
Research Article
Austin J HIV/AIDS Res. 2016; 3(2): 1027.
Prevalence and Factors Associated with Treatment Failure during Antiretroviral Therapy Atbobo-Dioulasso University Teaching Hospital (Burkina Faso) (2008- 2013)
Zoungrana J1,3*, Poda A1,3, Sondo KA4,5, Diallo I4,5, Bado G³, Ouedraogo AS¹, Koura M¹, Tougouma JB¹, Hema A³, Kaboré NF³, Soré I³, Yaméogo I³, Sawadogo AB3,4, Ouedraogo WT6, Savadogo M4,5, and Ouedraogo MS1,2
¹Polytechnic University of Bobo-Dioulasso, Higher Institute of Health Sciences, Bobo-Dioulasso, Burkina Faso
²Department of Internal Medicine of the University Hospital Souro Sanou, Bobo-Dioulasso, Burkina Faso
³Department of Infectious Diseases and Day hospital CHU Souro Sanou, Bobo-Dioulasso, Burkina Faso
4University of Ouagadougou, Training and Research Unit in Health Sciences (UFR / SDS)
5Service of Infectious Diseases at Yalgado Ouedraogo Hospital, Ouagadougou, Burkina Faso
6Regional Health Directorate of High Basins, Ministry of Health, Burkina faso
*Corresponding author: Zoungrana Jacques, Infectious Diseases and Day Hospital Service, Polytechnic University of Bobo-Dioulasso, Higher Institute of Health Sciences , 03 BP 4178 Bobo-Dioulasso 03, Burkina Faso
Received: June 13, 2016; Accepted: July 27, 2016; Published: July 29, 2016
Abstract
Summary: The objective of this study was to determine the prevalence and factors associated with therapeutic failure.
Materials and Methods: We have conducted a retrospective and analytical cohort study from January 2008 to December 2013, targeting out-patients under ambulatory consultation at the day hospital of Sanou Souro Teaching Hospital (SSTH). Were included in the study the entire patient’s sero positive for HIV-1 and under anti-retroviral treatment (ARVT), regularly followed in the active file of the Day hospital for at least twelve months and who have achieved a CD4 rate and a viral load in the systematic review of HIV infection.
Results: During the study period, 512 patients met our inclusion criteria. The median age was 37.5 years with (IQ 32.2 to 45.1) a sex ratio of 0.38 in favor of females. An opportunistic infection was the cause of the serology discovery with 57.6% of patients. According to WHO classification, 42.2% of patients were at stage 3 of WHO. At the start of the treatment, the median number of lymphocytes T CD4 was 179cells/μl (IQR: 85-258). The scheme two nucleoside reverse transcriptase inverse (NRTIs) and a non-nucleotide reverse transcriptase inhibitor (NNRTI) was the most used with 93.6%. The prevalence of treatment failure was (6.4%). Age and CD4 rate inferior to 200/mm3 at the start of the treatment were associated with treatment failure.
Conclusion: This study shows that treatment failure is common (6.4%) in the active line of the day hospital of Bobo-Dioulasso. A significant association between older age and a CD4 rate inferior to 200mm3 at the start of the treatment was recorded.
Keywords: Prevalence; Treatment failure; ARVT; Burkina Faso
Abbreviations
AIDS: Acquired Immune Deficiency Syndrome; ARV: Antiretroviral Drugs; ARVT: Antiretroviral Drug treatment; BMI: Body Mass Index; CD4: Cluster+D6:D28 of Differentiation 4; CHUSS: University Teaching Hospital Souro Sanou; ESOPE: Together for a Therapeutic Solidarity Hospital in Network; HDJ: Day hospital; HIV: Human Immunodeficiency Virus; IQR: Inter Quartile Range; M0: 0month; M12: 12-Months; NNRTI: Non-nucleotide Reverse Transcriptase Inhibitor; NRTIs: Nucleoside Reverse Transcriptase Inverse; WHO: World Health Organization
Introduction
Sub-Saharan Africa remains the area the most affected by HIV infection with 25.8 million people affected [1]. Many countries have set up strategies to stop the advance of the infection and improve patients’ comfort. These strategies focus on the availability of effective antiretroviral (ARV) drugs, whose use has significantly reduced mortality from HIV [2,3]. Indeed, these molecules cause a drop in viral load and consequently a restoration of immunity. Combined antiretroviral therapies has changed the management of HIV infection and transformed the perception of HIV infection which has become a chronic disease [4,5]. For that reason, new problems have emerged, especially treatment failure.
