
Research Article
Ann Hematol Oncol. 2016; 3(4): 1085.
Incidence and Severity of Oral Mucositis after High- Dose Melphalan in Stem Cell Transplant Patients: A Pilot Study of Oral Cryotherapy
Kanz BA¹, Savani BN² and Culos KA1,2*
¹Department of Pharmaceutical Services, Vanderbilt University Hospital, USA
²Department of Hematology/Oncology, Vanderbilt Ingram Cancer Center, USA
*Corresponding author: Culos KA, Department of Pharmaceutical Services and Department of Hematology/ Oncology, Vanderbilt University Hospital, Room 2639 The Vanderbilt Clinic, 1301 Medical Center Drive, Nashville, TN 37232, USA
Received: May 07, 2016; Accepted: June 20, 2016; Published: June 22, 2016
Abstract
Background: Oral mucositis (OM) results in morbidity for patients undergoing stem cell transplant (SCT) with a melphalan-based conditioning regimen. Oral Cryotherapy (OC) may prevent damage to the mucosa. Starting September 1, 2014, a 75-minute, standardized, OC protocol was implemented at Vanderbilt Ingram Cancer Center (VICC). This study evaluated the incidence and severity of OM before and after the institution of the protocol. A unique feature of this report is that the majority of patients underwent SCT in the outpatient setting.
Methods: This was a matched cohort retrospective chart review of adult patients who received an autologous SCT after a conditioning regimen containing high-dose melphalan (140-200 mg/m2) between September 1, 2013 and January 31, 2015. The primary outcomes were the incidence and severity of OM, measured using the National Cancer Institute Common Toxicity Criteria (NCI-CTC), from the administration of melphalan through engraftment. Secondary outcomes included hospitalizations, use of parenteral nutrition or patient controlled analgesia, and incidence of febrile neutropenia.
Results: Forty-one patients were in each cohort. The incidence of clinically relevant OM (=NCI-CTC grade 2) was lower in patients who received OC [10/41 (24%) vs. 29/41 (71%), p-value <0.05]. Patients who received OC but still developed OM were more likely to have grade 1 mucositis versus grade 2 or above as in the control cohort. There were no significant differences in secondary outcomes.
Conclusion: OC reduces OM in patients undergoing autologous SCT with melphalan conditioning. A75-minute OC protocol is effective and feasible for use in patients transplanted in multiple settings.
Keywords: Mucositis; Cryotherapy; Melphalan; Stem cell transplant; Regimen related toxicity
Abbreviations
ASCT: Autologous Stem Cell Transplant; ADL: Activities of Daily Living; EMR: Electronic Medical Record; FDA: Food and Drug Administration; GM-CSF: Granulocyte Macrophage Colony- Stimulating Factor; HD-Mel: High-dose Melphalan; NCI-CTC: National Cancer Institute Common Toxicity Criteria; OC: Oral Cryotherapy; OM: Oral Mucositis; SCT: Stem Cell Transplant; VICC: Vanderbilt Ingram Cancer Center
Introduction
High dose melphalan (HD-Mel, 140-200 mg/m²) is the standard of care conditioning regimen in patients with multiple myeloma preparing for autologous stem cell transplant (ASCT) [1]. While it is effective, it is associated with significant toxicity. With HD-Mel conditioning regimens, up to 80% of ASCT patients will experience oral mucositis (OM), ranging in severity from grade 1 through grade 5 per the National Cancer Institute Common Toxicity Criteria OM Scale (NCI-CTC) [2,3].
Clinical consequences of OM include dehydration, malnutrition, infection and reduced long-term survival [2]. Secondary consequences include increased use of parenteral narcotics and increased days requiring total parenteral nutrition. The typical time course of OM involves it’s development five to seven days after chemotherapy exposure with symptoms persisting until after engraftment (approximately day fourteen after transplant) [2].
A Cochrane Review from 2011 identified ten methods explored to prevent or reduce the severity of OM, and concluded only palifermin and oral cryotherapy (OC) had evidence to prevent mucositis [4]. Palifermin, a keratinocyte growth factor, is the only Food and Drug Administration (FDA) approved therapy for prevention of OM in SCT patients. Opponents of this therapy cite limitations of the approval studies for small sample sizes and lack of comparisons to other available mucositis prevention strategies [5,6].
