DG, Kim YH, Kim S, Oh HS, et al. 4-1BB signaling activates glucose and fatty acid metabolism to enhance CD8+ T cell proliferation. Cellular &Molecular Immunology. 2017; 14: 748-757.
- Kim C, Kim JG, Lee B, Choi M, Choi H, Kawada T, et al. Deficiency for Costimulatory Receptor 4-1BB Protects Against Obesity-Induced Inflammation and Metabolic Disorders. Diabetes. 2011; 60: 3159-3168.
- Tu TH, Kim C, Nam-Goong IS, Nam CW, Kim Y, Goto T, et al. 4-1BBL signaling promotes cell proliferation through reprogramming of glucose metabolism in monocytes/macrophages. The FEBS Journal. 2015; 282: 1468-1480.
- Tu TH, Kim C, Goto T, Kawada T, Kim B, Yu R. 4-1BB/4-1BBL Interaction Promotes Obesity-Induced Adipose Inflammation by Triggering Bidirectional Inflammatory Signaling in Adipocytes/Macrophages. Mediators of Inflammation. 2012; 2012: 972629.
- Jadhav A, Tiwari S, Lee P, Ndisang JF. The heme oxygenase system selectively enhances the anti-inflammatory macrophage-M2 phenotype, reduces pericardial adiposity, and ameliorated cardiac injury in diabetic cardiomyopathy in Zucker diabetic fatty rats. J Pharmacol Exp Ther. 2013; 345: 239-49.
- Tu TA-OX, Joe Y, Choi HS, Chung HA-O, & Yu RA-O (Induction of heme oxygenase-1 with hemin reduces obesity-induced adipose tissue inflammation via adipose macrophage phenotype switching. (1466-1861 (Electronic)).
- Kim CG, Lee H, Gupta N, Ramachandran S, Kaushik I, Srivastava S, et al. Role of Forkhead Box Class O proteins in cancer progression and metastasis. Seminars in cancer biology. 2018; 50: 142-151.
- Eijkelenboom A, Burgering BMT. FOXOs: signalling integrators for homeostasis maintenance. Nature Reviews Molecular Cell Biology. 2013; 14: 83-97.
- Semova I, Levenson AE, Krawczyk J, Bullock K, Gearing ME, Ling AV, et al. Insulin Prevents Hypercholesterolemia by Suppressing 12α-Hydroxylated Bile Acids. Circulation. 2022; 145: 969-982.
- Sarem Z, Bumke-Vogt C, Mahmoud AM, Assefa B, Weickert MO, Adamidou A, et al. Glucagon Decreases IGF-1 Bioactivity in Humans, Independently of Insulin, by Modulating Its Binding Proteins. The Journal of Clinical Endocrinology & Metabolism. 2017; 102: 3480-3490.
- Nakae J, Oki M, Cao Y. The FoxO transcription factors and metabolic regulation. FEBS Letters. 2008; 582: 54-67.
- Warburg O, Wind F, Negelein E. THE METABOLISM OF TUMORS IN THE BODY. The Journal of General Physiology. 1927; 8: 519-530.
- Malyshev IY. Epigenetic, post-transcriptional and metabolic mechanisms of macrophage reprogramming. Patologicheskaia fiziologiia i eksperimental’naia terapiia. 2015; 3: 118-127.
- Pascale RM, Calvisi DF, Simile MM, Feo CF, Feo F. The Warburg Effect 97 Years after Its Discovery. Cancers. 2020; 12: 2819.
- Li L, Liang Y, Kang L, Liu Y, Gao S, Chen S, et al. Transcriptional Regulation of the Warburg Effect in Cancer by SIX1. Cancer cell. 2018; 33: 368-385.e7.
- Curtis KD, Smith PR, Despres HW, Snyder JP, Hogan TC, Rodriguez PD, et al. Glycogen Metabolism Supports Early Glycolytic Reprogramming and Activation in Dendritic Cells in Response to Both TLR and Syk-Dependent CLR Agonists. Cells. 2020; 9: 715.
- Jones N, Blagih J, Zani F, Rees A, Hill DG, Jenkins BJ, et al. Fructose reprogrammes glutamine-dependent oxidative metabolism to support LPSinduced inflammation. Nature Communications. 2021; 12: 1209.
- M Saqcena, S Mukhopadhyay, C Hosny, A Alhamed, A Chatterjee, et al. Blocking anaplerotic entry of glutamine into the TCA cycle sensitizes K-Ras mutant cancer cells to cytotoxic drugs. Oncogene. 2015; 34: 2672-2680.
- Jha AK, Huang SC, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015; 42: 419-430.
- Peace CG, O’Neill LA. The role of itaconate in host defense and inflammation. The Journal of Clinical Investigation. 2022; 132: e148548.
- O’Neill LAJ. A critical role for citrate metabolism in LPS signalling. The Biochemical journal. 2011; 438: e5-e6.
- Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, Kasmi KCE, Smith AM, et al. Arginase-1–Expressing Macrophages Suppress Th2 Cytokine–Driven Inflammation and Fibrosis. PLoS Pathogens. 2009; 5: e1000371.
- Mills EL, Kelly B, Logan A, Costa AS, Varma M, Bryant CE, et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell. 2016; 167: 457-470.e13.
- Linton MF, Moslehi JJ, Babaev VR. Akt Signaling in Macrophage Polarization, Survival, and Atherosclerosis. International Journal of Molecular Sciences. 2019; 20: 2703.
- Vergadi E, Ieronymaki E, Lyroni K, Vaporidi K, Tsatsanis C. Akt Signaling Pathway in Macrophage Activation and M1/M2 Polarization. The Journal of Immunology. 2017; 198: 1006-1014.
- Meshkani R, Vakili S. Tissue resident macrophages: Key players in the pathogenesis of type 2 diabetes and its complications. Clinica chimica acta; international journal of clinical chemistry. 2016; 462: 77-89.
- Kanter JE, Hsu C, Bornfeldt KE. Monocytes and Macrophages as Protagonists in Vascular Complications of Diabetes. Frontiers in Cardiovascular Medicine. 2020; 7; 10.
- Lyudmila V Nedosugova, Yuliya V Markina, Leyla A Bochkareva, Irina A Kuzina, Nina A Petunina, et al. Inflammatory Mechanisms of Diabetes and Its Vascular Complications. Biomedicines. 2022; 10: 1168.
- Odegaard JI, Chawla A. Alternative macrophage activation and metabolism. Annual review of pathology. 2011; 6: 275-297.
- Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. European journal of pharmacology. 2020; 877: 173090.
- Huang SC, Smith AM, Everts B, Colonna M, Pearce EL, Schilling JD, et al. Metabolic Reprogramming Mediated by the mTORC2-IRF4 Signaling Axis Is Essential for Macrophage Alternative Activation. Immunity. 2016; 45: 817- 830.
- Ni Y, Zhuge F, Nagashimada M, Ota T. Novel Action of Carotenoids on Non-Alcoholic Fatty Liver Disease: Macrophage Polarization and Liver Homeostasis. Nutrients. 2016; 8: 391.
- Bang Y, Cutsem EV, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. The Lancet. 2010; 376: 687-697.
- Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo J, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nature medicine. 2013; 19: 1166-1172.
- Heidt T, Courties G, Dutta P, Sager HB, Sebas M, Iwamoto Y, et al. Differential Contribution of Monocytes to Heart Macrophages in Steady- State and After Myocardial Infarction. Circulation Research. 2014; 115: 284- 295.