In low income countries, there is little data on treatment failure and associated factors [6-9]. Several authors such as Fibriani in Indonesia [10] and Ahoua in Uganda [11] reported failure rates in their cohort studies with respectively 9.1% and 10.9%. In Burkina Faso, particularly at the day hospital (HDJ) of Bobo Dioulasso, there is, to our knowledge, little study on therapeutic failures. The objective of this study was to determine the prevalence and the factors associated with treatment failure.
Materials and Methods
We have conducted a retrospective and analytical cohort study from January 2008 to December 2012, targeting out-patients under ambulatory consultation at the Day Hospital (HDJ) of University Teaching Hospital Sanou Souro (CHU SS) of Bobo-Dioulasso which is the second capital of Burkina Faso after Ouagadougou.
Were included in the study all the patients HIV-1 positive under ARV treatment (ARVT) regularly followed in the active file of the HDJ for at least twelve months (M12) and who have achieved a CD4 proportion and a viral load in systematic checkup of HIV infection monitoring. For each selected patient, we have collected data during medical consultations thanks to ESOPE software (Together for Therapeutic Solidarity Hospital In Network) and in the medical record of patients. These data included socio-demographic, clinical, and biological data at the start of the antiretroviral therapy, the implementation of the treatment, the visiting dates of immunevirological monitoring and the observance of ARVT (LOGONE software). The data on the level of observance of our patients were extracted from ACCESS database after consulting observance support informed by nurses.
Treatment failure was defined as two successive measurements of viral load> 1000 copies /ml after a virological success. If the second viral load> 1000 copies/ml was missing, the following criteria were used to define treatment failure: a single viral load>1000 copies/ ml accompanied by an immunological failure as defined by WHO [12] or one viral load> 5000 copies /ml. A simple virologic rebound was defined as a viral load>1000 copies/ml without further criteria of virologic failure. Observance was measured by the ratio between the number of prescriptions made and the theoretical amount of expected prescriptions (which correspond to the number of months of treatment follow-up). The patient was considered as observing when this coefficient was strictly superior to 0.95. The management of treatment failure was based on enhancing observance and therapeutic change according to WHO algorithm for the adult [12].
The data collected from ESOPE database were transferred to Epi- Info 2000 version 3.5 for analysis. Demographic, clinical, therapeutic characteristics observance data, the evolutionary and biological profile of patients at inclusion (M0) and at month 12 were described basing on the descriptive statistics of variables. Quantitative variables were described by their medians and inter quartile intervals (IQR). All risk factors were analyzed through logistic regression. Were included in the model all the factors with a p value<0.25 in exploratory analysis. Variables maintained in the model were those whose p value was <0.05, as determined significance threshold in the overall analysis. In uni varied analysis, Pearson chi 2 test was used to compare proportions and Student’s test was used to test the equality of two averages in uni varied analysis.
Results
Epidemiological and clinical characteristics
During the study period, 2250 persons infected with HIV-1 were put on ARVT treatment between January 2008 and December 2013, of which 512 (4.4%) met our inclusion criteria. The median age was 37.5 years with (IQR 32.2 to 45.1). The Socio-demographic and clinical characteristics of the patients at enrollment are shown in Table 1.
Patients with married life represented (53.3%). Most patients lived within 10 kilometer from the center. Unschooled patients were 239, that is 46.7%. An opportunistic infection was the cause of the serology discovery of 57.6% of patients (n = 295). The median of BMI was 20.6 kg /m² (IQR 18.3 to 23.4) with extremes of 11.2 and 40.0 kg /m². The majority (58%; n = 270) had a normal body mass index. According to WHO classification 2006 (42.2%) of patients were at WHO stage 3. At the start of treatment, the median number T CD4 lymphocytes was 179 cells/μl (IQR: 85-258) with extremes of 1 and 399 cells/μl. Hemoglobin rate was not given with 13 patients. Hemoglobin median rate was 10.9 g /dl (IQR: 9.6 to 12) with extremes from 5.9 to 14,9g / dl; 31.5% (n = 158) of patients had a hemoglobin level inferior or equal to 10g/dl at the start of treatment. The median blood glucose (glycemia) was 4.9 mmol / l (IQR: 4.6 to 5.5) with extremes from 3.3 to 6,7mmol /l. The median of creatinine was 70mmol /l (IQR: 60 to 81) with extremes from 46 to 308μmol/l. The median of alanine aminotransferase (ALAT) was 19.5u /l (IQR: 12-30) with extremes from 12 to 30u/l.
