
Research Article
Ann Hematol Oncol. 2015; 2(8): 1059.
Are Fludarabine Based Regimens Still Adequate for Relapsed/Refractory Follicular Lymphoma? An 18-Year Single-Center Experience
Moita F¹*, Esteves S², Klose T², Koehler M¹ and Silva MG¹
¹Department of Hematology, Instituto Português de Oncologia de Lisboa Francisco Gentil, Portugal
²Clinical Research Unit, Instituto Português de Oncologia de Lisboa Francisco Gentil, Portugal
*Corresponding author: Moita F, Department of Hematology, Instituto Português de Oncologia de Lisboa Francisco Gentil, Rua Professor Lima Basto 1099-023 Lisboa, Portugal
Received: September 24, 2015; Accepted: November 10, 2015; Published: November 12, 2015
Abstract
There are no standard treatment recommendations in relapsed/refractory Follicular Lymphoma (R/R FL). Fludarabine, an effective but toxic agent, has been commonly used, but trials focusing on the risk-benefit balance in this setting are lacking and novel agents are available.
We conducted a single-center cohort study to evaluate the toxicity profile and supportive care needs of fludarabine-based regimens (FBR) administered in the first or second relapse. We retrospectively evaluated 116 R/R FL patients. Of these, 78 (67%) received FBR and 38 (33%), who were analyzed as an internal reference, received alkylating-based regimens (non-FBR). Similar disease control was obtained with both treatments. Treatment-related toxicities were high in FBR (74%) and non-FBR patients (68%). Growth factor use, transfusion requirements, short-term admissions to emergency room and prolonged hospitalization for toxicity were similar in FBR and non-FBR patients, but the latter were older and had different co-morbidities.
FBR patients over 60 years had higher incidences of grade ≥2 infections (46% vs. 18%; p=0.008). These regimens lead to prolonged hematological recovery, compromising subsequent treatments. With a median follow up of approximately 5 years, secondary malignancies were reported in 14% of patients.
High FBR toxicity and the availability of effective novel agents raise concerns about its adequacy in R/R FL setting. Therapeutic choices require a careful balance between efficacy, toxicity, cost and feasibility of subsequent therapies.
Keywords: Follicular lymphoma; Relapse; Therapy; Fludarabine; Toxicity
Abbreviations
R/R FL: Relapsed/refractory Follicular Lymphoma; FBR: Fludarabine-Based Regimens; non-FBR: Alkylating-Based Regimens; FL: Follicular Lymphoma; CLL: Chronic Lymphocytic Leukemia; R-FM: Rituximab, Fludarabine and Mitoxantrona; R-CHOP: Rituximab, Cyclophosphamide, Doxorubicin, Vincristine and Prednisolone; R-FC: Rituximab, Fludarabine and Cyclophosphamide; TRT: Treatment–Related Toxicities; TTNT: Time to Next Treatment; PFS: Progression-Free Survival; OS: Overall Survival; HSCT: Hematopoietic Stem Cell Transplant; ORR: Overall Response Rate; CR: Complete Remission
Background
Follicular lymphoma (FL), a common indolent non-Hodgkin lymphoma subtype [1], is characterized by high response rates to first line treatment, but almost universal relapses and progressively shorter subsequent remissions. Immunochemotherapy is standard for the treatment of advanced, high tumor burden cases [2]. While survival improved with the addition of the anti-CD20 monoclonal antibody rituximab to the clinical armamentarium [3-5], no chemotherapy regimen proved to be significantly superior to others in prospective, randomized studies [6,7].
FBR have been used for the past three decades in the treatment of indolent lymphomas. Their efficacy is well documented but their use has been hampered by frequent adverse events. The toxicity profile is mainly due to myelosuppression and lymphodepletion, with frequent, severe and unusual infections that can limit therapeutic benefits in elderly and fragile patients. A high incidence of second neoplasms was also reported [6,8,9].