OC is the practice of decreasing the temperature of the oral mucosa by swishing ice water or chewing ice chips prior to, during, and after chemotherapy administration. It is postulated to reduce OM by constricting blood vessels in the mucosa, decreasing exposure of the mouth to the offending agent [2,7]. A second proposed mechanism of action is that cryotherapy reduces the metabolic function of epithelial and basal cells providing a cytoprotective effect [7]. Multiple studies have shown OC to be an effective treatment to prevent OM in patients treated with HD-Mel [7-10]. While there are no head-to-head studies comparing OC to palifermin, OC does provide a financial benefit with limited safety implications and is recommended as a preventative therapy by both the National Comprehensive Cancer Network (NCCN) and the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ ISOO) in their respective guidelines [11,12].
Starting September 1, 2014, a standardized cryotherapy protocol was implemented in all ASCT patients receiving HD-Mel at Vanderbilt Ingram Cancer Center (VICC) (Figure 1). Patients at VICC are transplanted as outpatients, unless they have variables indicating a high-risk transplant warranting inpatient monitoring. Patients receive cryotherapy education and are instructed to chew ice for a total of seventy-five minutes, including thirty minutes prior to their infusion, during the fifteen-minute infusion, and thirty minutes after the infusion. This single center, retrospective, matched cohort study was conducted to evaluate the incidence and severity of OM before and after the institution of a standardized OC protocol.
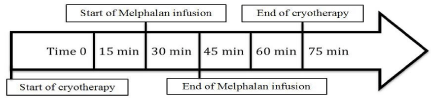
Figure 1: Timeline of cryotherapy administration.
Materials and Methods
Study population and design
The institutional review board at Vanderbilt University Medical Center approved this study. For this type of study, formal consent is not required. Patients were identified using the VICC SCT database and were included if they were 18 years of age or older and had undergone an ASCT with a conditioning regimen containing HDMel from September 2013 through January 2015. Patients who received their transplant after September 1, 2014 were included in the cryotherapy group. Patients who received their transplant in the year prior were matched preferentially based on their disease state, conditioning regimen, gender, race, age within a range of five years, weight within a range of ten kilograms, and number of prior chemotherapy regimens.
The primary objective of this study was to determine the incidence and severity of OM in patients who received OC versus historical controls who did not receive OC. Secondary outcomes included the incidence of febrile neutropenia, the incidence of febrile neutropenia specifically in patients with OM, total hospital admissions, hospital admissions for mucositis, hospitalizations where patients developed mucositis after admission, average days of patient controlled analgesia and average days of total parenteral nutrition.
Baseline demographics were collected from the electronic medical record (EMR). Outcomes data was collected from daily notes written by SCT clinicians. Mucositis was assessed and documented daily starting Day -3 by nursing staff and providers and continued until engraftment, defined as an absolute neutrophil count of 500 cells/uL for three consecutive days and greater than 20,000 platelets/ uL without transfusions. The NCI-CTC Mucositis Scale was utilized to grade mucositis, focusing on the functional grading scale, as shown in (Table 1) [2]. Clinically relevant mucositis was defined as Grade 2 or higher.
Clinical Scale
Functional Scale
Grade 1
Erythema
Minimal symptoms, normal diet; minimal respiratory symptoms but not interfering with function
Grade 2
Patchy ulcerations or pseudomembranes
Symptomatic but can eat and swallow modified diet; respiratory symptoms interfering with function but not with Activities of Daily Living (ADL)
Grade 3
Confluent ulcerations or pseudomembranes; bleeding with minor trauma
Symptomatic and unable to adequately aliment or hydrate orally; respiratory symptoms interfering with ADL
Grade 4
Tissue necrosis; significant spontaneous bleeding; life-threatening consequences
Symptoms associated with life-threatening consequences
Grade 5
Death
Death
Table 1: Summary of NCI-CTC OM Scale.