Therapeutic characteristics
The median duration of the treatment was 2.7 years (IQR: 1.7 to 4.2) with extremes from 1.2 years to 4.9 years. The scheme two nucleoside reverse transcriptase inverse (NRTIs) and a nonnucleotide reverse transcriptase inhibitor (NNRTI) was the most used with 93.6% (479). Therapeutic combinations containing zidovudine (AZT) were the most prescribed 63.9% (327). The most used treatment protocols were zidovudine/lamivudine/nevirapine (AZT/3TC/NVP) with a frequency of 31.8% and (zidovudine /lamivudine/efavirenz) (AZT/3TC/EFV) prescribed for 28.3% of patients at treatment start. Within 12-month follow-up, the level of patients’ observance to ARV was assessed 6 times and 63.7% of patients were found to observant to all the six assessments. The proportion of observing patients was increasing with ARV exposure time; the maximum of observing patients was noted at the 12th month (93.2%) (Figure 1). In 12 months of follow up, the median number of CD4 T lymphocytes increased from 179 to 312 cells / μll, that is a gain of 133 cells.
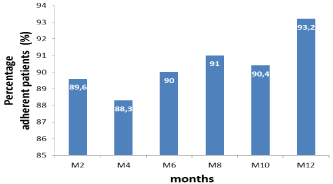
Figure 1: Evolution of the proportion of observing patients through time.
Prevalence
The prevalence of treatment failure was (6.4%). The majority of patients, 29 (87.9%) who failed had a viral load higher than 10,000 copies/ml. Among patients who didn’t succeed, 63.6% (21/33) have respected their appointment for routine consultations. For all cases of treatment failure encountered, 44 (72.1%) went to second-line treatment according to WHO algorithm used with 3 patients in treatment failure, ARVs were replaced for intolerance and pregnancy. At twelve month of follow up of the 512 patients, 78 (15.2%) had a simple rebound during their follow-up.
Factors associated with treatment failure
Only age and a CD4 level inferior to 200/mm3 at treatment administering was associated with failure with statistically significant associations as reported in Table 1.
Characteristics
Effectifs N=512
Pourcentages
Sexe
Males
females
141
371
27,5
72,5
Ages
< 37,5 ans
= 37,5 ans
393
119
76,8
23,2
Education
Primarylevel or less
Secondary or post-secondary
239
273
46,7
53,3
Occupation:
Jobles
Working
291
221
56,8
43,2
BMI in kg/ m2
< 18,5
=18.5
242
270
47,3
52,7
Residence
Urban
Rural
415
97
81,1
18,9
Current CD4+ count (cells/μl)
< 200
=200
354
158
69,1
30,9
Table 1: Socio-demographic and clinical characteristics of the patients at enrollment.
Discussion
This study was conducted at the day hospital of Bobo-Dioulasso, a reference center of the West and South -west sub-region of Burkina Faso in the comprehensive care of HIV-positive people. With nearly 4,500 people followed as ambulatory patients at the Day Hospital and nearly 350 annual inclusions. Patients admitted to the Day Hospital are representative of HIV+ diagnosed patients in Burkina Faso large cities.
Epidemiological and clinical characteristics
The median age of our patients is super passable to the results of other authors met in the literature [13,14]. This median age, very young, reflects the high frequency of HIV-AIDS infection, which is considered as a disease of young people [15].
The 0.38 sex ratio (M / F) shows a female predominance, which is identical to that of the study of Thompson and al. [13]. This is probably explained by the fact that in Africa, women are more affected, due to a larger heterosexual contamination, when, in the North, homosexual transmission or transmission due to intravenous drug abuse are more common.
