
Special Article - Multiple Myeloma
Ann Hematol Oncol. 2016; 3(7): 1100.
A Secondary B Acute Lymphoblastic Leukemia in Known Case of Multiple Myeloma Treated with Lenalidomide- A Rare Case Report
Karapetyan L1, Hough B2, Geynisman DM3, Nason KS4, Luketich JD4,Jobe B5, Zaidi A5 and Gibson MK1*
1Department of Pathology, Tata Memorial Centre, India
2Department of cancer cytogenetics, Tata Memorial Centre, India
3Medical oncology, Tata Memorial Centre, India
*Corresponding author: Prashant Tembhare, Department of Pathology, Hematopathology Laboratory, Tata Memorial Centre 7th Floor, Annex building, Dr E Borges Road, Parel, Mumbai- 400012, India
Received: June 23, 2016; Accepted: August 09, 2016; Published: August 11, 2016
Abstract
Therapy-related or secondary acute myeloid leukemias in Multiple Myeloma (MM) are well documented; however, secondary B-acute lymphoblastic leukemia (B-ALL) in MM is rarely reported. Chromosome 11q23 abnormality is frequently seen in secondary acute myeloid leukemia; but secondary-ALL without 11q23 abnormality is extremely rare and associated with poor survival. Although, the role of lenalidomide causing secondary malignancies in MM has not been fully understand and explained, we are presenting a case of secondary B-ALL in known case MM treated with Lenalidomide without association of 11q23 abnormality. A 52-years old lady, a diagnosed and treated case of MM with Lenalidomide achieved a complete remission (CR). She was on routine follow and presented with fever and reduced appetite. Complete blood count (CBC) showed thrombocytopenia & peripheral-blood-smear (PBS) examination showed 60% blasts. Blasts were negative for cytochemical myeloperoxidase (MPO). Flowcytometric immunophenotyping (FCI) of blasts population showed B ALL phenotype. Cytogenetics evaluation did not reveal translocations involving 11q23, the KMT2A gene locus. Although extremely rare, secondary-ALL is one of the therapy-related neoplasms in MM without translocations involving 11q23, the KMT2A gene locus. In addition to CR with lenalidomide therapy in our patient, and absence of B-ALL clone at the time of initial diagnosis by FCI indicates that the secondary B-ALL was de novo disease. Chronic lenalidomide use in the treatment of MM may have resulted in the development of s-ALL, however, the exact association between MM, chronic therapy with lenalidomide and s-ALL in this patient remains unknown and needs further work up.
Keywords: Secondary B acute lymphoblastic leukemia; Multiple myeloma; Lenalidomide
Introduction
Therapy-related or secondary acute myeloid leukemias (AML) in multiple myeloma (MM) are well documented; however, secondary B acute lymphoblastic leukemia (B-ALL) in MM is rarely reported [1]. The frequently found chromosomal abnormality in secondary AML as well as secondary B-ALL is translocations involving 11q23, the KMT2A gene locus. Secondary B-ALL without 11q23 abnormality is extremely rare and associated with poor survival. In addition to that there are only a few published reports describing occurrence of secondary B-ALL in MM patients. We report a case of secondary B-ALL in known case MM treated with Lenalidomide without association of 11q23 abnormality who had achieved complete remission.
Case Presentation
A 52-years old lady, presented with fatigue and lower backache and being investigated for the same. Meanwhile MRI spine was done and it revealed wedge compression of D8 vertebra. Serum immunoelectrophoresis showed abnormal electrophoretic pattern with presence of monoclonal band IgG/lambda (1.4 G/dl) type. The renal function test and complete blood count (CBC) levels were within normal ranges except for anemia with hemoglobin (Hb) of 7 gm/dL (range: 13-17 gm/dL). Serum calcium was 9.2 mg/dL (range: 8.5-10.2 mg/dL) and beta-2 microglobulin was 3.8 mg/L (< 2 mg/L). The bone marrow (BM) aspiration revealed 38% plasma cells and infiltration in sheets by plasma cells in trephine biopsy. FCI of BM aspiration revealed clonal plasma cells. Skeletal survey showed many lytic lesions of the skull and lumbar-sacral area. A diagnosis of MM was made in 2010. After that she was treated with Lenalidomide, dexamethasone and zolendronic acid for two years. She had achieved complete remission in 2012 and was on routine follow up till July 2014. Subsequently, she presented with fever, weakness and reduced appetite. CBC revealed total leukocyte count (TLC) - 5.42x109/L (range: 4-11x109/L), Hb - 12.4 gm/dL (range: 13-17 gm/dL), and platelets - 7x109/L (range: 150-410x109/L). Peripheral-bloodsmear (PBS) examination showed 60% blasts and was negative for cytochemical myeloperoxidase (MPO) staining.