In chronic lymphocytic leukemia (CLL) [10,11] and mantle cell lymphoma [5,12] the results of prospective clinical trials lead to precise recommendations on the use of fludarabine. In contrast, in relapsed FL, where this drug has been commonly used, there are no standard treatment recommendations. Trials focusing on the riskbenefit balance of FBR in this setting are lacking but a high incidence of hematological toxicity has been documented [13,14].
In frontline, a randomized trial showed that rituximab, fludarabine and Mitoxantrone (R-FM) was as effective as rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone (R-CHOP). However, fludarabine use was compromised by excessive toxicity [6]. Similar results were documented in a phase 2 trial where rituximab, fludarabine and cyclophosphamide (R-FC) lead to high response rates but also excessive deaths and premature study termination [15].
Among treatment options for R/R FL, bendamustine, an alkylating agent sharing similarities with the purine-analog structure, has recently been widely adopted [2]. Its toxicity profile compares favorably with anthracycline-and fludarabine-based regimens [16] but long term results have not been published. Restricted availability in some European countries, including Portugal, limited its use as an alternative to fludarabine. Novel agents that may prove beneficial in terms of efficacy and toxicity are becoming available, although cost issues are of concern. We believe it is still relevant to characterize the risks and benefits of FBR in this setting.
Our aim was to evaluate the toxicity profile and associated supportive care of FBR regimens in the real life setting of relapsed FL, profiting from the 18-year experience of a single center where other alternatives were limited.
Methods
We conducted a single-center cohort study. Fifty percent of all patients with grade 1, 2 or 3a FL referred to our tertiary cancer center in Portugal between 1994 and 2011were randomly selected for retrospective review. Patients undergoing second or third line treatment until 2014 were included in the analysis. Patients receiving first line FBR, and relapsed patients receiving oral cyclophosphamide, chlorambucil, rituximab alone, Y90 ibritumomab tiuxetan or intensive regimens (ESHAP) were excluded.
FBR were recommended during this period for relapsed disease and included mainly FC (fludarabine 25-30 mg/m²/day, x3 days; cyclophosphamide 200-300 mg/m²/day x3 days every 28 days, 6 cycles), FMC (fludarabine 20-25 mg/m²/day x3 days; Mitoxantrone 8-10 mg/m²/day x1 day; cyclophosphamide 200-300 mg/m²/day x3 days every 28 days, 4 cycles). Rituximab 375 mg/m² was added on day 1 after 2003. Maintenance with rituximab 375 mg/m² every 8 weeks started in 2012.
According to local practice, patients not receiving FBR were treated with6 to 8 CHOP or CVP cycles ±Rituximab [6].
Growth factors were allowed at the physician’s discretion. Chemotherapy cycles were delayed or suspended according to toxicities.
The cut-off date for analysis was May 2015. Demographic and clinical variables were recorded. For internal reference, patients receiving non-FBR were also analyzed.
Relevant treatment–related toxicities (TRT) included grade 3-4 hematological events, grade ≥2 infections and allergies, and second neoplasms. The frequency and severity of side effects were recorded according to the NCI-CTCAE v4.0 [17]. Response to therapy, time to next treatment (TTNT), progression-free survival (PFS) and overall survival (OS) (calculated from the start of second or third line treatment) [18] was also evaluated. Patients undergoing consolidation with autologous or allogeneic hematopoietic stem cell transplant (HSCT) were censored for PFS at the time of transplant.
Time-to-event endpoints were analyzed by Kaplan-Meier method. Group comparisons were performed with Pearson’s Chisquared test or Fisher’s exact test for categorical variables; t-test or Wilcoxon rank sum test were used for quantitative variables. All tests were two-sided with a 5% significance level. All analyses were done using R software [19].
Results
Patient population, treatment and effectiveness
Of 449 randomly selected patients, 116 fulfilled the inclusion criteria (Figure 1). Seventy-eight (67%) patients received FBR as second or third line while 38 (33%) received other regimens. Most patients were treated at first relapse (76% in the FBR and 87% in the non-FBR group). First line treatment was mostly CHOP or CVP (93%) with or without rituximab.