The median of body mass index (BMI) at the implementation of antiretroviral therapy was 20.6 kg /m2 with extremes of 11.2 and 40 kg/m2. This result is lower than that reported by ELIRA Dokekias A and al. [16] who found a median BMI of 26.6 kg [extremes 16 and 95]. In our study, as in other similar studies in Africa [16,17] and elsewhere [18,19], nearly the majority of patients started the treatment at an advanced stage of the disease, that is 42.2% at stage 3 and 18.38% at stage 4 of WHO clinical classification. These results could be explained partly by the fact that there is a refusal to early HIV testing in resource-limited countries because of stigma and secondly by the fact that most patients only come to hospital after having used modern or traditional self-medication. The late care of individuals infected with HIV has a high risk of treatment failure. As a matter of fact, it is obvious, people infected by HIV who come late to care centers have a higher risk of developing early or late treatment failure. These patients are also more at risk of multiple opportunistic infections in a short period of time and be hospitalized [20]. These findings could explain most of the failures in Africa.
Therapeutic characteristics
The most commonly used therapeutic combinations were within the framework of the recommendations of the National Program for the Management of HIV in Burkina Faso [21] in accordance with WHO guidelines for resource-limited countries. Most patients had received a frontline ARVT 93.6%: 31.8% in the form of combination and 28.3% in the form of three or two separate medicines. In twelve month of follow-up observance was satisfactory at the level of all of our patients. This result is consistent with other studies in Africa [6,7,8]. The therapeutic response was generally good with a satisfactory clinical response, but marked by a relatively high prevalence of treatment failure.
Prevalence of failure
The prevalence of treatment failure in Burkina Faso population, not to say in African population is weak. Among the 512 patients who engaged in antiretroviral therapy, 78 (15.2%) had a simple rebound during the follow-up of 12 months and 33 (6.4%) had treatment failure. It affects a significant proportion of the young female population in Bobo-Dioulasso. This prevalence was similar to that previously reported by Pouget et al [22] in France, (5.3%) in HIV patients under ARVT in the cohort of a provincial COREVIH. These results contrast with higher values reported by Beaudrap [23] which found a prevalence of 11% in the cohort Senegalese Initiative for access to ARV drugs on the immuno-virologic response and resistance to antiretroviral. Other authors such as Fibriani in Indonesia [10] and Ahoua in Uganda [11] reported higher rates of treatment failure in their cohort studies with respectively (9.1%) and 10.9%. The role of caregivers and associations in the regular follow-up of the level of observance, observance strengthening talks, the active search for lost patients, the prevention of opportunistic infections are all factors that might explain in part the low failure rate in our sample.
Factors associated with failure
Age: In our series, age over 35 years (p <10-4) was statistically associated with treatment failure. Several authors have identified it as a risk factor. Moore in Canada [24] noted that patients with an advanced age during HIV experienced more treatment failure than young patients. Gazzola [25] in Italy has made the same observation. This could be explained by the deficit of immune reconstitution functions which gradually increases with age. These results are different from those found by Anude [26] and Marimoutou [27]. Anude found a significant association with age being inferior to 30 years, a hemoglobin level inferior to 10 g/dl, the low level of observance and treatment failure. While Marimoutou [27] found an association with a high viral load at treatment implementation (Table 2).
Complete Model
Final Model
Characteristics
Treatment failure
n (%)
OR ajusté
[IC 95%]
p
OR adjusted
[IC 95%]
p
Age
• =36
4(12,1)
1
1
• 37-46
19(57,6)
4,37[1,58;12,08]
4.10-3
4,59[1,67;12,61]
3.10-3
• >46
10(30,3)
3,82[1,27;11,42]
0,02
3,88[1,31;11,54]
0,01
Sex
• Female
21(63,6)
0,71[0,33 ; 1,54]
0,39
• Male
12(36,4)
1
Location
• <10
25(75,8)
0,90[0,25 ; 3,29]
0,87
• 10-50
2(6,1)
1
• 51-100
2(6,1)
2,43[0,39;15,20]
0,34
• >100
4(12,1)
2,99[0,53;16,82]
0,21
Circumstance of HIV discovery
• Voluntary testing
22(66,7)
1,29[0,60 ; 2,79]
0,51
• Clinical suspicion
11(33,3)
1
CD4 initial
• <100
14(42,4)
1
1
• 100-200
2 (6,1)
0,10[0,02;0,44]
3.10-3
0,11[0,02 ;0,49]
4.10-3
• 201-300
14(42,4)
0,90[0,42 ;1,91]
0,78
0,95[0,46 ;1,99]
0,90
• >300
3(9,1)
0,41[0,11 ;1,48]
0,17
0,39[0,10 ;1,43]
0,16
Initial viral load
• = 100000
• > 100000
25 (75,8)
08(24,2)
1[0,92-1]
1
0,99
Regime
• With AZT*
19(57,6)
0,72[0,35 ;1,48]
0,37
• Without AZT
14(42,4)
1
Observance
• = 95%
28(84,8)
0,08[0,03 ; 0,21]
10-4
1[0,4- 0,99]
0,64
• > 95%
05(15,2)
1
1
Duration of treatment ARV**
• =2,7 years
21(63,6)
0,37|0,23-0,59]
10-4
0,98[0,1-0,99]
0,99
• > 2,7 years
12(36,4)
1
1
Table 2: Factors associated with treatment failure (multivaried analysis).
