
Special Article - Acute and Chronic Myeloid Leukemia
Ann Hematol Oncol. 2016; 3(12): 1126.
Treating Acute Myeloid Leukemia with Sequential Autologous Leukemia Antigen-Loaded DC-CIK Cells after Chemotherapy: A Retrospective Study
Si-han Lai, Deng R, Fu L, Yi H, Fang-yi Fan, Wang Y, Qiu L, Yi-lan Liu, Ye-cheng Li, Xiao-juan Miao, Yan-rong Shuai, Guang-cui He and Su Y*
Department of Hematology and Hematopoietic Stem Cell Transplantation and Cell Immunotherapy Center, Cheng Du Military General Hospital of PLA, Cheng Du, Sichuan 610083, P. R, China
*Corresponding author: Yi Su, Department of Hematology and Hematopoietic Stem Cell Transplantation and Cell Immunotherapy Center, Cheng Du Military General Hospital of PLA, Rong Du Avenue 270#, Cheng Du, Sichuan, 610083, P. R, China
Received: November 11, 2016; Accepted: December 27, 2016; Published: December 30, 2016
Abstract
To retrospectively analyze the clinical safety and effectiveness of treating acute myeloid leukemia (AML) with sequential autologous leukemia antigenloaded DC-CIK cells after chemotherapy. Four hundred AML patients were divided into treatment (n=210) and control (n=190) groups, and categorized into favorable, intermediate, and poor risk sub-groups according to NCCN guidelines. DC-CIK cellular parameters were measured by flow cytometry. Treatmentrelated adverse responses were observed, and the clinical efficacies were compared between groups and among the favorable, intermediate, and poor risk sub-groups. Patients in the treatment group underwent at least four DCCIK infusions. The CD3+:CD8+ and CD3+:CD56+ ratio of the DC-CIK cells was 78.3±4.3% and 35.1±3.5%, respectively, which was significantly higher than in CIK cells 52.1±3.8% and 19±3.1% (both P<0.05). Patient overall survival (OS) (54.8% vs. 37.9%, P<0.05) and disease-free survival (DFS) (49.5% vs. 32.1%, P<0.05) in the treatment group was significantly higher than the control group. OS of treatment group patients in the favorable, intermediate, and poor sub-groups was 73.4%, 52.9%, and 10.3%, and was 51.5%, 36.1%, and 8.0% in the control group, respectively. Treatment group patient OS in the favorable and intermediate risk sub-groups was significantly higher the control group (P<0.05). however, the outcomes of the poor risk AML patients could not be improved (P>0.05). Thirty-one (14.7%) treatment group patients had only transient minimal adverse responses. Sequential infusion of DC-CIK cells could significantly increase DFS and long-term survival of favorable and intermediate risk AML patients. For poor risk AML patients, we suggest immediate HSCT therapy after induction of remission.
Keywords: AML; DC-CIK; Immunotherapy
Introduction
Traditional therapy of treating acute myeloid leukemia (AML) includes predominantly chemotherapy and hematopoietic stem cell transplantation (HSCT). The relatively high relapse rate and incidence of multidrug resistance (MDR) are major challenges in treating AML. In recent years, using targeted therapy, demethylation therapy, and immunotherapy to treat AML has been acknowledged as an intense area of recent research.
Immunotherapy based on dendritic cell (DC)-activated Cytokine-induced killer (CIK) has been increasingly used in clinical practice. Cytokine-induced killer cells (CIKs) are a group of heterogeneous cells obtained by the in vitro culture of peripheral blood mononuclear cells (PBMCs) under the co-stimulation of IFN-γ, anti-CD3 mAb, and IL-2. CIKs have the advantages of both the powerful anti-tumor activities of T cells and the restricted tumor killing effects of the major histocompatibility complex (MHC) of NK cells, complemented by a powerful proliferation capacity and high cytotoxicity [1].
Dendritic cells (DCs) are the most potent professional antigen-presenting cells that play important roles in inducing the primary immune response. Co-culture of CIKs that display a potent cell-mediated killing capacity and DCs that exhibit powerful antigen presenting capacity could significantly increase the proliferation and killing capacity of CIKs [2].
Multiple studies have shown that treating various tumors by adoptive immunotherapy based on a strategy of using DCCIKs display very acceptable prospects in clinical practice, and especially in the elimination of minimal residual disease (MRD) of leukemia [3-6]. In the present study, autologous leukemia antigenloaded DC-CIKs were used for the treatment of AML patients. Additionally, the safety profiles and clinical efficacies were investigated, and the results reported herein.