Patient characteristics are summarized in Table 1. Non-FBR patients were older at the start of second/third line treatment (p=0.022) and had a higher incidence of chronic kidney disease (p=0.010). This group additionally had longer response duration to first line therapy (median 2.3 versus 1.3 years, p=0.001). Groups were balanced for gender, other co-morbidities, FLIPI risk and response to first treatment.
FBR (n=78)
Non-FBR (n=38)
p-value
Age at diagnosis, years
Median [min-max]
Age >60 years, n (%)
56 [26-76]
30 (39%)
61 [24-78]
20 (53%)
0.087
0.213
Age at start of 2nd/3rd line treatment
Median [min-max]
Age >60 years, n (%)
60.5 [29-77]
39 (50%)
66 [31-83]
26 (68%)
0.022
0.094
Gender, n (%)
0.838
Female
Male
40 (51%)
38 (49%)
21 (55%)
17 (45%)
Comorbidities at start of 2nd/3rd line treatment, n (%)
No comorbidities
Diabetes mellitus
Cardiac
Peripheralneuropathy
Renal failure
High infectious risk1
Unknown
47 (60%)
13 (17%)
7 (9%)
2 (3%)
0 (0%)
6 (8%)
1 (1%)
25 (66%)
4 (11%)
4 (11%)
2 (5%)
4 (11%)
6 (16%)
0 (0%)
0.709
0.550
0.748
0.596
0.010
0.203
Year of diagnosis
Median [min-max]
2002 [1994-2011]
2004 [1995-2010]
0.524
Year of start of 2nd/3rd line treatment
Median [min-max]
2005 [1996-2014]
2009 [1998-2014]
0.003
Ann Arbor stage at diagnosis, n (%)
I/II
III/IV
Unknown
6 (8%)
71 (91%)
1 (1%)
9 (24%)
29 (76%)
0 (0%)
0.036
Extranodal involvement at diagnosis, n (%)
Yes
Number of sites Median [min-max]
Bone marrow involvement, n (%)
50 (64%)
1 [1-3]
39 (50%)
20 (53%)
1 [1-2]
16 (42%)
0.326
0.779
1.000
FLIPI, n (%)
Low risk (FLIPI 0-1)
Intermediate risk (FLIPI 2)
High risk (FLIPI 3-5)
Unknown
5 (6%)
19 (24%)
42 (54%)
12 (15%)
3 (8%)
13 (34%)
20 (53%)
2 (5%)
0.765
First line treatment regimen, n(%)
RCHOP/RCVP
CHOP/CNOP
CVP
Other
Alkylating agents + corticosteroids
Rituximab
32 (41%)
31 (40%)
10 (13%)
5 (6%)
4 (5%)
1 (1%)
17 (45%)
6 (16%)
12 (32%)
3 (8%)
3 (8%)
0 (0%)
0.023
Number of cycles administered in 1st line
Median [min-max]
8 [3-10]
6.5 [3-11]
0.812
Best response to first line treatment, n (%)
Complete response
Partial response2
Stable disease / progression
20 (26%)
45 (58%)
13 (17%)
15 (40%)
18 (47%)
5 (13%)
0.1913
Time to first relapse/progression, years
Median [min-max]
1.3 (0.2-11.4)
2.3 (0.2-10.3)
0.001
1Prior grade 3/4 infections, repeated infections, or clinical conditions leading to increased infectious risk.
2Includes response without bone marrow reevaluation.
3Comparison of complete response vs. non-complete response.
Table 1: Patient characteristics and first line treatment.
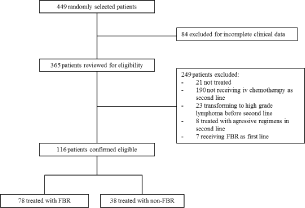
Figure 1: Patient flow diagram. (FBR: Fludarabine-Based Regimens).