Statistical methods
Statistical analyses were completed using Stata Version 14. Nonparametric, nominal data was analyzed using Chi-Square and Fisher’s Exact Tests. Non-parametric, ordinal data was analyzed using the Mann-Whitney U test. Descriptive statistics were used as appropriate to report percentages, medians, and inter quartile ranges. An alpha significance level of 0.05 was used for all tests.
Results and Discussion
Patients
One hundred eighty-one patients were identified for inclusion in this study from September 2013 through January 2015. Forty-six patients were initially excluded because they did not receive HD-Mel based conditioning regimens. The remaining 41 patients who received cryotherapy constituted the cryotherapy cohort. An additional 53 patients were excluded who did not match the cryotherapy group, leaving 41 matched patients in the control group.
Baseline characteristics are shown in (Table 2). The cohorts were matched preferentially, indicating that the confounders were weighted based on their perceived importance as defined in the methods. There were no significant differences in baseline characteristics, indicating that the potential confounders were appropriately controlled through the matching process. Cohorts were both exposed to granulocyte macrophage colony-stimulating factor (GM-CSF). Most patients were Caucasian males in their sixth decade of life with multiple myeloma who received full-dose HD-Mel after a median of two prior regimens.
Characteristic
Cryotherapy Group
No. (%)
Control Group
No. (%)
P-value
Disease
1
Multiple Myeloma
34 (83%)
34 (83%)
Amyloid
5 (12%)
5 (12%)
Other
2 (5%)
2 (5%)
Conditioning Regimen
0.82
Melphalan (200mg/m2)
24 (59%)
27 (66%)
Melphalan (140mg/m2)
16 (39%)
13 (32%)
TEAM (200mg/m2)
1 (2%)
1 (2%)
Gender (Female)
14 (34%)
18 (44%)
0.497
Race (Caucasian)
37 (90%)
32 (78%)
0.23
Mean Age ( ±SD)
58.5 ±9.8 years
59± years
0.86
Mean weight ( ±SD)
86.6 ±16 kg
85.9 ±16 kg
0.83
Median number of prior regimens (IQR)
2 (1-3)
2 (1-3)
1
Table 2: Baseline Characteristics.
Primary and secondary outcomes
The incidence of clinically relevant OM in the cryotherapy group was significantly reduced [10/41 (24%) vs. 29/41 (71%), p-value < 0.05]. The median severity of mucositis in the cryotherapy group was also significantly less than in the control group [Grade 1 (IQR 1-2) vs Grade 2 (IQR 2-3), p< 0.05].
There were no significant findings in the secondary outcomes (Table 3). The incidence of febrile neutropenia in the cryotherapy versus control groups was 54% versus 73%, respectively [22/41 (54%) vs 30/41 (73%), p=0.1] and 27% versus 56% specifically in patients who experienced OM compared to those who did not [11/41 (27%) vs 23/41 (56%), p=0.08].The total number of hospitalizations in the control group was 36 compared to 31 in the cryotherapy group, with 23/31 (74%) in the cryotherapy group versus 34/36 (94%) in the control group associated with mucositis.
Cryotherapy Group
Control Group
p-value
Incidence of febrile neutropenia
22 (54%)
30 (76%)
0.1
Patients with OM
11 (48%)
23 (74%)
0.08
Total hospital admissions
31 (76%)
36 (88%)
0.15
Hospitalization for mucositis
4 (13%)
7 (19%)
0.44
Concurrent mucositis
19 (61%)
27 (75%)
0.3
PCA use (average days)
8 (5.5)
9 (5.8)
0.9
TPN use (average days)
4 (6)
7 (5.6)
0.47
Table 3: Secondary Outcomes.
Discussion
OM is a significant cause of morbidity and mortality in patients receiving HD-Mel prior to ASCT. Both the MASCC/ISOO Clinical Practice Guidelines for Oral Mucositis and the NCCN Task Force Report on the Prevention and Management of Mucositis in Cancer Care cite cryotherapy as an appropriate preventative intervention in these patients [11,12]. Despite the recommendations for OC, the data remains limited with few randomized trials occupying the space [13].