Patients and Methods
Patients
Four hundred AML patients (PML/PARα positive AML patients were excluded) that were diagnosed at our hospital between January 2009 and July 2015 were randomly included. AML was diagnosed according to the AML French-American-British (FAB) and the WHO classification criteria [7-8].
The present study was approved by the Ethics Committee of the General Hospital of Chengdu Military Region. The inclusion criteria were as follows: 1) patients were confirmed as favorable, moderate, or high risk AML. The risk status of the patients were classified according to the NCCN AML guidelines (2016); 2) the induction chemotherapy for patients <60 years of age used the DA or IDA regimen (D: daunorubicin; A: cytarabine; ID: idarubicin), while for patients =60 years of age, the induction chemotherapy regimen using CAG was employed (C: Aclarubicin; A: cytarabine; G: granulocyte colonystimulating factor). The consolidation and maintenance therapy of patients used cytarabine-based regimens; and 3) patients in the treatment group signed informed consent documentation prior to adoptive transfer of DC-CIKs.
The exclusion criteria were as follows: 1) combined with severe chronic consumptive diseases, or combined with severe liver, heart, or renal disease; 2) could not complete four or more cycles of chemotherapy due to any reason; and 3) the families of the patients required receipt of other treatment methods.
The patients were divided into two groups according to treatment or no treatment by adoptive transfer of DC-CIK. For patients in the treatment group, sequential DC-CIK infusion was performed after chemotherapy, while for patients in the control group, only chemotherapy was performed. The age, sex, disease type, and risk status were not significantly different between either group (Table 1).
Characteristics
Treatment group (n, 100%)
Control group (n, 100%)
Total
210
190
Age
>60 years
58 (27.6)
47 (24.7)
=60 years
152 (72.4)
143 (75.3)
Sex
Male
121 (57.6)
107 (56.3)
Female
89 (42.4)
83 (43.7)
Diagnosis
M0
4 (1.9)
2 (1.0)
M1
16 (7.6)
9 (4.7)
M2
96 (45.7)
93 (48.9)
M4
39 (18.5)
27 (14.2)
M5
52 (24.7)
58 (30.5)
M6
3 (1.4)
1 (0.5)
Risk status
Favorable-risk
79 (37.6)
68 (35.7)
Intermediate risk
102 (48.5)
97 (52.0)
Poor risk
29 (13.8)
25 (13.1)
The age, sex, disease type, and risk status were not significantly different between the two groups (P>0.05).
Table 1: The clinical characteristics of the 400 recruited AML patients.
Reagents and equipment
RPMI-1640 complete culture medium was obtained from Hyclone Inc., lymphocyte separation medium was obtained from the second pharmaceutical factory in Shanghai, China. The cytokines/ growth factors rhIL-4, rhGM-CSF, rhIL-2, rhIL-1α, IFN-γ, and rhTNF-α were obtained from Peprotech Inc. Mouse-anti-human CD3 mAb was obtained from SBA Inc. FITC labeled anti-human CD80, CD83, and CD86 mAb were all obtained from Serotec. The centrifuge (model number: KDC-2046) used in these studies was obtained from Zhongjia, University of Science and Technology of China. The fully humidified CO2 cell culture incubator was obtained from Shanghai Lishen, and the flow cytometer (BD FACS Calibur) was obtained from BD (USA).
Preparation of autologous leukemia antigen
Before the initial treatment, 20 mL of bone marrow was collected from each patient, 0.83% ammonium chloride was used to lyse the red blood cells. After the debris of the red blood cells was removed, discontinuous gradient centrifugation (360g×10 min) was performed to remove the mononuclear cells. The cell precipitate was then washed with normal saline and centrifugation was repeated. Then repeated freezing and thawing (-80 °C to 42 °C, which was repeated five times) of the cell precipitate was performed, followed by sterilization by filtration through a 0.22 μm membrane. The protein concentration in the cell lysate was measured, and stored at -80 °C as the purified antigen until subsequent use.