Sixty-seven FBR patients (86%) received a backbone of FC. Rituximab was added in 35 patients (45%) and Mitoxantrone in 37 (47%). Five patients received further maintenance treatment with rituximab and four proceeded to high dose chemotherapy with autologous HSCT (n=2) or allogeneic HSCT (n=2). Non-FBR patients were treated with CVP (71%, n=27) or CHOP (29%, n=11), associated with rituximab in 30 cases (79%). Fourteen received rituximab maintenance. Only one patient proceeded to autologous HSCT. Median number of cycles was 4.5 in FBR and 7 in non-FBR patients, as expected per intent-to-treat.
Response rates were high (overall response rate (ORR)/complete remission (CR) 87%/38% in FBR and 81%/42% in non-FBR). With a median follow-up of 56.6 months for FBR and 25.2 months for non- FBR, 2-year PFS was 50% and 60%, respectively. 2-year OS were also identical, 75% in both groups; median TTNT was 30.5 months for FBR and 33.3 months for non-FBR.
Toxicity and supportive care
Chemotherapy delay rates (26% for FBR; 24% for non-FBR) and premature treatment discontinuation (28% for FBR; 26% for non- FBR) were similar between the two groups and mainly due to TRT. These were documented in 74% of FBR and 68% of non-FBR patients. No grade 5 toxicities occurred.
Hematological toxicity predominated, with grade 3/4 events similarly reported in the two groups (69% in FBR and 50% in non- FBR patients). The most common toxicity in the FBR group was lymphopenia (Figure 2A). Neutropenia occurred in 49% FBR and 34% non-FBR. There were 4 cases of hemolytic anemia associated to fludarabine. The hematological recovery to grade <3 occurred within 1 month for all non-FBR patients and no persistent cytopenias were seen in this group. In contrast, FBR patients experiencing hematological toxicity had a median time to neutrophil, platelet and hemoglobin recovery of 0.5, 3 and 1.5 months, respectively. Three patients never recovered 1000 neutrophils/μL (follow-up 3-42 months) and one had persistent grade 3/4 thrombocytopenia (follow-up 7 months). The association of rituximab to FBR resulted in higher rates of grade ≥3 neutropenia (60% vs. 40% without rituximab, p=0.07). In contrast, advanced age did not increase grade 3-4 hematological toxicity in this group (p=0.326, Figure 3).
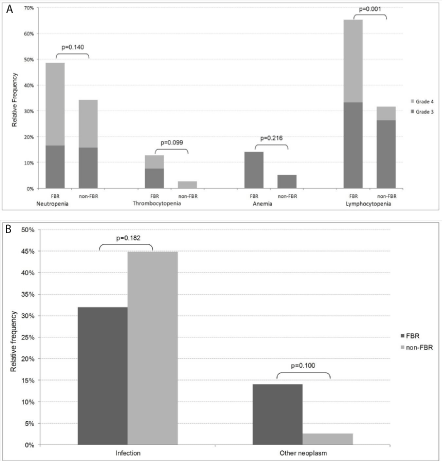
Figure 2: Treatment related toxicities in fludarabine based regimens (FBR) and non-FBR (A) Grade 3 and 4 hematological toxicity (B) Grade ≥2 infections and
second neoplasm.
Grade ≥2 infections were reported in 32% of FBR-receiving patients and 45% in the non-FBR group (p=0.182, Figure 2B). Age over 60 was associated with a significantly higher incidence of grade ≥2 infections in the FBR group (46% versus 18% if age <60, p=0.008, Figure 3).
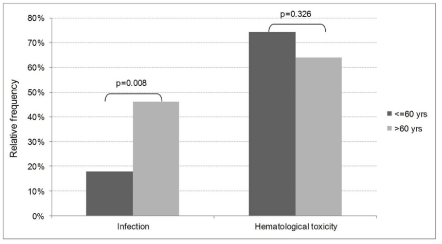
Figure 3: Infection (grade ≥2) and hematological toxicity (grade ≥3) in fludarabine based regimens (FBR) according to age.
