
Rapid Communication
Ann Hematol Oncol. 2019; 6(7): 1260.
Vitamin E Inhibits Apoptosis of Human CD34+-Derived Erythroid Progenitor Cells Obtained From Healthy Adults
Jilani T1*, Khan I2, Salim A2, Bilwani F1, Moiz B3, Azam I4 and Iqbal MP1
1Department of Biological and Biomedical Sciences, Aga Khan University, Pakistan
2Dr. Panjwani Center for Molecular Medicine and Drug Research, International Centre for Chemical and Biological Sciences, University of Karachi, Karachi, Pakistan
3Department of Pathology and Laboratory Medicine, Aga Khan University, Pakistan
4Department of Community Health Sciences, Aga Khan University, Pakistan
*Corresponding author: Jilani T, Department of Biological and Biomedical Sciences, Aga Khan University, Stadium Road, P.O. Box 3500, Karachi, Pakistan
Received: May 30, 2019; Accepted: July 08, 2019; Published: July 15, 2019
Abstract
Inhibition of proapoptotic proteins by various intra- and extracellular factors have been shown to cause increased survival of EPCs. The aim of the present study was to investigate the effect of vitamin E on Tumor Necrosis Factor-Alpha (TNF-a)-induced apoptosis of CD34+- derived EPCs of healthy human adults. CD34+- derived EPCs were isolated from human Peripheral Blood Mononuclear Cells (PBMNCs) and treated in vitro with various concentrations of TNF-a to determine the dose inducing maximum apoptosis. EPCs were incubated at various concentrations of vitamin E or Erythropoietin (EPO) as control for 4 hours before adding 100 ng/mL of TNF-a (a dose producing maximum apoptosis). After 24-hour incubation, quantification of percentage of EPCs undergoing apoptosis was carried out by FITC-Annexin V and PI staining followed by flow cytometry. In a dose-dependent manner, TNF-a alone increased the percentage of early and late apoptosis of treated CD34+- derived EPCs. There was a significant difference in the mean percentage of TNF-a-induced apoptotic CD34+-derived EPCs in early and late apoptosis by vitamin E treatment (p value= 0.080) which was higher in early as compared to late apoptosis. There was a significant difference in the percentages of TNF-a induced early and late apoptosis of CD34+- derived EPCs by vitamin E treatment or EPO treatment when analyzed for a statistical interactive effect on early and late apoptosis and drug concentration (p value < 0.001).The study suggests a positive role of vitamin E in decreasing TNF-a induced apoptosis of CD34+- derived EPCs from healthy human adults.
Keywords: Human erythroid progenitor cells; Vitamin E; Apoptosis; Erythropoiesis
Introduction
The maintenance of human erythroid homeostasis is dependent primarily upon a physiological balance between normal erythropoiesis, survival and programmed cell death (apoptosis) of Erythroid Progenitor Cells (EPCs) and erythrocytes [1]. The apoptosis of EPCs is regulated by a number of pro-apoptotic factors including B-cell lymphoma-2 (Bcl-2)-associated x (Bax), Bcl-2-associated death promoter (Bad), BH3-interacting domain (Bid), Bcl-2-interacting mediator of cell death (Bim), Bcl-2-antagonist killer (Bak), Bcl-2- interacting killer (Bik), Cytochrome c, cysteine-dependent aspartatespecific proteases (caspases), Caspase Activated Dnase (CAD), interferon gamma (INF-γ) and tumor necrosis factor-alpha (TNF-a) [2-5]. The major known factors that inhibit EPCs’ apoptosis include Bcl-2, B cell lymphoma extra-large (Bcl-Xl), intracellular Inhibitor of Apoptosis (IAP), Interleukin-3 (IL-3), Stem Cell Factor (SCF) and Erythropoietin (EPO) [6-9].