Preparation of autologous leukemia antigen-loading DC
Before the initiation of chemotherapy, 60 mL of peripheral blood was obtained from each patient. Discontinuous gradient centrifugation (360g×10 min) was used to obtain the mononuclear cells, which were seeded into a flask containing 10% RPMI-1640 culture median with normal human AB serum. After incubation at 37 °C for 2 h in the 5% CO2 cell incubator, the suspended cells were obtained for the culture of CIK cells. In this process, 20 mL of fresh culture medium (RPMI-1640 culture medium, 10% normal human AB serum, 125 ng/mL rh-GM-CSF, and 125 ng/mL rhIL-4) was added to the attached cells, and then cultured at 37 °C in the 5% CO2 cell incubator. Fqresh cytokines were added on days 3 and 5 to obtain a final concentration of 125 ng/mL for rhGM-CSF and 125 ng/ mL for rhIL-4. In addition, autologous leukemia cell lysate was added on day 5 to obtain a final concentration of 100 mg/mL, and rhTNF-α was added on day 6 of culture to obtain a final concentration of 1000 U/mL. The cells were harvested on day 7, and the immunophenotypes of the cells were determined, following which, the cells were cocultured with CIK cells.
Preparation of CIK cells
The suspended cells that were previously harvested were added into RPMI-1640 culture medium, and centrifuged at 360 g for 15 min to remove the supernatant. Then the cells were re-suspended in RPMI- 1640 culture medium. After counting the cells, the cell suspension was transferred into a 75 cm2 flask, and then IFN-γ (final concentration of 1000 U/mL) was added, and the cells were cultured at 37 °C in the incubator for 24 h. At the end of this phase of cell culture, a final concentration of 70 mg/mL mouse-anti-human CD3 mAb, 1000 U/ mL of rhIL-2, and 200 U/mL of rhIL-1α was added, and the cells were further cultured. During this time, cell growth was observed, and the culture medium was changed every three days, at which moment fresh culture medium containing IL-2 was added to the cell cultures.
Co-culture of DC-CIK
The DCs were harvested after 7d of culture, counted, and five volumes of CIKs were added, and then co-cultured for 3-5 additional days.
Immunophenotypic analysis of DC, CIK, and DC-CIKs
On day 7 of the cell culture, laser scanning flow cytometry was used to analyze the immunophenotypic expression of cell-surface expressed CD83, CD80, and CD86 by DCs. On day 12 of the cell culture, laser scanning flow cytometry was again used to determine the phenotypic expression and ratiometric frequency of CD3+:CD8+ and CD3+:CD56+ expression of the CIKs and DC-CIKs.
Back-infusion of DC-CIK by adoptive transfer
Detection of bacteria, fungi, and endotoxin in the DC-CIK cell mixture showed negative results. Microbial and fungal culture also showed negative results, suggesting that the mixture was not contaminated by microbial organisms. The DC-CIKs were centrifuged and washed on day 12 of culture, and then 100 mL normal saline, 25 mL 20% human serum albumin, and 200, 000U rhIL-2 was used to resuspend the cells. The mixture was back-infused to the patients within 2h. Intravenous injection of 5mg dexamethasone was performed before the back-infusion to prevent allergic cross-reactivity. The time of the back-infusion was the agranulocytosis phase after chemotherapy. The count of the cells that was back-infused was (1.0- 9.0)×109, and 3-5 back-infusions were adoptively transferred to each patient. Subcutaneous injection of IL-2 (200, 000 U/day) was also performed for 10 days during the back-infusion process.
Monitoring the toxic response and evaluation of clinical efficacies
The vital signs and any toxic responsiveness were observed during and after the DC-CIK back-infusion until the toxic response disappeared or until such time that the patients received other treatments. The items observed, and the staging of the toxic responses were performed according to the WHO criteria of acute and subacute toxic effects [9]. The patients were followed up until the relapse of leukemia or up to December 31, 2014, whichever arrived first. Cytometry of MRD, bone marrow cytological examinations, and chromosomal examinations were also performed. The overall survival (OS) and disease-free survival (DFS) of the patients in each follow-up duration were calculated.
Statistical analysis
SPSS version 19.0 software was used for statistical analysis. Independent Student’s t-test was used for the comparisons between two groups. The Kaplan-Meier curve was used for the analysis of OS and DFS. Chi-square test was used for the comparison of OS among the groups. An alpha value of P<0.05 was considered statistically significant.