Growth factor use, transfusion requirements, short-term emergency room admissions during chemotherapy and prolonged hospitalization for toxicity were similar in FBR and non-FBR patients (Table 2). 74% of FBR patients received anti-infective prophylaxis as per local recommendations, with oral acyclovir (74%) and oral co- trimoxazole (56%). Antifungals and other antibiotics were rarely administered. Only 21% non-FBR patients received anti-infective prophylaxis. Oral or intravenous antibiotherapy was required at least once during treatment in 54% of FBR and 47% in non-FBR patients.
FBR (n=78)
Non-FBR (n=38)
Admissions for toxicity
Patients with ≥1 admission, n (%)
Duration, days
Median [min-max]
Mean (SD)
19 (24%)
10 [3-47]
13.5 (9.9)
10 (26%)
14 [2-28]
12.9 (11.4)
Emergency room
Patients with ≥1 occurrence, n (%)
Number of episodes
Median [min-max]
Mean (SD)
37 (47%)
1 [1-5]
1.7 (1.2)
15 (40%)
1 [1-4]
1.8 (1.1)
Antibiotic treatment, n (%)
Yes
Oral
Parenteral
42 (54%)
26 (33%)
16 (21%)
18 (47%)
11 (29%)
7 (18%)
Transfusions, n (%)
Yes
Number of cycles with transfusion
Median [min-max]
Mean (SD)
16 (21%)
1 [1-3]
1.3 (0.7)
4 (11%)
1 [1-1]
1 (0)
Growth factors, n (%)
Yes
Number of cycles with growth factors
Median [min-max]
Mean (SD)
32 (41%)
2 [1-8]
2.5 (1.8)
15 (40%)
5 [1-8]
4.5 (2.3)
Table 2: Supportive care associated with treatment related toxicity.
Subsequent lymphoma treatment was needed in half of the patients (51% in FBR; 47% in non-FBR). At the start of treatment, 11/40 FBR patients had grade ≥2 cytopenias (9 thrombocytopenia, 1 neutropenia, 1 both) that were attributed to delayed hematological toxicity in 4 and bone marrow (BM) infiltration in 3; 4patients had no BM evaluation. In contrast, all non-FBR patients had normal hematological counts at the start of next chemotherapy. Also, grade ≥3 hematological toxicity and toxicity-related treatment discontinuation were more frequent during subsequent treatment in FBR than non- FBR patients (40% vs. 17%, p=0.079 and 13% vs. 6%, p=0.655).
Secondary malignancies were almost exclusively seen in FBR patients. Four had myelodysplasia and 7 other secondary neoplasms (2 breasts, 2 skins, 1 lung and 1 head and neck carcinoma and 1 GIST). Only one non-FBR patient developed a high-grade sarcoma.
Discussion
In this series comprising R/R FL patients treated with FBR between 1996 and 2014, we confirmed the efficacy of these regimens and the high incidence of hematological and other toxicities (including secondary malignancies), with detrimental impact on subsequent therapies. Short-term toxicities and supportive care requirements in non-FBR patients were also high but these results need to be interpreted with caution given the important differences between the two groups. In fact, non-FBR patients were older and had a higher incidence of kidney disease. Non-FBR patients also had longer responses to first line. This agrees with our policy of repeating alkylating-containing regimens for patients with long responses or for those considered unfit for FBR due to age and specific co-morbidities.
We found that patients older than 60 years-old receiving FBR had a significantly higher incidence of grade ≥2 infections. Increasing age has been previously associated with excessive fludarabine toxicity in lymphomas [20,21], including CLL, where toxicity over 65 years results in the loss of survival benefit seen in younger groups [10,11].
Although acute myelosuppression was similar between groups, FBR resulted in prolonged recovery times and in some cases persistent cytopenias, as previously described [12]. The tolerability to subsequent chemotherapy was also compromised with high incidence of grade ≥3 hematological toxicity and premature discontinuation.