Some of the fat-soluble vitamins including vitamin K and alltrans- retinoic acid had been suggested to prevent apoptosis of human peripheral blood EPCs and adult bone marrow CD34+ve cells in vitro [10,11]. Some of the earlier human and animal studies had shown the beneficial role of antioxidants in suppressing the oxidative stressmediated apoptosis of hematopoietic progenitor stem cells derived from bone marrow and peripheral venous blood samples [12,13]. Moreover, abnormally increased levels of pro-oxidants and/ or decreased levels of anti-oxidants had been shown to inhibit human erythropoiesis and promote apoptosis of EPCs [14,15].
Some of the experimental animal studies showed that vitamin E treatment decreased radiation-induced apoptosis in bone marrow and peripheral blood hematopoietic stem cells [16,17] and in jejunal tissue slides [18]. Vitamin E supplementation had also been suggested to stimulate the proliferation and survival of hematopoietic stem cells [19,20] and promote the differentiation and maturation of EPCs in marrow of experimental animals [21,22]. All of the above mentioned studies are suggestive of a protective role of vitamin E on EPCs. However, there has been no reported study to determine the effect of vitamin E on CD34+-derived EPCs isolated from human PBMNCs.
Therefore, the objective of this study was to investigate any protective effect of vitamin E on TNF-a-induced apoptosis of CD34+- derived EPCs isolated from PBMNCs obtained from apparently healthy adult human volunteers.
Materials and Methods
Ethics statement
The present study was approved by the Ethical Review Committee of the Aga Khan University, Karachi, Pakistan. A written informed consent was obtained from all the participants included in this study.
Participants’ enrollment
Volunteers included in this study were healthy adult males, 18- 45 years old who had no previous history of any systemic disease or injury, acute or chronic infection and blood loss during last six months. They were non-smokers and had no history of any alcohol use and had not taken vitamins or iron supplements during the last six months.
Separation of PBMNCs from human peripheral blood
Venous whole blood sample (10-20 mL) was collected at Stem cell laboratory of Dr. Panjwani Center for Molecular Biology and Drug Research, University of Karachi, Pakistan from healthy adult human volunteers. PBMNCs were separated from the blood sample using Ficoll-paque density centrifugation method [5]. Dulbecco’s Modified Eagle Medium (DMEM, Stemcell Technologies Inc., Canada) was added along with penicillin/streptomycin (Gibco, USA) into the tube containing PBMNCs.
Isolation and expansion of CD34+-derived EPCs from PBMNCs
CD34+- cells were isolated from PBMNCs using CD34+ selection kit (Stemcell Technologies Inc., Canada) through magnetic assisted cell sorting (MACS) [5,23].
The isolated CD34+ human PBMNCs were differentiated and expanded in vitro for 7-14 days in serum free DMEM supplemented with Stem Span TM erythroid selective expansion and differentiation supplement (Stemcell Technologies Inc., Canada), containing penicillin/streptomycin and 10% fetal bovine serum (FBS, Stemcell Technologies Inc., Canada) [24]. The density of the CD34+-derived EPCs was maintained at 2-4 x 105 cells/mL and the cultured cells were observed daily for any changes in the morphology [4,5].
The isolated CD34+-derived EPCs were stained with 4,6-Diamidino-2-Phenylinode (DAPI) and mouse anti human- CD34+-Phycoerythrin (PE), (Becton Dickinson Holdings, USA), mouse anti human-CD74+-PE (Becton Dickinson Holdings, USA) and mouse anti human-Glycophorin A+-PE (Becton Dickinson Holdings, USA), mouse anti human-CD3+ antibody (Becton Dickinson Holdings, USA) and mouse anti human-CD14+ antibody (Becton Dickinson Holdings, USA). The stained cells were then examined using the immunofluorescence microscope (Nikkon TE 2000, Japan) [25-27].