Lilleby, et al. published one of the larger, randomized trials evaluating cryotherapy in 2006. Patients receiving HD-Mel were randomized to either chew ice chips or swish room temperature normal saline rinses before, during and after melphalan infusions for a total time of six hours [7]. The primary outcome assessed was the development of grades 3-4 OM, as defined by the NCI-CTC, with secondary outcomes including number of days of intravenous narcotic use and total parenteral nutrition. Results indicated a significant decrease in grades 3-4 OM in patients who received OC. There were also significant differences in number of days of total parenteral nutrition and intravenous narcotics. However, the burden of a sixhour OC session on both the patient and transplant center limits the feasibility of this regimen in an outpatient transplant setting. Followup studies evaluated varying time periods with similarly efficacious results, but the guidelines do not recommend a specific time period [14-16]. In this study, 75 minutes was deemed to be most appropriate.
Multiple studies have shown strict oral care guidelines may play a role in the prevention of OM [11-13]. Outpatient ASCT removes this potential confounder. The majority of patients in this trial received a transplant as an outpatient, meaning that they came to clinic to receive their treatments but spent the majority of their time in unmonitored environments. This may have allowed for more exposure to variables that impact the incidence of mucositis such as the discovery and reporting of mucositis without frequent physical exams, the development of mucositis due to the lack of encouraged optimal oral care or lack of monitoring of nutrition after the development of mucositis. The successful results of this study demonstrate OC without strict inpatient oral care guidelines is still a worthwhile initiative to reduce OM. This will be important as more centers move towards outpatient transplants, although it should be noted that the effect of these confounding factors may have been minimal.
A large concern with the use of OC is compliance. Some patients do not tolerate the cooling sensation in their mouth over an extended period of time and others develop an aversion to ice chips after the OC experience [13]. Due to the novel nature of this intervention at the institution, staff did not document the actual administration of cryotherapy so the level of compliance was unable to be assessed and represents a major limitation of this study.
There are other notable limitations with this study. First, it was a single center study with a limited sample size. Therefore, we may not have captured some differences in the population, resulting in a type II error where a difference exists but was not found. This also indicates our external validity is limited and our results may not be applicable to populations that are not similar to the patients in this study or in a different part of the country or world.
Next, this was a retrospective chart review that relied on documentation to determine the incidence and severity of mucositis, as well as administration of cryotherapy. This would add to our inability to capture a difference, if one existed, due to missing or inadequate data. Further, there are limitations to utilizing historical controls as a comparator [17]. By using historical controls, we essentially chose to not observe an active contemporary control group in this study due to the initiation of the protocol across all groups and therefore the lack of control group at the institution. However, this may mean that there was something inherently different about the historical control group that would have changed the results had we used a contemporary control group. We are encouraged by the fact that patients were only included from 2013-2015 and supportive care and dosing of melphalan did not change significantly during that time. Next, historical sampling may bias results due to drift, which essentially means an investigator and their hypothesis are influenced by the prior knowledge they have of the intervention in the past. In this study, this was combated by borrowing the controls prospectively so they were not evaluated prior to the intervention group to provide background information or help to formulate our hypothesis.
The strength of a single arm historical trial is that it has power when the historical control rate and the contemporary control rate are equivalent but has inflated type I error or reduced power if the true control rate drifts in either direction [17]. By using a weighted match to pick the historical controls, we increased our chance of making correct inferences by accounting for as many covariates as possible. But there is still a distinct possibility that there was something inherently different about the historical cohort that was unaccounted for in the statistics and influenced these results and so is a limitation of this study.
Overall, our study confirms previous reports that cryotherapy reduces the incidence and severity of OM with HD-Mel prior to ASCT [7-10]. This investigation reviewed a unique patient population, as the majority of patients underwent ASCT in the outpatient setting. This is an important distinction as our study showed consistent efficacy of OC outside the controlled environment of an inpatient facility. The 75-minute cryotherapy protocol utilized was sufficient to reduce clinically relevant mucositis and feasible for the duration of an outpatient visit, allowing for versatility in location of transplant while maintaining a consistent cryotherapy protocol.
Conclusion
OC is an effective intervention to reduce the incidence and severity of OM in patients receiving HD-Mel based conditioning regimens undergoing ASCT. This investigation provides a validated 75-minute cryotherapy protocol to be implemented in both the outpatient and inpatient setting.