Cytogenetic analysis was performed by using common B ALL probes and includes BCR/ABL1 by using Locus Specific Identifier (LSI) BCR/ABL1 dual fusion translocation probe, KMT2A by using LSI KMT2A (11q23) break apart rearrangement probe, TCF3/PBX1; t(1;19) and ETV6/RUNX1; t(12;21) fusion translocation probes and revealed negative results for all these translocation, however showed tri-tetrasomy of chromosomes 1, 4, 9, 10, 11, 17, 19 & 22 in 90-95% cells and ploidy analysis revealed triploidy in 100% of cells. In addition to this, no other common MM related abnormalities were detected.
BM specimen was further processed for FCI using a lyse-stainwash technique and a comprehensive eight-color antibody-panel for acute leukemia on Navios (Beckman Coulter; BC) and analyzed using kaluza-1.3 software (BC). The acute leukemia panel included monoclonal antibodies against CD1a, CD2, CD3, CD4, CD5, CD8, CD10, CD11b, CD13, CD15, CD16, CD19, CD20, CD22, CD33, CD34, CD38, CD45, CD56, CD58, CD117, CD123, HLADR, cytoplasmic CD79a, cytoplasmic CD3 and cytoplasmic MPO. Data were collected and analyzed using a CD45–side scatter based gating strategy and it revealed 21% of lymphoblasts. These blasts express moderate CD19, CD20, CD34, CD38, HLADR and CD58, dim CD10, sCD22, CD45, and CD123. They were also positive for cytoplasmic CD79a but negative for cytoplasmic myeloperoxidase and other myeloid/T cell markers (Figure 1). Further she was planned for chemotherapy ALL protocol but she succumbed to death after one week of diagnosis.
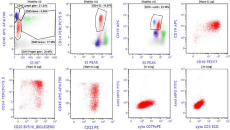
Figure 1: Immunophenotypic analysis of bone marrow specimen. The dot plots show blasts (red dots) with positive expression for CD10, CD19, CD20, CD34, coexpression
of CD22 and cytoplasmic CD79a. The blasts were negative for cytoplasmic CD3 and myeloperoxidase.
Abbreviations: B-ALL: B-acute lymphoblastic leukemia; MM: Multiple Myeloma; CBC: Complete Blood Count; CR: Complete Remission; FCI: Flowcytometric
Immunophenotyping; PBS: Peripheral-Blood-Smear; MPO: Myeloperoxidase; AML: Acute Myeloid Leukemias; Hb: Hemoglobin; BM: Bone Marrow; TLC: Total
Leukocyte Count; PBS: Peripheral-Blood-Smear; LSI: Locus Specific Identifier; FDA: Food and Drug Administration
Discussion
Therapy-related acute leukemia is a heterogeneous disease that may occur especially after treatment with chemotherapy (alkylating agent/topoisomerase II inhibitor) or radiotherapy [1]. These alkylating agent-related acute leukemias are mostly AML with antecedent myelodysplasia with a mean latency period of 5 to 7 years [1-3]. On the other hand, secondary leukemias caused by DNA topoisomerase II inhibitors had relatively short latent periods (1-5 years) without antecedent myelodysplasia [1-3]. Translocations involving 11q23, the KMT2A gene locus are the most common characteristic chromosomal aberrations seen in therapy-related or secondary acute myeloid leukemias [4]. Incidence of therapy-related acute lymphoblastic leukemias is very much less than that of the therapy related AML and represents approximately 12% of all therapyrelated acute leukemias and 1.2% to 4% of adult ALLs [4,5]. However, similar to that of therapy related AML chromosomal abnormalities in KMT2A gene (11q23) being the commonest ones. The risk of developing secondary AML in MM patients after treatment with alkylating agent therapy has been calculated to be 3-5% at 3 years and 10-15% at 10 years [6] while that of secondary ALL is extremely rare, about 0.5-1% of treated patients [6]. There were few case reports of treated MM cases terminated into s- B ALL published in the literature [7-13] and very few of them had documented absence of 11q23 cytogenetic abnormality (Table 1). Chen, et al. [11] also showed that, s-ALL cases with an 11q23 abnormality compared to cases without an 11q23 abnormality had a longer latency period (median, 36 vs. 19 months). There is an increased risk of developing secondary malignancies including ALL after the chronic use of lenalidomide to treat MM have been reported by Food and Drug Administration (FDA) [12] and also showed the median time span to develop the secondary malignancy was 2 years. Recently, Palumbo, et al. [14] also showed similar findings and stated that increased risk of developing hematological second primary malignancies after use of lenalidomide in newly diagnosed MM patients.