Treatment of CD34+-derived EPCs with TNF-a, and vitamin E or EPO
In order to examine the effects of vitamin E and EPO on TNF- a-induced apoptosis of CD34+-derived EPCs, the purified CD34+- derived EPCs were treated in vitro with various concentrations of TNF-a (zero, 10, 50 and 100 ng/mL), (Thermo Fisher Scientific, USA) in serum free DMEM in the presence or absence Stem SpanTM erythroid selective expansion and differentiation supplement (three independent experiments) for 24 hours to induce apoptosis in EPCs [28,29]. The measurement of percent apoptosis of the treated CD34+- derived EPCs was carried out by using FITC-Annexin V apoptosis detection kit (Becton Dickinson Holdings, USA) and propidium iodide ( PI, Sigma-Aldrich, USA) staining followed by flow cytometry (Biosciences, USA) [30-31]. The results were analyzed using the CellQuest software (Biosciences, USA). 10,000 events were recorded for each sample. Gated cells were then analyzed for the presence of Annexin V-FITC+ve and PI+ve cells. The maximum percent early and late apoptosis of CD34+-derived EPCs was observed at the TNF-a concentration of 100 ng/mL, and this dose of TNF-a was selected to study any protective effect of vitamin E on apoptosis of CD34+-derived EPCs. The EPCs were then treated in vitro with vitamin E (10, 50 and 100 μg/mL, Sigma-Aldrich, USA) or EPO (10, 50 and 100 IU/mL, RG Pharmaceutica PVT LTD, Pakistan) in three separate wells for 04 hours followed by addition of TNF-a (100 ng/ mL) in each of the respective wells [19,32-34]. The CD34+-derived EPCs treated with vitamin E or EPO and TNF-a were then incubated in DMEM for 24 hours at 37oC in 5% CO2. Percentage values of early and late apoptosis of CD34+-derived EPCs treated with vitamin E or EPO was determined by using Annexure V-FITC and PI staining through flow cytometry [30,31].
Statistical analysis
The mean differences of the effects of treatment with increasing concentrations of vitamin E and EPO on TNF-a-induced early and late apoptosis and the interaction of these drugs and apoptotic status of CD34+-derived EPCs were statistically analyzed using Two-way analysis of variance (Two-way ANOVA) with replacement. A p value < 0.05 was considered statistically significant.
Results
Purification of isolated and cultured CD34+-derived EPCs
The purified CD34+-derived EPCs were positive for CD34, CD71 and Glycophorin A (GPA). These cells were found to be negative for CD3 and CD14. One limitation of this experiment is use of PE conjugated to all surface markers which did not allow us to analyze for the presence of all antigen on one cell simultaneously. Figure 1 Effect of increasing concentrations of TNF-a on mean cell apoptosis of CD34+ -derived human EPCs on early and late apoptosis Quantification of percent apoptosis of CD34+-derived EPCs after incubation with increasing concentrations of TNF-a. This shows the effect of in vitro treatment (24 hours) of cultured CD34+-derived EPCs with increasing dosages of TNF- a (zero, 10, 50 and 100 ng/ mL) alone on early and late apoptosis of EPCs. In a dose-dependent manner, TNF-a alone increased percent early apoptosis of treated EPCs from 4.5% to 58.7%, while the increase in late apoptosis was from 2.3% to 8.9%.
The effect of increasing concentrations of vitamin E (10, 50 and 100 μg/mL) or EPO (10, 50 and 100 IU/mL) on TNF-a (100 ng/mL)- induced mean percent cell apoptosis of CD34+-derived EPCs were statistically analyzed by using Two-way analysis of variance (Twoway ANOVA) with replacement as shown in (Table 1).
Drug
Concentration
Apoptosis (%± SD)
F statistic (p value)*
Early
Late
Concentration
Apoptosis status
Concentration* Apoptosis status
EPO
0 (IU/mL)
58.7±4.87
8.9±3.43
1.55 (0.363)
10.1 (0.050)
73.2 (< 0.001)
10 (IU/mL)
55.3±5.80
3.5±0.88
50 (IU/mL)
35.9±1.94
3.5±0.79
100 (IU/mL)
5.3±1.81
0.8±0.70
Vit. E
0 (μg/mL)
58.7±4.87
8.9±3.43
1.91 (0.303)
38.3 (0.008)
17.6 (< 0.001)
10 (μg/mL)
47.1±1.25
5.3±1.10
50 (μg/mL)
38.3±3.33
6.8±1.49
100 (μg/mL)
25.2±4.70
2.7±1.93
*F statistic and p value were obtained by using Two-way analysis of variance (Two-way ANOVA) with replacement to study the effect of increasing concentrations of drugs/ physiological compounds (EPO or vitamin E) on early and late apoptosis and statistical interaction between increasing concentrations of drugs/ physiological compounds and early or late apoptosis.