Initial CD4lymphocytes: Treatment failure was frequently seen with patients who had an initial CD4 rate inferior to 200 cells /mm3 (p <10-4). The same result was reported by Florence [28] Suisse. What is more, Gazzola and Anude [25,26] have confirmed in their study that persistent physical pain, the presence of disabling symptoms at physical or social level and the existence of co-infections are associated with treatment failure. For other authors like Anude [26] and Florence [28], the failure was associated with a higher CD4 rate at treatment implementation. The non-observance of ARV treatment, persistent viral replication, the occurrence of opportunistic infections or co-infections could explain this failure for these authors.
Conclusion
This study shows that treatment failure is common (6.4%) in the active line of the Day Hospital of Bobo-Dioulasso. A significant association between older age and a CD4 rate inferior to 200 mm3 at therapeutic treatment was recorded. Such a correlation suggests a higher failure rate with patients who are old and who consult doctors late. But further studies are needed to confirm this trend.
References
- WHO. World Health Statistics 2014. UNAIDS.
- Brooks JT, Kaplan JE, Masur H. What’s new in the 2009 US guidelines for prevention and treatment of opportunistic infections among adults and adolescents with HIV. HIV Med. 2009; 17: 109-114.
- Jeffrey Eaton, Leigh Johnson, Joshua Salomon. HIV Treatment as Prevention: SystematicComparison of Mathematical Models of the Potential Impact of Antiretroviral Therapy on HIV Incidence in South Africa. PLoS Med. 2012; 9: 1123-1198.
- Bassett IV, Chetty S, Giddy J, Reddy S, Bishop K, Lu Z, et al. Screening for acute HIV infection in South Africa: Finding acute and chronic disease. HIV Med. 2011; 12: 46-53.
- World Health Organization (WHO) Geneva: WHO; Improving Access to Antiretroviral Therapy in Resource-Limited Settings: Guidelines for a Public Health Approach. 2002.
- Oumar AA, Dao S, Diamoutene A, Coulibaly S, Koumare B, Maiga II, et al. Factors associated with antiretroviral treatment observance at Point “G”hospital. Mali Med. 2007; 22: 18–21.
- Oku AO, Owoaje ET, Ige OK, Oyo-ita A. Prevalence and determinants of adherence to HAART amongst PLHIV in a tertiaryhealthfacility in south-south Nigeria. BMC Infectious Diseases. 2013; 13: 401.
- Williams AB, Amico KR, Bova C, Womack JA. A proposal for quality standards for measuring medication adherence in research. AIDS Behav. 2013; 17: 284-297.
- Sharma S, Khadga P, Dhungana GP, Chitrakar U. Medication adherence to antiretroviral therapy among patients visiting antiretroviral therapy center at Tribhuvan University Teaching Hospital, Kathmandu Nepal. Kathmandu Univ. Med J (KUMJ). 2013; 11: 50-53.
- Fibriani A, Wisaksana R, Indrati A, Hartantri Y, Van de Vijver D, Schutten M and al. Virological Failure and Drug Resistance During First Line Anti- Retroviral Treatment in Indonesia. Journal of Medical Virology. 2013; 85: 1394-1401.
- Ahoua L, Guenther G, Pinoges L, Anguzu P, Chaix ML, Tiec CL et al. Risk factors for virological failure and sub therapeutic antiretroviral drug concentrations in HIV-positive adults treated in rural northwestern Uganda. BMC Infectious Diseases. 2009; 9: 1471-2334.
- WHO. Antiretroviral therapy for HIV infection in adults and adolescents. Recommendations for a public health approach. 2010.
- Thompson T, Lee MG, Clarke T, Mills M, Wharfe G, Walter C. Prevalence of gastrointestinal symptoms among ambulatory HIV patients and a control population. Ann Gastroenterol. 2012 ; 25 : 243-248.