Study
No. of cases
Treatment Received
Latency period (Years)
KMT2A (11q23) aberration
Alkylating agent
Lenalidomide
[7]
1
Yes
No
One
Detected
[9]
1
Yes
No
One
Not detected
[8]
1
Yes
No
Nine
Not detected
[10]
1
No
Yes
Two
Not available
[11]
2
Yes
No
Five to six
Not detected
[12]
3
No
Yes
Two
Not available
[13]
3
Not available
Yes
Two to seven
Not detected
Present study
1
No
Yes
Four
Not detected
Table 1: Different studies published in the literature.
Our patient had received only a lenalidomide in MM treatment and developed secondary BALL with a no detectable translocations involving 11q23, the KMT2A gene locus. The latency period was four years from diagnosis of MM and was comparable with the mean latency period described in the literature for secondary ALL related to lenalidomide treatment [12,14] (Table 1).
Conclusion
Although extremely rare, secondary ALL is one of the therapyrelated neoplasms in MM without translocations involving 11q23, the KMT2A gene locus. In addition to CR with lenalidomide therapy in our patient, and absence of B-ALL clone at the time of initial diagnosis by FCI indicates that the secondary B-ALL was de novo disease. Chronic lenalinomide use in the treatment of MM may have resulted in the development of s-ALL, however, the exact association between MM, chronic therapy with lenalidomide and s-ALL in this patient remains unknown and needs further work up. Detailed immunophenotypic and cytogenetics evaluation is important for further categorization and risk stratification of such cases.
References
- Swerdlow S, Campo E, Harris N, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; Lyon: IARC; 2008.
- Zhang Y, Poetsch M, Weber-Matthiesen K, Rohde K, Winkemann M, Haferlach T, et al. Secondary acute leukaemias with 11q23 rearrangement: clinical, cytogenetic, FISH and FICTION studies. Br J Haematol. 1996; 92: 673-680.
- Hawkins MM, Wilson LM, Stovall MA, Marsden HB, Potok MH, Kingston JE, et al. Epipodophyllotoxins, alkylating agents, and radiation and risk of secondary leukemia after childhood cancer. BMJ. 1992; 304: 951-958.
- Ishizawa S, Slovak ML, Popplewell L, Bedell V, Wrede JE, Carter NH, et al. High frequency of pro-B acute lymphoblastic leukemia in adults with secondary leukemia with 11q23 abnormalities. Leukemia. 2003; 17: 1091-1095.
- Pagano L, Pulsoni A, Tosti ME, Annino L, Mele A, Camera A, et al. Acute lymphoblastic leukaemia occurring as second malignancy: report of the GIMEMA archive of adult acute leukaemia. Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto. Br J Haematol. 1999; 106: 1037-1040.
- Leone G, Voso MT, Sica S, Morosetti R, Pagano L. Therapy related leukemias: susceptibility, prevention and treatment. Leuk Lymphoma. 2001; 41: 255-276.
- Ueda K, Yamamoto G, Shinohara A, Hangaishi A, Kurokawa M. Early onset of acute lymphoblastic leukemia with MLL rearrangement after autologous stem cell transplantation for multiple myeloma. Ann Hematol. 2009; 88: 813-814.
- Piszcz J, Bolkun L, Cichocka E, Kloczko J. Secondary acute lymphoblastic leukaemia in a multiple myeloma patient. Contemp Oncol Pozn Pol. 2012; 16: 593-595.
- Lau LG, Tan LK, Koay ESC, Liu TC. Acute lymphoblastic leukemia after tandem autologous stem cell transplantations for multiple myeloma. Leukemia. 2005; 19: 299-301.
- Gonzalez MM, Kidd L, Quesada J, Nguyen N, Chen L. Acute myelofibrosis and acute lymphoblastic leukemia in an elderly patient with previously treated multiple myeloma. Ann Clin Lab Sci. 2013; 43: 176-180.
- Chen W, Wang E, Lu Y, Gaal KK, Huang Q. Therapy-related acute lymphoblastic leukemia without 11q23 abnormality: report of six cases and a literature review. Am J Clin Pathol. 2010; 133: 75-82.
- FDA Drug Safety Communication: Safety Review Update of Cancer Drug Revlimid (lenalidomide) and Risk of Developing New Types of Malignancies. 2012.
- Tan M, Fong R, Lo M, Young R. Lenalidomide and secondary acute lymphoblastic leukemia: a case series. Hematol Oncol. 2015.
- Palumbo A, Bringhen S, Kumar SK, Lupparelli G, Usmani S, Waage A, et al. Second primary malignancies with lenalidomide therapy for newly diagnosed myeloma: a meta-analysis of individual patient data. Lancet Oncol. 2014; 15: 333-342.