Table 1: Effect of increasing concentrations of vitamin E or EPO on TNF-a-induced mean early and late cell apoptosis of EPCs using Two-way analysis of variance (Two-way ANOVA) with replacement.
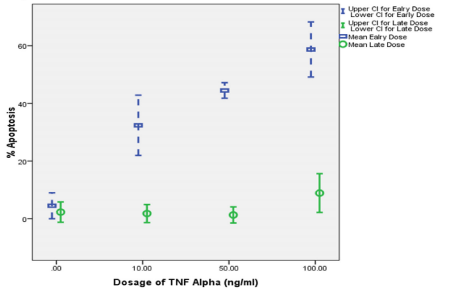
Figure 1: Effect of increasing concentrations of TNF-a on early and late
apoptosis of CD34+-derived EPCs.
It shows the effect of in vitro treatment (24 hours) of cultured CD34+-derived
EPCs with increasing dosages of TNF- a increased percent early apoptosis
of treated EPCs from 4.5% to 58.7%. However, treatment of cultured EPCs
resulted in an increased percent late apoptosis from 2.3% to 8.9%. Since
the maximum percent apoptosis of CD34+-derived EPCs was at TNF-a
concentration of 100 ng/mL, this concentration was chosen to study the
effects of vitamin E and EPO on apoptosis of CD34+-derived EPCs.
Vitamin E decreased the TNF-a-induced early apoptosis of CD34+-derived EPCs from 58.7% to 25.2% and late apoptosis from 8.9% to 2.7% at its maximum concentration of 100 μg/mL, while EPO (used as a control) decreased the mean percentage of early apoptosis from 58.7% to 5.3%, and of late apoptosis from 8.9% to 0.8%. There was a marginal difference in the mean percentage of TNF-a-induced apoptotic CD34+-derived EPCs by EPO treatment (p value= 0.050), which was slightly higher in early as compared to late apoptosis. There was a significant difference in the mean percentage of TNF- a-induced apoptotic CD34+-derived EPCs by vitamin E treatment (p value= 0.008), which was higher in early apoptosis as compared to late apoptosis. Moreover, there was a significant difference in the mean percentage of TNF-a-induced apoptotic CD34+-derived EPCs by vitamin E treatment or EPO treatment, when analyzed for a statistical interactive effect on early and late apoptotic phases and drug concentration as analyzed by Two-way ANOVA with replacement (p value ‹ 0.001).
Supplementary Figure 1; In order to further confirm our results, the experiment was repeated using CD34+ -derived EPCs from a different donor and employing dosages of vitamin E and EPO which were found to give maximum protective effects and studying the early and late apoptosis. Supplementary Figure 1 illustrates the effects of vitamin E (100 μg/mL) and EPO (100 IU/mL) onTNF-a (100 ng/ mL)-induced apoptosis on CD34+ -derived EPCs isolated from human PBMNCs.
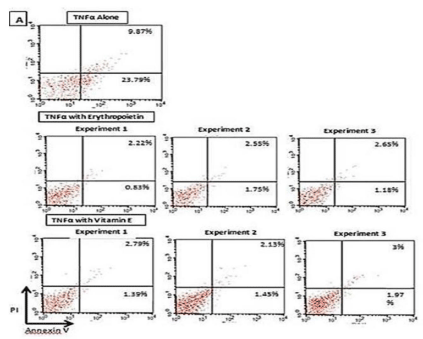
Supplementary Figure 1: Effect of vitamin E (100 μg/ mL) and EPO (100
IU/mL) on TNF-a (100 ng/mL)-induced apoptosis on CD34+-derived EPCs
(reconfirmation of previous results).