Results
Phenotypic analysis of the DC and CIK
The cell body of the DCs from the patients in the treatment group increased by day 7 of the culture, the surfaces were evident as DC with large numbers of sheet-like processes and blur-shaped structures. The percentage of the typical DCs was 60%-70%, and half of the cells were in a status of semi-attachment. The results of the immunophenotypic analyses of the DCs were as follows: (82±7.6) % was CD80+ cells, (61±7.1) % was CD83+ cells, and (80±8.4) % was CD86+ cells. All DCs from the patients in the treatment group were expressed with mature DC surface markers by day 7 of culture. CIK cells in culture for day3 began to proliferate, the day 5-6 of rapid growth, the cell volume larger, irregular shape, grow in clusters (Figure 1). On day 12 of culture, flow cytometry showed that the ratio of CD3+CD8+ and CD3+CD56+ was (78.3±4.3) % and (35.1±3.5) % in the DC-CIKs, which was significantly higher than was found in the CIKs [(52.1±3.8) % and (19±3.1) %] (t=24.1 and 26.6, respectively, P<0.05) (Table 2). These findings showed that after sensitization with DC, the proliferation and activity of the CIKs increased significantly.
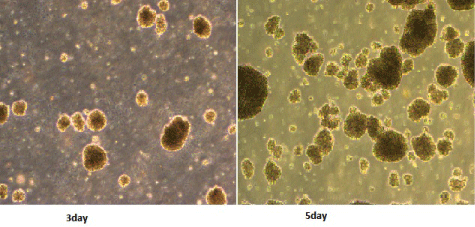
Figure 1: Morphology of CIK cells (1:40).
CIK
DC-CIK
CD3+CD8+
CD3+CD56+
CD3+CD8+
CD3+CD56+
0d
19.3±2.1
3.2±1.5
28.3±4.2
3.81±1.1
12d
52.1±3.8
19±3.1
78.3±4.3
35.1±3.5
Table 2: Changes of cell immune phenotypes after Co-cultured Dendritic cell with CIK (%).
Clinical efficacy evaluation
The patients were followed up for a period of 6-52 months (median: 28 months). Thirty-four (14.7%) patients in the treatment group and 21 (11.0%) patients in the control group dropped out due to the diagnosis of refractory leukemia. The other patients in the treatment group completed 4-6 cycles of autologous leukemia antigenloaded DC-CIK adoptive transfers. Figures 2-6 show the results of the patients that had received, or had not received DC-CIK therapy. The OS (54.8% vs. 37.9%, P<0.05) and DFS (49.5% vs. 32.1%, P<0.05) of the patients was significantly higher in the treatment group than in the control group. The OS of the patients in the favorable, intermediate, and poor risk sub-groups was 73.4%, 52.9%, and 10.3% in the treatment group, and 51.5%, 36.1%, and 8.0% in the control group, respectively. The OS in the favorable and intermediate risk sub-groups were significantly higher in the treatment group than were found in the control group (P<0.05). However, the difference in the poor risk sub-group was not statistically significant.
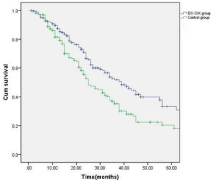
Figure 2: Overall survival of the patients in the DC-CIK and control group
(P<0.05).
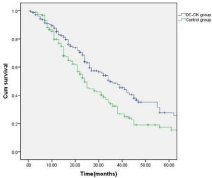
Figure 3: Disease-free survival of the patients in the DC-CIK and control
group (P<0.05).
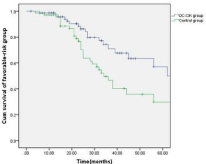
Figure 4: Comparison of the overall survival of the patients in the favorable
sub-group between the treatment and control group.
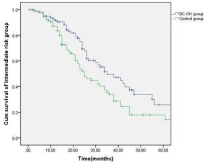
Figure 5: Comparison of the overall survival of the patients in the intermediate
risk sub-group between the treatment and control group.
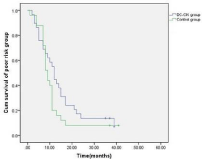
Figure 6: Comparison of the overall survival of the patients in the poor risk
sub-group between the treatment and control group.
Adverse response of the patients after DC-CIK adoptive transfer infuions
Thirty-one (14.7%) patients in the treatment group presented with transient fever and shivering, with the temperature of about 37.5-39.1 °C. These responses mainly occurred within 0.5-2 hours after the DCCIK infusion. No drug therapy was required for most patients, and the symptoms regressed after approximately 1-2 hours. For some patients that could not tolerate the symptoms, commonly used anti-pyretics were used for the treatment, and the symptoms were effectively improved.