- Bouchaud O, Matheron S. Particularité de l’infection par le VIH en zone tropicale. In: Sida. Paris: Doin. 1998 ; 6 : 57-65.
- Mbopi-Keou FX Dempouo Djomassi L, Monebenimp F. Study of factors related to adherence to antiretroviral therapy in patients followed at the outlet unit in charge of HIV / AIDS of the Hospital District of Dschang, Cameroon. The Pan AfricanMedical Journal. 2012; 12: 55.
- Elect A Dokekias, Atipo Galiba Fo, Dzia Lepfoundzou Bokilo A Ntsimba P Nsitou Mb, Malanda F et al. Evaluation of antiretroviral therapy in adults infected with HIV, Followed in the Hematology Service of the University Hospital of Brazzaville, Congo. Bull Soc PatholExot. 2008; 2: 109-112.
- Laurent C, NgomGueye NF, Diakhate N, Gueye PM, Diouf M, Laniere I and al. Efficacy and tolerability of antiretroviral therapy in the context of the Senegalese initiative of access to antiretroviral drugs. in: A DesclauxLanièce I N’doye Tavern B eds. The Senegalese initiative of access to antiretroviral drugs. Paris: Social Sciences Collection and AIDS. 2002; 143-533.
- Mugyenyi P, S Walker, Hakim J, and al. Clinically driven versus routine laboratory monitoring of HIV antiretroviral therapy in Africa (DART): a randomized non-inferiority trial. Lancet. 2010; 375: 123-131.
- Castilla J, Sobrino P, De La Fuente L, Noguer I, Guerra L, Parras F. Late diagnosis of HIV infection in the era of highly active antiretroviral therapy: consequences for AIDS incidence. AIDS. 2002; 16: 1945-1951.
- Chadborn TR, Baster K, Delpech VC, Sabin CA, Sinka K, Rice BD, et al. No time to wait: how many HIV-infected homosexual men are diagnosed late and consequently die? (England and Wales, 1993–2002). AIDS. 2005; 19: 513-520.
- Faso Presidency / National Council for the Fight against AIDS and STIs. Standards and management protocols in medical care for people living with HIV in Burkina Faso. 2008 ; 54-202.
- Pouget-Abadie X, Hocqueloux L, G Gras, .RoncatoSaberan-M, T .Prazuck, .Bernard L and al. Causes and risk factors for treatment failure in HIV patients on ART in the cohort of a provincial COREVIH. 16th National Infectious Diseases Day, 10,11,12 June 2015, Nancy.
- Beaudrap P, M Thiam, Diouf A, Toure Kane-C, NgomGueye N-F, Vida N. immuno-virological response and viral resistance to treatment. in HIV- 1 patients treated for 10 years in the ISAARV - Cohort ANRS 1215. Final Report. 2012.
- Moore DM, Hogg RS, Yip B, Wood E, Tyndall M, Braitstein P, et al. Discordant Immunologic and Virologic Responses to Highly Active Antiretroviral Therapy Are Associated With Increased Mortality and Poor Adherence to Therapy. JAcquir Immune DeficSyndr. 2005; 40: 288-293.
- Gazzola L, Tincati C, The Absence of CD4+ T Cell Count Recovery Despite Receipt of Virologically Suppressive Highly Active Antiretroviral Therapy: Clinical Risk, Immunological Gaps, and Therapeutic Options HIV/AIDS. CID. 2009; 48: 328-337.
- Anude CJ, Eze E, Onyegbutulem H C, Charurat M, Etiebet M-A, Ajayi S, et al. Immuno-virologic outcomes and immunovirologic discordance among adults alive and on anti-retroviral therapy at 12 months in Nigeria. BMC Infectious Diseases. 2013 ; 13: 1471-2334.
- MarimoutouC , Chêne G, Mercié P , Neau D, Farbos S, MorlatP, et al. Prognostic factors of combined viral load and CD4+ cell count responses under triple antiretroviral therapy, Aquitaine cohort, 1996-1998, JAIDS. 2001; 27: 161-167.
- Florence E, Lundgren J, Dreezen C, Fisher M, Kirk O, Blaxhult A and al. Factors associated with a reduced CD4 lymphocyte count response to HAART despite full viral suppression in the EuroSIDA study. Rev HIV Med. 2003; 4: 255-262.