In contrast to what has been reported after first line CLL treatment [10], in our series the combination of rituximab with FBR was only marginally associated with an increased incidence of grade 3/4 neutropenia. This is in agreement with results from a randomized trial comparing FCM to R-FCM [5], where the incidence of grade 3/4 hematological toxicity was not influenced by rituximab.
Late toxicity of FBR should also be taken into account when deciding treatment, given the expected long-term survival of FL. With a median follow up of approximately 5 years, we confirmed a high incidence (14%) of secondary malignancies. There was only one secondary tumor reported in the non-FBR group, but caution should be taken in comparing these results, as the duration of follow-up was much shorter in this group.
This association was previously reported by Sacchi et al. [9], where indolent lymphoma patients treated with FBR had a higher incidence of secondary tumors compared to other treatments. Carney et al. [8] also reported a 10.8% incidence of secondary myelodysplasia/ acute myeloid leukemia following FBR. Recently, in the FOLL05 trial [6], the incidence of secondary malignancies in patients receiving R-FM was superior to R-CHOP/R-CVP (8% versus 3% and 2.4%, respectively), and secondary acute myeloid leukemia was only seen after fludarabine.
The retrospective, single-center design and low patient numbers are limitations of this analysis. Nevertheless these results reflect the real life setting of R/R FL treatment. Given the lack of randomized trials comparing different regimens, the choice of treatment is often guided by data derived from phase 2 trials combined with the individual clinical experience and availability of different drugs. Attempts have been made to identify subpopulations at high risk for fludarabine toxicity and a predictive model for infectious complications has been proposed [20] but not widely validated. As there are no established predictors for toxicity, the subjective evaluation of individual patients often remains the determining factor in selecting fludarabine for the treatment of relapsed FL.
In conclusion, although FBR were associated with adequate disease control in relapsed FL, toxicity was frequent and impacted on further therapies. These results are consistent with previous reports in other lymphoma types and should be taken into account for decisionmaking. With the advent of effective novel agents targeting recently identified biological mechanisms of disease [22-25], the therapeutic choices in tumors as indolent as FL requires a careful balance between efficacy, toxicity, cost and feasibility of subsequent therapies.
References
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissue. 2008; 439.
- Dreyling M, Ghielmini M, Marcus R, Salles G, Vitolo U, Ladetto M. Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol [Internet]. 2014; 25: 76–82.
- Marcus R, Imrie K, Solal-Celigny P, Catalano JV, Dmoszynska A, Raposo JC, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol. 2008; 26: 4579-4586.
- Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005; 106: 3725-3732.
- Forstpointner R, Dreyling M, Repp R, Hermann S, Hänel A, Pott C, et al. cyclophosphamide, mitoxantrone ( FCM ) significantly increases the patients with relapsed and refractory follicular and mantle cell lymphomas?: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group The addition of rituxim. 2013; 104: 3064–3071.
- Federico M, Luminari S, Dondi A, Tucci A, Vitolo U, Rigacci L, et al. R-CVP versus R-CHOP versus R-FM for the initial treatment of patients with advanced-stage follicular lymphoma: Results of the FOLL05 trial conducted by the fondazione italiana linfomi. J Clin Oncol. 2013; 31: 1506–1513.
- Rummel MJ, Niederle N, Maschmeyer G, Banat GA, von Grünhagen U, Losem C, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013; 381: 1203-1210.
- Carney DA, Westerman DA, Tam CS, Milner A, Prince HM, Kenealy M, et al. Therapy-related myelodysplastic syndrome and acute myeloid leukemia following fludarabine combination chemotherapy. Leukemia. 2010; 24: 2056-2062.
- Sacchi S, Marcheselli L, Bari A, Marcheselli R, Pozzi S, Luminari S, et al. Secondary malignancies after treatment for indolent non-Hodgkin's lymphoma: a 16-year follow-up study. Haematologica. 2008; 93: 398-404.
- Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010; 376: 1164-1174.