Discussion
AML is the most common acute leukemia in adults, and the prognoses of the patients are very poor. With the universal application of morphological, immunological, cytogenetic, and molecular biological technologies, AML is now classified as favorable, intermediate, and poor risk types according to the chromosomal karyotypes identified, mutant genes, physical conditions, ages, complications, and other special medical histories. The stratification of the prognostic prediction has been widely accepted over recent years. After the patients were stratified according to the risk status, more precise and individualized treatments for AML could be performed. After treatment by chemotherapy, HSCT, or other new drugs, it was found that some favorable and intermediate risk AML patients could achieve complete remission after induction therapy, and remain in a state of continued complete remission. However, the treatment efficacy in poor risk leukemia patients is relatively poor, and most of them show insensitivity to routine chemotherapy, with low remission rates, clinical relapse in the short term, and progression to refractory leukemia in clinical practice.
Currently, there is no universally accepted standard mode of treatment for the treatment of adults with poor risk AML. For young patients with poor risk AML that could tolerate chemotherapy, HSCT should be performed as soon as possible after remission. While for those patients where such treatment modalities are unavailable, high dose chemotherapy should be used to induce remission, consolidation and intensification, and maintenance of therapy. For the poor risk patients that could not tolerate chemotherapy and HSCT, aggressive supportive treatment plus experimental drug therapy should be performed [10]. Disease relapse and MDR remain major challenges for the treatment for both the favorable/intermediate and poor risk AML patients. Further, elimination of minimal residual leukemia after complete remission is also a key challenge during therapy. Currently, the precise approaches on how to perform low-toxic but highly effective treatment of leukemia is considered crucial research in the clinical management and therapy of leukemia.
Adoptive cellular immunotherapy is an important component of cancer therapy. This treatment method infuses autologous or homologous immunologic effector cells into the patients with the intention of killing malignant tumor cells, and minimal residual disease following chemotherapy in cancer patients. Currently, the clinical applications of natural killer cells (NK), cytokineinduced killer cells (CIK), DC-CIK, cytotoxic lymphocyte (CTL), and CAR-T have achieved significant outcomes [11-14]. As the applications of CIKs are economic and safe, with multiple cellular resources available, and the preparation of such cellular resources being relatively robust and quite basic in design and implementation, CIKs remain one of the most widely used cells in adoptive cellular immunotherapy [15]. Our results also showed that DCs could promote the proliferation and activation of CIKs. Flow cytometry showed that the ratios of CD3+CD8+ and CD3+CD56+ ratios in the DC-CIKs were significantly higher than in the CIKs on day 12 of culture, which were in agreement with other studies.
In vitro evidence showed that DC-CIKs could eliminate minimal residual leukemia. In a study performed by Liu, et al. [16], 48 patients with acute leukemia or minimal residual leukemia that achieved morphological complete remission but not molecular complete remission, were divided into a chemotherapy group and DC-CIKs plus chemotherapy group. It was found that DC-CIKs could inhibit the leukemia-related genes, promote the negative-conversion of the immunophenotypes of the minimal residual leukemia, increase the elimination rate of the minimal residual leukemia, improve the immune functions of the patients, and prolong the remission stage of the patients. DC-CIKs could also eliminate the leukemia stem cells (LSCs). Zhang, et al. [17] applied DC-CIKs to the AML cell-line KG- 1a, and found that DC-CIKs could induce apoptosis of the KG-1 cells, suggesting that DC-CIKs display potent LSC-killing effects.
DC-CIKs could also reverse the MRD of leukemia cells. Therefore, DC-CIKs have been increasingly applied in clinical practices due to the significant anti-leukemia effects [18]. In the present study, 400 AML patients that achieved a CR1 phase after routine chemotherapy were chosen, and sequential infusion of autologous leukemia antigenloaded DC-CIKs was performed after consolidation chemotherapy. All 210 patients in the treatment group completed four to six infusions of DC-CIKs, and only 31 of them (14.7%) were found with transient fever and shivering, while no other adverse response was found. The OS in the treatment group was 54.8%, which was significantly higher than in the control group (37.9%). The DFS in the treatment group (49.5%) was also significantly higher than in the control group (32.1%). In addition, we also found that the OS in the favorable and intermediate risk sub-groups were significantly higher in the treatment group than was found in the control group, while the OS in the poor risk sub-group was not significantly different between either groups. Therefore, we concluded that DC-CIK is an important treatment method that is both safe and efficacious in the therapy of AML. Although few patients were found with transient fever, which could be associated with the secretion of inflammatory cytokines, and anti-tumor effects from the CIKs [3], these symptoms could be improved after symptomatic treatment. Additionally, DCCIKs could also significantly improve the quality of life and survival time of the favorable and intermediate risk AML patients; however, the outcomes of the poor risk AML patients could not be improved. For poor risk AML patients, we suggest immediate HSCT therapy after induction of remission.