Human CD34+-derived EPCs cells isolated from human PBMNCs were
treated with vitamin E and/or EPO for 4 hours followed by the addition of
TNF-a (100 ng/mL) in three independent experiments. Cells were then
stained with annexin V-FITC and Propidium Iodide (PI) followed by analysis
for early and late apoptotic cells by flow cytometry.
Treatment of human CD34+-derived EPCs with vitamin E (100 μg/mL) resulted
in a significant decrease in the percent cell early apoptosis to 1.60% ±0.18%
(mean±SD from three independent experiments) and another significant
decrease in percent cell late apoptosis to 2.6%±0.26% (mean±SD from three
independent experiments).
The main findings include:
The percentage of early apoptotic CD34+-derived EPCs after treatment with TNF-a alone was 23.8%, while the percentage of late apoptotic CD34+ cells was 9.9% (Supplementary Figure 1).
Treatment of human CD34+-derived EPCs with EPO (100 IU/ mL) resulted in a decrease in the percent cell early apoptosis to 1.25% ± SD from three independent experiments) and a decrease in percent cell late apoptosis to 2.4% ± 0.22% (mean ± SD from three independent experiments) (Supplementary Figure 1).
Discussion
In the present study, we were able to show that vitamin E in an in vitro culture inhibited the TNF-a induced apoptosis of CD34+- derived EPCs. A number of earlier studies had proposed the role of TNF-a as an inducer of apoptosis [35,36] and EPO as a potent antiapoptotic agent for human EPCs [37,38]. Therefore, in the present study, we used TNF-a for inducing apoptosis in EPCs and EPO as a drug to determine whether vitamin E has a role in decreasing apoptosis of CD34+-derived EPCs (positive control in context of antiapoptotic action of vitamin E). Vitamin E treatment was observed to reduce the percent apoptosis of EPCs obtained from apparently healthy adult humans even at its minimum concentration of 10 μg/ mL (Table 1).
The normal plasma vitamin E levels in healthy adult humans had been reported to be between 5.0 μg/mL to15.0 μg/mL [39]. Thus, it seems that inhibition of apoptosis of human EPCs by vitamin E treatment might be possible at the normal plasma vitamin E levels. However, maximum inhibition of early apoptosis (from 58.7% to 25.2%) and late apoptosis (from 8.9% to 2.7%) of CD34+-derived EPCs was observed at a dose of 100 μg/mL of vitamin E in the present study. These findings are corroborated by the results of a previous study by Yano et al, who have shown that incubation of human monocytic U937 cells with vitamin E for 24 hours decreased TNF-a (0.5 μg/L, 02 μg/L and 100 μg/L)-mediated percent cell apoptosis from 20.9% to 13.5% at 0.5 μg/L, 28.8% to 22.2% at 02 μg/L and 15.3% to 8.5% at 100 μg/L by vitamin E treatment [40].
However, the results of another study by Bergman et al. carried out to find out the in vitro effect vitamin E (0.125 mg/mL) on apoptosis of PBMNCs from healthy human volunteers failed to show any significant change in percent apoptosis of the treated PBMNCs [41]. The possible reason for difference in the results of the present study and the referred study [41] could be that we investigated the anti-apoptotic effect of vitamin E on relatively well defined population of CD34+-derived EPCs, while Bergman et al, examined the anti-apoptotic effects of vitamin E on PBMNCs. Vitamin E had been suggested to inhibit experimentally-induced apoptosis in animal tissues by decreasing the activity of some of the pro-apoptotic caspases [42-45]. The possible mechanism of vitamin E in rescuing EPCs undergoing TNF-a-mediated apoptosis in the present study might be inhibition of some of the pro-apoptotic proteins and/ or stimulation of some of the anti-apoptotic proteins as depicted in (Figure 2).
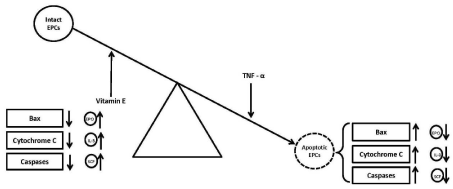
Figure 2: Effect of vitamin E on anti- and pro-apoptotic proteins.