References
- Moreau P, Facon T, Attal M, Hulin C, Michallet M, Maloisel F. Comparison of 200mg/m (2) melphalan and 8 Gy total body irradiation plus 140 mg/m (2) melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myeloma 9502 randomized trial. Blood. 2002; 99: 731-735.
- Batlle M, Morgades M, Vives S, Ferrà C, Oriol A, Sancho JM, et al. Usefulness and safety of oral cryotherapy in the prevention of oral mucositis after conditioning regimens with high-dose melphalan for autologous stem cell transplantation for lymphoma and myeloma. European Journal of Haematology. 2014; 93: 487-491.
- Tuncer HH, Rana N, Milani C, Darko A, Al-Homsi SA. Gastrointestinal and hepatic complications of hematopoietic stem cell transplantation. World J of Gastroenterol. 2012; 18: 1851-1860.
- Worthington HV, Clarkson JE, Bryan G, Furness S, Glenny AM, Littlewood A, et al. Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev. 2011; 13.
- Loprinzi CL, Martenson JA. Keratinocyte Growth Factor: Not Ready for Prime Time. J Clin Oncol. 2003; 21: 1429-1430.
- Loprinzi C, Barton DL, Sloan JA. Whose Opinion Counts? J Clin Oncol. 2006; 24: 5183-5185.
- Lilleby K, Garcia P, Gooley T, McDonnnell P, Taber R, Holmberg L, et al. A prospective, randomized study of cryotherapy during administration of high-dose melphalan to decrease the severity and duration of oral mucositis in patients with multiple myeloma undergoing autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 2006; 37: 1031-1035.
- Spielberger R, Stiff P, Bensinger W, Gentile T, Weisdorf D, Kewalramani T, et al. Palifermin for Oral Mucositis after Intensive Therapy for Hematologic Cancers. N Engl J Med. 2004; 351: 2590-2598.
- Salvador P, Azusano C, Wang L, Howell D. A randomized controlled trial of an oral care intervention to reduce mucositis severity in stem cell transplant patients. J Pain Symptom Manage. 2012; 44: 64-73.
- Mori T, Aisa Y, Yamazaki R, Mihara A, Ikeda Y, Okamoto S. Cryotherapy for the prevention of high-dose melphalan-induced oral mucositis. Bone Marrow Transplant. 2006; 38: 637-638.
- Lalla RV, Bowen J, Barasch A, Elting L, Epstein J, Keefe DM, et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2014; 120: 1453-1461.
- Bensinger W, Schubert M, Ang KK, Brizel D, Brown E, Eilers JG, et al. NCCN Task Force Report. prevention and management of mucositis in cancer care. J Natl Compr Canc Netw. 2008.
- Peterson DE, Ohrn K, Bowen J, Fliedner M, Lees J, Loprinzi C, Mori T, et al. Systematic review of oral cryotherapy for management of oral mucositis caused by cancer therapy. Support Care Cancer. 2013; 21: 327-332.
- Mori T, Yamazaki R, Aisa Y, Nakazato T, Kudo M, Yashima T, et al. Brief oral cryotherapy for the prevention of high-dose melphalan-induced stomatitis in allogeneic hematopoietic stem cell transplants recipients. Support Care Cancer. 2006; 14: 392-395.
- Aisa Y1, Mori T, Kudo M, Yashima T, Kondo S, Yokoyama A, et al. Oral cryotherapy for the prevention of high-dose melphalan-induced stomatitis in allogeneic hematopoietic stem cell transplant recipients. Support Care Cancer. 2005; 13: 266-269.
- Tartarone A, Matera R, Romano G, Vigliotti ML, Di Renzo N. Prevention of high-dose melphalan-induced mucositis by cryotherapy. Leuk Lymphoma. 2005; 46: 633-634.
- Viele K, Berry S, Neuenschwander B, Amzal B, Chen F, Enas N, Hobbs B, et al. Use of historical control data for assessing treatment effects in clinical trials. Pharm Stat. 2014; 13: 41-54.