In recent years, and with the identification of many leukemiarelated abnormal signaling pathways and the alterations of leukemiarelated genes, the etiologies of leukemia are gradually being uncovered. The consequent new treatment methods also change the perception of leukemia as being a chronic and not malignant disease. Consequential infusion of autologous leukemia antigen-loaded DC-CIKs for favorable and intermediate risk patients after chemotherapy could better eliminate the minimal residual leukemia, improve survival and disease survival rates than simple chemotherapy. This method has encouraging clinical prospects due to highly acceptable safety profiles. The findings of the present study could provide novel ideas for the clinical treatment of human leukemia.
References
- Nagaraj S, Ziske C, Ingo GH Schmidt-Wolf. Human cytokine-induced killer cells have enhanced in vitro cytolytic activity via non-viral interleukin-2 gene transfer. Genet Vaccines Ther. 2004; 2: 12.
- Wang QJ, Wang H, Pan K, Li YQ, Huang LX, Chen SP, et al. Comparative study on anti- tumor immune response of autologous cytokine-induced killer (CIK) cells, dendriticcells-CIK (DC-CIK), and semi-allogeneic DC-CIK. Chin J Cancer. 2010; 29: 641-648.
- Jianan P, Jiuwei G, Jiuwei C. Progress of cytokine-induced killer cells therapy in cancer. Chin J Clinicians (Electronic Edition). 2014; 8: 2889-2893.
- Zhang YJ,Dong M,Ping CQ. The Research Progress of Autologous DC and CIK Cells in the Treatment of Acute Myeloid Leukemia. Progress in Modern Biomedicine. 2015; 15: 760-763.
- Bai Y, Zheng JE, Wang N, Cai HH, Zhai LN, Wu YH, et al. Effects of dendritic cell-activated and cytokine-induced killer cell therapy on 22 children with acute myeloid leukemia after chemotherapy. J Huazhong Univ Sci Technolog Med Sci. 2015; 35: 689-693.
- Dong M, Kong DS, Zhao HL. Recent Clinical Observation on the DC and CIK Cell in the Treatment of Refractory and Recurrent Acute Myeloid Leukemia. Progress in Modern Biomedicine. 2014; 14: 159-162.
- Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al: Proposed revised criteria for the classification of acute myeloid leukemia: a report of the French-American-British Cooperative Group. Ann Intern Med. 1985; 103: 620-625.
- Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002; 100: 2292-2302.
- Miller AB, Hoogstraten BB, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981; 47: 207-217.
- MS Lippl, K Spiekermann. Acute myeloid leukemia. Info Onkologie. 2015; 18: 30-37.
- Tian H, Chen GH, Xu Y, Qiao M, Liu HW, Wu DP. Current research advance on cellular immunotherapy for leukemia-review. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2013; 21: 1326-1330.
- Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric Antigen Receptor–Modified T Cells in Chronic Lymphoid Leukemia. N Engl J Med. 2011; 365: 725-733.
- Pizzitola I, Anjos-Afonso F, Rouault-Pierre K, Lassailly F, Tettamanti S, Spinelli O, et al. Chimeric antigen receptors against CD33 /CD123 antigens efficiently target primary acute myeloid leukemia cells in vivo. Leukemia. 2014; 28:1596-1605.
- Mardiros A, Dos Santos C, McDonald T, Brown CE, Wang X, Budde LE, et al. T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and antitumor effects against human acute myeloid leukemia. Blood. 2013; 122: 3138-3148.
- Mao Q, Li L, Zhang C, Sun Y, Liu S, Cui S. Clinical effects of immunotherapy of DC-CIK combined with chemotherapy in treating patients with metastatic breast cancer. Pak J Pharm Sci. 2015; 28: 1055-1058.
- Liu QC, Zhang HM, Zhang YN. Clinical study of dendritic cells and cytokine-induced killer cells for eliminating minimal residual leukemia. Chin J Cancer Biother. 2013; 20: 398-403.
- Zhang Y, Zhang LS, Zeng PY, Chong-yang WU, Liang-cai YI, Jun BAI, et al. Cytotoxicity effect of DC-CIK on acute myelogenous leukemic stem cells. Chin J of Cancer Biother. 2011; 18: 404-408.
- Yang Y, Yang B, Cai LL, Ran HH, Yu RL, Chi XH, et al. Clinical Study of Autologous Cytokine Induced Killer Cells Combined with Chemotherapy for Elderly Patients with Acute Myeloid Leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2014; 22: 58-63.