The findings of the present study suggest a new role of vitamin E in promoting erythropoiesis by altering the balance between proand anti-apoptotic proteins affecting EPCs which needs further investigations. The results of the present study should be viewed within the context of a limitation of the study. Our study is limited to the TNF- a induced apoptosis of isolated human CD34+-derived EPCs, while there are a number of other pro-apoptotic and antiapoptotic biomolecules which could be influencing the overall balance of apoptosis of EPCs. For example, many molecules in addition to TNF-a can alter the effect of pro-apoptotic proteins (like Bax, Cytochrome C, Caspases, Interferon-gamma) and anti-apoptotic proteins (like stem cell factor SCF and IL-3 etc) on EPCs.
Conclusion
We report that vitamin E enhances erythropoiesis through inhibition of apoptosis of CD34+ -derived EPCs isolated from PBMNCs in healthy adult human subjects. It appears that certain proapoptotic factors such as TNF- a could be increasing the apoptosis of EPCs, and vitamin E and EPO have a role in decreasing the early and late apoptosis of these cells, thereby resulting into increased levels of mature erythrocytes.
Acknowledgements
The study was supported by the Departmental funds of the Aga Khan University. We gratefully acknowledge the technical help provided by Ms. Farhat Abajani, Ms. Khalida Iqbal and Ms. Naseema Mehboobali.
References
- Pellegrin S, Heesom, KJ, Satchwell TJ. Differential proteomic analysis ofhuman erythroblasts undergoing apoptosis induced by EPO-withdrawl. PLoS ONE. 2012; 7: e38356.
- Koulnis M, Porpiglia E, Porpiglia PA. Contrasting dynamic response in vivo of the Bcl-xL and Bim erythropoietic survival pathways. Blood. 2012; 119: 1228-1239.
- Diwan A, Koesters AG, Capella D, Geiger H, Kalfa TA, Dorn II GW. Targeting erythroblast-specific apoptosis in experimental anemia. Apoptosis. 2008; 13: 1022-1030.
- Kheansaard W, Masoodi S, Nilganuwong S, Tanyoung DI. Interferon-gamma induced nitric oxide-mediated apoptosis of anemia of chronic disease in rheumatoid arthritis. Rheumatol Int. 2013; 33: 151-156.
- Tanyong DI, Panichob P, Kheansaard W, Fucaroen S. Effect of tumor necrosis factor-alpha on erythropoietin and erythropoietin-induced erythroid progenitor cell proliferation in β-thalassemia/ hemoglobin E patients. Turk J Hematol. 2015; 32: 304-310.
- Mori M, Uchida M, Watanabe T. Activation of extracellular signal-regulated kinases ERK1 and ERK2 induces Bcl-xL up-regulation via inhibition of caspase activities in erythropoietin signaling. J Cell Physiol. 2003; 195: 290- 297.
- Tanyong DI, Winichagoon P, Siripin D, Seevakool W, Fucarroen S. Role of interleukin-3 and signaling pathways on beta-thalassemia/ HBE erythroid progenitor cell in culture. Southeast Asian J Trop Med Pub Health. 2007; 38: 897-903.
- Joo Chung IK, Dai C, Krantz SB. Stem cell factor increase the expression of FLIP that inhibits IF-γ-induced apoptosis in human erythroid progenitor cells. Blood. 2003; 101: 1324-1328.
- Deng H, Zhang J, Yoon T, Song Di, Li D, Lin A. Phosphorylation of Bcl- associated death protein (Bad) by erythropoietin-activated c-Jun N-terminal protein kinase 1 contributes to survival of erythropoietin-dependent cells. Int J Biochem Cell Biol. 2011; 43: 409-415.
- Sada E, Abe Y, Ohba R. Vitamin K2 modulates differentiation and apoptosis of both myeloid and erythroid lineages. Eur J Haematol. 2010; 85: 538-548.