- Eichhorst B, Fink AM, Busch R, Kovacs G, Maurer C, Lange E, et al. Frontline Chemoimmunotherapy with Fludarabine (F), Cyclophosphamide (C), and Rituximab (R) (FCR) Shows Superior Efficacy in Comparison to Bendamustine (B) and Rituximab (BR) in Previously Untreated and Physically Fit Patients (pts) with Advanced Chronic …. Blood. 2014; 124: 19.
- Kluin-Nelemans HC, Hoster E, Hermine O, Walewski J, Trneny M, Geisler CH, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med. 2012; 367: 520-531.
- Morschhauser F, Mounier N, Sebban C, Brice P, Solal-Celigny P, Tilly H, et al. Efficacy and safety of the combination of rituximab, fludarabine, and mitoxantrone for rituximab-naive, recurrent/refractory follicular non-Hodgkin lymphoma with high tumor burden: A multicenter phase 2 trial by the Groupe d’Etude des Lymphomes de l'Adult. Cancer. 2010; 116: 4299–4308.
- Sacchi S, Pozzi S, Marcheselli R, Federico M, Tucci A, Merli F, et al. Rituximab in combination with fludarabine and cyclophosphamide in the treatment of patients with recurrent follicular lymphoma. Cancer. 2007; 110: 121-128.
- Tomás JF, Montalbán C, De Sevilla AF, Martínez-López J, Díaz N, Canales M, et al. Frontline treatment of follicular lymphoma with fludarabine, cyclophosphamide, and rituximab followed by rituximab maintenance: toxicities overcome its high antilymphoma effect. Results from a Spanish Cooperative Trial (LNHF-03). Leukemia & lymphoma. 2011; 52: 409-416.
- Robinson KS, Williams ME, van der Jagt RH, Cohen P, Herst JA, Tulpule A, et al. Phase II multicenter study of bendamustine plus rituximab in patients with relapsed indolent B-cell and mantle cell non-Hodgkin's lymphoma. J Clin Oncol. 2008; 26: 4473-4479.
- Services H. Common Terminology Criteria for Adverse Events (CTCAE). 2010; 2009: 0–71.
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007; 25: 579-586.
- Dean CB, Nielsen JD. Generalized linear mixed models: a review and some extensions. Lifetime Data Anal. 2007; 13: 497-512.
- Tam CS, Wolf MM, Januszewicz EH, Grigg AP, Prince HM, Westerman D, et al. A new model for predicting infectious complications during fludarabine-based combination chemotherapy among patients with indolent lymphoid malignancies. Cancer. 2004; 101: 2042-2049.
- Polizzotto MN, Tam CS, Milner A, Januszewicz EH, Prince HM, Westerman D, et al. The influence of increasing age on the deliverability and toxicity of fludarabine-based combination chemotherapy regimens in patients with indolent lymphoproliferative disorders. Cancer. 2006; 107: 773-780.
- Sehn LH, Goy A, Offner FC, Martinelli G, Caballero MD, Gadeberg O, et al. Randomized Phase II Trial Comparing Obinutuzumab (GA101) With Rituximab in Patients With Relapsed CD20+ Indolent B-Cell Non-Hodgkin Lymphoma: Final Analysis of the GAUSS Study. J Clin Oncol. 2015.
- Westin JR, Chu F, Zhang M, Fayad LE, Kwak LW, Fowler N, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol. Elsevier; 2015; 15: 69–77.
- Tuscano JM, Dutia M, Chee K, Brunson A, Reed-Pease C, Abedi M, et al. Lenalidomide plus rituximab can produce durable clinical responses in patients with relapsed or refractory, indolent non-Hodgkin lymphoma. Br J Haematol. 2014; 165: 375-381.
- Flinn IW, Kahl BS, Leonard JP, Furman RR, Brown JR, Byrd JC, et al. Idelalisib, a selective inhibitor of phosphatidylinositol 3-kinase-δ, as therapy for previously treated indolent non-Hodgkin lymphoma. Blood. 2014; 123: 3406-3413.