- Herault O, Domenech J, Georget MT, Clement N, Colombat P, Binet C. Alltrans retinoic acid prevents apoptosis of human marrow CD34+ cells deprived of haematopoietic growth factors. Br J Haematol. 2002; 118: 289-295.
- Xie F, Zhao MF, Zhu HB. Effects of oxidative stress on hematopoiesis of hematopoietic stem and progenitor cells with iron overload. Zhonghua Yi Xue Za Zhi. 2011; 19: 3284-3288.
- Mosca L, Marcellini S, Perluigi M. Modulation of apoptosis and improved redox metabolism with the use of a new antioxidant formula. Biochem Pharmacol. 2002; 63: 1305-1314.
- Taoka K, Kumano K, Nakamura F. The effect of iron overload and chelation on erythroid differentiation. Int J Hematol. 2012; 95: 149-159.
- Dallalio G, Means RT. Effects of oxidative stress on human erythroid colony formation: Modulation by gamma-interferon. J Lab Clin Med. 2003; 141: 395- 400.
- Satyamitra M, Ney PH, Graves III J, Mullaney CP, Srinivasan V. Mechanism of radioprotection by d-tocotrienol: pharmacokinetics, pharmacodynamics and modulation of signaling pathways. Brit J Radiol. 2012; 85: e1093-e1103.
- Wambi C, Sanzari J, Wan XS. Dietary antioxidants protect hematopoietic cells and improve animal survival after total-body radiation. Radiat Res. 2008; 169: 384-396.
- Singh VK, Sing PK, Wise SY, Posarac A, Fatanmi OO. Radioprotective properties of tocopherol succinate against ionizing radiation in mice. J Radiat Res. 2013; 54: 210-220.
- Bichay TJ, Roy RM. Modification of survival and hematopoiesis in mice by tocopherol injection following irradiation. Strahlenther Onkol. 1986; 162: 391- 399.
- Roy RM, Malick MA, Clark GM. Increased haematopoietic stem cell survival in mice injected with tocopherol after X-irradiation. Strahlentherapie. 1982; 158: 312-318.
- Nogueira PA, Barbosa CMV, Segreto HRC. a-Tocopherol induces hematopoietic stem/progenitor cell expansion and ERK1/ 2-mediated differentiation. J Leukoc Biol. 2011; 90: 1111-1117.
- Kulkarni S, Ghosh SP, Satyamitra M. d-tocotrienol protect haematopoietic stem and progenitor cells in mice after total body irradiation. Radiat Res. 2010; 173: 738-747.
- Guzman FP, Rodriguez GM, Mayani H. In vitro proliferation, expansion and differentiation of a CD 34+ cell-enriched hematopoietic cell population from human umbilical cord blood in response to recombinant cytokines. Arch Med Res. 2002; 33: 107-114.
- Spiropoulos A, Goussetis E, Margeli A. Effect of inflammation induced by prolonged exercise on circulating erythroid progenitors and markers of erythropoiesis. Clin Chem Lab Med. 2010; 48: 199-203.
- Filippone C, Franssila R, Kumar A. Erythroid progenitor cells expanded from peripheral blood without mobilization or pre-selection: Molecular characteristics and functional competence. PLoS ONE. 2010; 5: e9496.
- Fujimi A, Matsunaga T, Kobune M. Ex vivo large-scale generation of human red blood cells from cord blood CD34+ cells by co-culturing with macrophages. Int J Hematol. 2008; 87: 339-350.
- Xio W, Koizumi K, Nishio M. Tumor necrosis factor-a inhibits generation of Glycophorin A+ cells by CD34+ cells. ExpHematol. 2002; 30: 1238-1247.
- Johnson CS, Cook CA, Furmanski, P. In vivo suppression of erythropoiesis by Tumor Necrosis Factor-Alpha (TNF-alpha): reversal with exogenous Erythropoietin (EPO). Exp Hematol. 1990; 18: 109-113.
- Wisniewski D, Strife A, Atzpodien J, Clarkson BD. Effects of recombinant human tumor necrosis factor on highly enriched hematopoietic progenitor cell populations from normal human bone marrow and peripheral blood and bone marrow from patients with chronic myeloid leukemia. Cancer Res. 1987; 47: 4788-4794.
- Vermes I, Haanen C, Nakken HS, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labeled Annexin V. J Immunolog Methods. 1995; 184: 39-51.
- Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, Van Osers MH. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994; 84: 1415-1420.
- Singh VK, Parekh VI, Brown DS, Kao TC, Mog SR. Tocopherol succinate: modulation of antioxidant enzymes and oncogene expression and hematopoietic recovery. Int J Radiol Oncol Biol Phys. 2011; 79: 571-578.
- Asadi E, Jahanshahi M, Golalipour MJ. Effect of vitamin E on oocyte apoptosis in nicotine-treated mice. Ir J Bas Med Sci. 2012; 15: 880-884.
- Horvathova M, Kapralova K, Zidova Z, Dolezal D, Popisilova D, Divoky V. Erythropoietin-driven signaling ameliorates the survival defect of DMT1- mutant erythroid progenitors and erythroblasts. Hematological. 2012; 97: 1480-1488.
- Jacobs-Helber SM, Roh K, Bailey D. Tumor necrosis factor-alpha expressed constitutively in erythroid cells or induced by erythropoietin has negative and stimulatory roles in normal erythropoiesis and erythroleukemia. Blood. 2003; 101: 524-531.
- Papadaki HA, Kritikos HD, Valatas V. Anemia of chronic disease in rheumatoid arthritis is associated with increased apoptosis of bone marrow erythroid cells: improvement following anti-tumor necrosis factor-a antibody therapy. Blood. 2002; 100: 474-482.
- Malik J, Kim AR, Tyre KA, Anjuli R, Cherukuri AR, Palis J. Erythropoietin critically regulates the terminal maturation of murine and human primitive erythroblasts. Haematologica. 2013; 98: 1778-1787.
- Lui JC, Kong SK. Erythropoietin activates caspase-3 and down regulates CAD during erythroid differentiation in TF-1 cells- A protection mechanism against DNA fragmentation. FEBS Letters. 2006; 580: 1965-1970.
- Farrell PM, Levine SL, Murphy MD, Adams AJ. Plasma tocopherol levels and tocopherol-lipid relationships in a normal of population of children as compared to healthy adults. Am J Clin Nutr. 1978; 31: 1720-1726.
- Yano M, Kishida E, Iwasaki M, Shosuke K, Masuzawa Y. Docosahexanoic acid and vitamin E can reduce human monocytic U937 cell apoptosis induced by Tumor necrosis factor. J Nutr. 2000; 130: 1095-1101.
- Bergman M, Salman H, Djaldetti M, Fish L, Punsky, Bessler H. In vitro immune response of human peripheral blood cells to vitamins C and E. J Nutr Biochem. 2004; 15: 45-50.
- Petrova GV, Donchenko GV. Effect of alpha-tocopherol and its derivatives on actinomycin D induced apoptosis of rat thymocytes. Ukr Biokhim Zh. 2005; 77: 72-77.
- Uemura M, Manabe H, Yoshida N, Fujita N, Ochiai J, Matsumoto N. Alphatocopherol prevents apoptosis of vascular endothelial cells via a mechanism exceeding that of mere antioxidation. Eur J Pharmacol. 2002; 456: 29-37.
- Makpol S, Rahim NA, Hui CK, Ngah WZW. Inhibition of mitochondrial cytochrome c release and suppression of caspases by gamma-tocotrienol prevent apoptosis and delay aging in stress-induced premature senescence of skin fibroblasts. Oxid Med Cell Longiv. 2012; 785743.
- Do MH, Kim SN, Seo SY, Yeo EJ, Kim SY. d-Tocopherol prevents methylglycoxal-induced apoptosis by reducing ROS generation and inhibiting apoptotic signaling cascades in human umbilical vein endothelial cells. Food Funct. 2015; 6: 1568-1577.